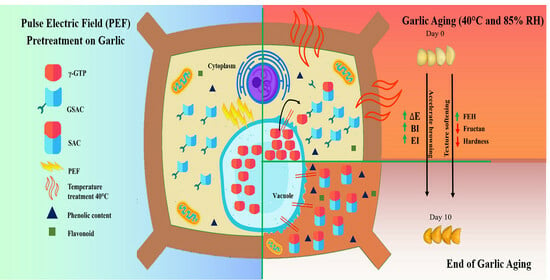
Promoting the Aging Process and Enhancing the Production of Antioxidant Components of Garlic through Pulsed Electric Field Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. Appearance and Browning Degree
2.3. Texture Measurement
2.4. Fructan Content
2.5. FEH Activity
2.6. TPC and TFC
2.6.1. Extraction of Polyphenols
2.6.2. Determination of TPC
2.6.3. Determination of TFC
2.7. Electrolyte Leakage (EI)
2.8. γ-GTP Activity
2.9. SAC Content
2.10. Statistical Analysis
3. Results and Discussion
3.1. Effects of PEF Pretreatment on Garlic’s Appearance, Browning Index (BI), and Relative Leakage (EI) during Aging
3.2. Effects of PEF Pretreatments on TPC and TFC of Garlic during Aging
3.3. Effects of PEF Pretreatments on Texture and Fructan Content of Garlic during Aging
3.4. Effects of PEF Pretreatments on SAC Content of Garlic during Aging
3.5. Analysis of the Relationship between PEF Pretreatments and Composition Changes during Garlic Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.-C.; Jang, H.-D.; Lin, W.-D. Biochemical properties of black garlic aged under different temperatures of commercial rice wine extracts in Taiwan. J. Food Meas. Charact. 2021, 15, 509–518. [Google Scholar] [CrossRef]
- Chan, K.-H.; Chang, C.-K.; Gavahian, M.; Yudhistira, B.; Santoso, S.P.; Cheng, K.-C.; Hsieh, C.-W. The Impact of Different Pretreatment Processes (Freezing, Ultrasound and High Pressure) on the Sensory and Functional Properties of Black Garlic (Allium sativum L.). Molecules 2022, 27, 6992. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.J.; Malunga, L.N.; Eskin, M.; Eck, P.; Thandapilly, S.J.; Thiyam-Hollander, U. Valorization of Heat-Treated Brewers’ Spent Grain Through the Identification of Bioactive Phenolics by UPLC-PDA and Evaluation of Their Antioxidant Activities. Front. Nutr. 2021, 8, 634519. [Google Scholar] [CrossRef] [PubMed]
- Baron, G.; Ferrario, G.; Marinello, C.; Carini, M.; Morazzoni, P.; Aldini, G. Effect of Extraction Solvent and Temperature on Polyphenol Profiles, Antioxidant and Anti-Inflammatory Effects of Red Grape Skin By-Product. Molecules 2021, 26, 5454. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.E.; Baghdadi, A.; Ahmad, I. Changes in Phytochemical Synthesis, Chalcone Synthase Activity and Pharmaceutical Qualities of Sabah Snake Grass (Clinacanthus nutans L.) in Relation to Plant Age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef]
- Xu, X.; Miao, Y.; Chen, J.Y.; Zhang, Q.; Wang, J. Effective production of S-allyl-L-cysteine through a homogeneous reaction with activated endogenous γ-glutamyltranspeptidase in garlic (Allium sativum). J. Food Sci. Technol. 2015, 52, 1724–1729. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-A.; Tsai, J.-C.; Cheng, K.-C.; Liu, K.-F.; Chang, C.-K.; Hsieh, C.-W. Extracts of black garlic exhibits gastrointestinal motility effect. Food Res. Int. 2018, 107, 102–109. [Google Scholar] [CrossRef]
- Park, J.-M.; Han, Y.-M.; Kangwan, N.; Lee, S.-Y.; Jung, M.-K.; Kim, E.-H.; Hahm, K.-B. S-allyl cysteine alleviates nonsteroidal anti-inflammatory drug-induced gastric mucosal damages by increasing cyclooxygenase-2 inhibition, heme oxygenase-1 induction, and histone deacetylation inhibition. J. Gastroenterol. Hepatol. 2014, 29, 80–92. [Google Scholar] [CrossRef]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. An analysis of the changes on intermediate products during the thermal processing of black garlic. Food Chem. 2018, 239, 56–61. [Google Scholar] [CrossRef]
- Qiu, Z.; Qiao, Y.; Zhang, B.; Sun-Waterhouse, D.; Zheng, Z. Bioactive polysaccharides and oligosaccharides from garlic (Allium sativum L.): Production, physicochemical and biological properties, and structure–function relationships. Compr. Rev. Food Sci. Food Saf. 2022, 21, 3033–3095. [Google Scholar] [CrossRef]
- Rivera-Huerta, M.; Lizárraga-Grimes, V.L.; Castro-Torres, I.G.; Tinoco-Méndez, M.; Macías-Rosales, L.; Sánchez-Bartéz, F.; Tapia-Pérez, G.G.; Romero-Romero, L.; Gracia-Mora, M.I. Functional Effects of Prebiotic Fructans in Colon Cancer and Calcium Metabolism in Animal Models. BioMed Res. Int. 2017, 2017, 9758982. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition analysis and antioxidant properties of black garlic extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Chua, L.S.; Abdullah, F.I.; Lim, S.H. Physiochemical changes and nutritional content of black garlic during fermentation. Appl. Food Res. 2022, 2, 100216. [Google Scholar] [CrossRef]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Effects of thermal treatment on polysaccharide degradation during black garlic processing. LWT 2018, 95, 223–229. [Google Scholar] [CrossRef]
- Aoudeh, E.; Oz, E.; Kelebek, H.; Brennan, C.; Ahmad, N.; Elobeid, T.; Oz, F. Black garlic production: The influence of ageing temperature and duration on some physicochemical and antioxidant properties, and sugar content. Int. J. Food Sci. Technol. 2023, 58, 3580–3590. [Google Scholar] [CrossRef]
- Cheong, K.L.; Yan, F.; Huang, X. Enymologic Characterization of Garlic Fructan Exohydrolase. J. Food Biochem. 2012, 36, 248–253. [Google Scholar] [CrossRef]
- Bae, S.E.; Cho, S.Y.; Won, Y.D.; Lee, S.H.; Park, H.J. Changes in S-allyl cysteine contents and physicochemical properties of black garlic during heat treatment. LWT Food Sci. Technol. 2014, 55, 397–402. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Lee, C.-H.; Chen, Y.-A.; Wu, J.-T.; Tsai, M.-S.; Cheng, K.-C.; Hsieh, C.-W. Preparation of S-allyl cysteine-enriched garlic by two-step processing. LWT 2020, 124, 109130. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chen, Y.-A.; Lee, C.-H.; Wu, J.-T.; Cheng, K.-C.; Hsieh, C.-W. A strategy for promoting γ-glutamyltransferase activity and enzymatic synthesis of S-allyl-(L)-cysteine in aged garlic via high hydrostatic pressure pretreatments. Food Chem. 2020, 316, 126347. [Google Scholar] [CrossRef]
- Masztalerz, K.; Dróżdż, T.; Nowicka, P.; Wojdyło, A.; Kiełbasa, P.; Lech, K. The Effect of Nonthermal Pretreatment on the Drying Kinetics and Quality of Black Garlic. Molecules 2023, 28, 962. [Google Scholar] [CrossRef] [PubMed]
- Naliyadhara, N.; Kumar, A.; Girisa, S.; Daimary, U.D.; Hegde, M.; Kunnumakkara, A.B. Pulsed electric field (PEF): Avant-garde extraction escalation technology in food industry. Trends Food Sci. Technol. 2022, 122, 238–255. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.-A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the application of pulsed electric fields (PEF) technology for food processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, B.; Punthi, F.; Gavahian, M.; Chang, C.-K.; Hazeena, S.H.; Hou, C.-Y.; Hsieh, C.-W. Nonthermal technologies to maintain food quality and carbon footprint minimization in food processing: A review. Trends Food Sci. Technol. 2023, 141, 104205. [Google Scholar] [CrossRef]
- Gavahian, M.; Pallares, N.; Al Khawli, F.; Ferrer, E.; Barba, F.J. Recent advances in the application of innovative food processing technologies for mycotoxins and pesticide reduction in foods. Trends Food Sci. Technol. 2020, 106, 209–218. [Google Scholar] [CrossRef]
- Wiktor, A.; Lammerskitten, A.; Barba, F.; Michalski, M.; Toepfl, S.; Parniakov, O. 1.15—Drying Processes Assisted by PEF for Plant-Based Materials. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 271–280. [Google Scholar]
- Punthi, F.; Yudhistira, B.; Gavahian, M.; Chang, C.-K.; Cheng, K.-C.; Hou, C.-Y.; Hsieh, C.-W. Pulsed electric field-assisted drying: A review of its underlying mechanisms, applications, and role in fresh produce plant-based food preservation. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5109–5130. [Google Scholar] [CrossRef]
- Chang, C.-K.; Lung, C.-T.; Gavahian, M.; Yudhistira, B.; Chen, M.-H.; Santoso, S.P.; Hsieh, C.-W. Effect of pulsed electric field-assisted thawing on the gelling properties of pekin duck meat myofibrillar protein. J. Food Eng. 2023, 350, 111482. [Google Scholar] [CrossRef]
- Lung, C.-T.; Chang, C.-K.; Cheng, F.-C.; Hou, C.-Y.; Chen, M.-H.; Santoso, S.P.; Yudhistira, B.; Hsieh, C.-W. Effects of pulsed electric field-assisted thawing on the characteristics and quality of Pekin duck meat. Food Chem. 2022, 390, 133137. [Google Scholar] [CrossRef]
- Subhashree, S.N.; Sunoj, S.; Xue, J.; Bora, G.C. Quantification of browning in apples using colour and textural features by image analysis. Food Qual. Saf. 2017, 1, 221–226. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, Y.-T.; Kim, M.; Noh, B.-S.; Choi, W.-S. Effect of high hydrostatic pressure (HHP) treatment on flavor, physicochemical properties and biological functionalities of garlic. LWT Food Sci. Technol. 2014, 55, 347–354. [Google Scholar] [CrossRef]
- Krivorotova, T.; Sereikaite, J. Determination of fructan exohydrolase activity in the crude extracts of plants. Electron. J. Biotechnol. 2014, 17, 329–333. [Google Scholar] [CrossRef]
- ISO 14502-1: 2005; Determination of Substances Characteristic of Green and Black Tea—Part 1: Content of Total Polyphenols in Tea-Colorimetric Method using Folin-Ciocalteu Reagent. International Organization for Standardization Switzerland: Geneva, Switzerland, 2005; p. 10.
- Kaya, C.; Kirnak, H.; Higgs, D.; Saltali, K. Supplementary calcium enhances plant growth and fruit yield in strawberry cultivars grown at high (NaCl) salinity. Sci. Hortic. 2002, 93, 65–74. [Google Scholar] [CrossRef]
- Li, N.; Lu, X.; Pei, H.; Qiao, X. Effect of Freezing Pretreatment on the Processing Time and Quality of Black Garlic. J. Food Process Eng. 2015, 38, 329–335. [Google Scholar] [CrossRef]
- Lin, Z.-R.; Zeng, X.-A.; Yu, S.-J.; Sun, D.-W. Enhancement of Ethanol–Acetic Acid Esterification Under Room Temperature and Non-catalytic Condition via Pulsed Electric Field Application. Food Bioprocess Technol. 2012, 5, 2637–2645. [Google Scholar] [CrossRef]
- Liu, T.; Burritt, D.J.; Oey, I. Understanding the effect of Pulsed Electric Fields on multilayered solid plant foods: Bunching onions (Allium fistulosum) as a model system. Food Res. Int. 2019, 120, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Vorobiev, E.; Lebovka, N. Pulsed-Electric-Fields-Induced Effects in Plant Tissues: Fundamental Aspects and Perspectives of Applications. In Electrotechnologies for Extraction from Food Plants and Biomaterials; Springer: New York, NY, USA, 2008; pp. 39–81. [Google Scholar]
- Won, Y.-C.; Min, S.C.; Lee, D.-U. Accelerated Drying and Improved Color Properties of Red Pepper by Pretreatment of Pulsed Electric Fields. Dry. Technol. 2015, 33, 926–932. [Google Scholar] [CrossRef]
- Yang, N.; Huang, K.; Lyu, C.; Wang, J. Pulsed electric field technology in the manufacturing processes of wine, beer, and rice wine: A review. Food Control 2016, 61, 28–38. [Google Scholar] [CrossRef]
- Niu, D.; Ren, E.-F.; Li, J.; Zeng, X.-A.; Li, S.-L. Effects of pulsed electric field-assisted treatment on the extraction, antioxidant activity and structure of naringin. Sep. Purif. Technol. 2021, 265, 118480. [Google Scholar] [CrossRef]
- Marsellés-Fontanet, Á.R.; Martín-Belloso, O. Optimization and validation of PEF processing conditions to inactivate oxidative enzymes of grape juice. J. Food Eng. 2007, 83, 452–462. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Z.; Aila, R.; Hou, Y.; Carne, A.; Bekhit, A.E.-D.A. Effect of pulsed electric fields (PEF) on physico-chemical properties, β-carotene and antioxidant activity of air-dried apricots. Food Chem. 2019, 291, 253–262. [Google Scholar] [CrossRef]
- González-Ramírez, P.J.; Pascual-Mathey, L.I.; García-Rodríguez, R.V.; Jiménez, M.; Beristain, C.I.; Sanchez-Medina, A.; Pascual-Pineda, L.A. Effect of relative humidity on the metabolite profiles, antioxidant activity and sensory acceptance of black garlic processing. Food Biosci. 2022, 48, 101827. [Google Scholar] [CrossRef]
- Lu, R.; Hou, W.; Zhao, W.; Zhao, S.; Wang, P.; Zhao, X.; Wang, D. New insights on low-temperature storage regulating garlic greening and the accumulation of pigment precursors via glutathione metabolism and energy cycles. Food Chem. 2023, 417, 135848. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Sun, L.; Chen, M.; Wang, J. The Comparison of the Contents of Sugar, Amadori, and Heyns Compounds in Fresh and Black Garlic. J. Food Sci. 2016, 81, C1662–C1668. [Google Scholar] [CrossRef]
- Li, F.; Cao, J.; Liu, Q.; Hu, X.; Liao, X.; Zhang, Y. Acceleration of the Maillard reaction and achievement of product quality by high pressure pretreatment during black garlic processing. Food Chem. 2020, 318, 126517. [Google Scholar] [CrossRef]
- Galindo, F.G.; Dejmek, P.; Lundgren, K.; Rasmusson, A.G.; Vicente, A.; Moritz, T. Metabolomic evaluation of pulsed electric field-induced stress on potato tissue. Planta 2009, 230, 469–479. [Google Scholar] [CrossRef]
- Van den Ende, W.; Michiels, A.; De Roover, J.; Verhaert, P.; Van Laere, A. Cloning and functional analysis of chicory root fructan1-exohydrolase I (1-FEH I): A vacuolar enzyme derivedfrom a cell-wall invertase ancestor? Mass fingerprint of the 1-FEH I enzyme. Plant J. 2000, 24, 447–456. [Google Scholar] [CrossRef]
- Ueno, K.; Yokoshima, S.; Sasajima, Y.; Ishiguro, Y.; Yoshida, M.; Shiomi, N.; Onodera, S. Two Fructan 1-Exohydrolase Isoforms Hydrolyze Fructans in Edible Burdock (Arctium lappa L.) during Storage at a Low Temperature. J. Appl. Glycosci. 2015, 62, 65–72. [Google Scholar] [CrossRef]
- Bian, H.; Zhou, Q.; Du, Z.; Zhang, G.; Han, R.; Chen, L.; Tian, J.; Li, Y. Integrated Transcriptomics and Metabolomics Analysis of the Fructan Metabolism Response to Low-Temperature Stress in Garlic. Genes 2023, 14, 1290. [Google Scholar] [CrossRef] [PubMed]
- Holland, V.; Koller, S.; Lukas, S.; Brüggemann, W. Drought- and frost-induced accumulation of soluble carbohydrates during accelerated senescence in Quercus pubescens. Trees 2016, 30, 215–226. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, H.; Zhang, G.; Wang, J.; Tian, J. Gene Co-Expression Analysis Reveals the Transcriptome Changes and Hub Genes of Fructan Metabolism in Garlic under Drought Stress. Plants 2023, 12, 3357. [Google Scholar] [CrossRef]
- van Arkel, J.; Sévenier, R.; Hakkert, J.C.; Bouwmeester, H.J.; Koops, A.J.; van der Meer, I.M. Tailor-made fructan synthesis in plants: A review. Carbohydr. Polym. 2013, 93, 48–56. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, Z.; Liu, L.; Mu, D.; Wu, J.; Li, X.; Wu, X. Changes of Physicochemical Properties in Black Garlic during Fermentation. Fermentation 2022, 8, 653. [Google Scholar] [CrossRef]
- Karnjanapratum, S.; Supapvanich, S.; Kaewthong, P.; Takeungwongtrakul, S. Impact of steaming pretreatment process on characteristics and antioxidant activities of black garlic (Allium sativum L.). J. Food Sci. Technol. 2021, 58, 1869–1876. [Google Scholar] [CrossRef]
- Moens, L.G.; Van Wambeke, J.; De Laet, E.; Van Ceunebroeck, J.-C.; Goos, P.; Van Loey, A.M.; Hendrickx, M.E.G. Effect of postharvest storage on potato (Solanum tuberosum L.) texture after pulsed electric field and thermal treatments. Innov. Food Sci. Emerg. Technol. 2021, 74, 102826. [Google Scholar] [CrossRef]
- Kang, M.-J.; Kang, J.-R.; Woo, M.S.; Kang, D.; Shin, J.-H. Alterations in the Physicochemical Properties and Antioxidant Activity during Aging of Stored Raw Garlic. Foods 2022, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Yudhistira, B.; Punthi, F.; Lin, J.-A.; Sulaimana, A.S.; Chang, C.-K.; Hsieh, C.-W. S-Allyl cysteine in garlic (Allium sativum): Formation, biofunction, and resistance to food processing for value-added product development. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2665–2687. [Google Scholar] [CrossRef] [PubMed]
- Kodera, Y.; Kurita, M.; Nakamoto, M.; Matsutomo, T. Chemistry of aged garlic: Diversity of constituents in aged garlic extract and their production mechanisms via the combination of chemical and enzymatic reactions (Review). Exp. Ther. Med. 2020, 19, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Poojary, M.M.; Roohinejad, S.; Koubaa, M.; Barba, F.J.; Passamonti, P.; Režek Jambrak, A.; Oey, I.; Greiner, R. Impact of Pulsed Electric Fields on Enzymes. In Handbook of Electroporation; Miklavčič, D., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 2369–2389. [Google Scholar]
- Aguiló-Aguayo, I.; Odriozola-Serrano, I.; Quintão-Teixeira, L.J.; Martín-Belloso, O. Inactivation of tomato juice peroxidase by high-intensity pulsed electric fields as affected by process conditions. Food Chem. 2008, 107, 949–955. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Chen, Q.; Liu, X.-H.; Chen, Z.-X. Inactivation of soybean lipoxygenase in soymilk by pulsed electric fields. Food Chem. 2008, 109, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Van Loey, A.; Verachtert, B.; Hendrickx, M. Effects of high electric field pulses on enzymes. Trends Food Sci. Technol. 2001, 12, 94–102. [Google Scholar] [CrossRef]
- Gao, C.; Tello, E.; Peterson, D.G. Identification of coffee compounds that suppress bitterness of brew. Food Chem. 2021, 350, 129225. [Google Scholar] [CrossRef] [PubMed]





| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 | PC9 | |
|---|---|---|---|---|---|---|---|---|---|
| γ-GTP | 0.951 | 0.049 | −0.255 | −0.117 | 0.111 | 0.001 | 0.030 | 0.003 | −0.037 |
| SAC | 0.948 | 0.047 | −0.271 | −0.115 | 0.107 | 0.007 | 0.016 | 0.000 | 0.037 |
| FEH | 0.257 | 0.911 | 0.299 | 0.052 | 0.097 | 0.029 | 0.042 | 0.001 | 0.001 |
| Fructan | −0.668 | −0.687 | 0.192 | 0.048 | 0.197 | 0.037 | 0.043 | 0.002 | 0.001 |
| TPC | 0.962 | −0.186 | 0.168 | 0.028 | −0.021 | −0.054 | 0.009 | 0.084 | 0.002 |
| TFC | 0.940 | −0.061 | 0.032 | 0.305 | 0.096 | −0.055 | −0.075 | −0.030 | −0.001 |
| ΔE | 0.947 | −0.155 | 0.189 | −0.154 | −0.016 | 0.111 | −0.081 | 0.001 | −0.002 |
| BI | 0.904 | −0.245 | 0.272 | −0.193 | −0.053 | −0.068 | 0.047 | −0.051 | 0.001 |
| Hardness | −0.929 | 0.193 | 0.071 | −0.263 | 0.116 | −0.075 | −0.076 | 0.010 | 0.000 |
| Eigenvalue | 6.700 | 1.470 | 0.408 | 0.256 | 0.098 | 0.031 | 0.025 | 0.011 | 0.003 |
| Variability (%) | 74.47 | 16.30 | 4.53 | 2.84 | 1.09 | 0.34 | 0.28 | 0.12 | 0.03 |
| Cumulative value (%) | 74.47 | 90.77 | 95.30 | 98.14 | 99.23 | 99.57 | 99.85 | 99.97 | 100.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-K.; Tsai, S.-Y.; Tsai, M.-S.; Tu, A.-T.; Hou, C.-Y.; Cheng, K.-C.; Zhu, W.-L.; Mulyani, R.; Hsieh, C.-W. Promoting the Aging Process and Enhancing the Production of Antioxidant Components of Garlic through Pulsed Electric Field Treatments. Antioxidants 2024, 13, 374. https://doi.org/10.3390/antiox13030374
Chang C-K, Tsai S-Y, Tsai M-S, Tu A-T, Hou C-Y, Cheng K-C, Zhu W-L, Mulyani R, Hsieh C-W. Promoting the Aging Process and Enhancing the Production of Antioxidant Components of Garlic through Pulsed Electric Field Treatments. Antioxidants. 2024; 13(3):374. https://doi.org/10.3390/antiox13030374
Chicago/Turabian StyleChang, Chao-Kai, Sheng-Yen Tsai, Ming-Shiun Tsai, An-Ting Tu, Chih-Yao Hou, Kuan-Chen Cheng, Wei-Lun Zhu, Rizka Mulyani, and Chang-Wei Hsieh. 2024. "Promoting the Aging Process and Enhancing the Production of Antioxidant Components of Garlic through Pulsed Electric Field Treatments" Antioxidants 13, no. 3: 374. https://doi.org/10.3390/antiox13030374