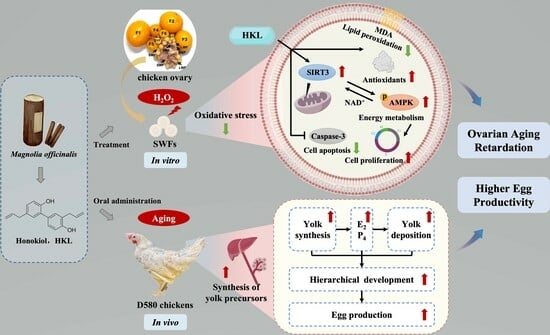
Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Materials
2.2. Culture of SWFs and Treatments of Chemicals
2.3. Animal Experiment
2.4. Staining and Morphological Observation
2.5. Biochemical Analysis
2.6. RNA Extraction and qRT-PCR
2.7. Western Blot
2.8. Statistical Analysis
3. Results
3.1. Alleviating Effect of HKL on Imbalance of Cell Proliferation and Apoptosis in the H2O2-Induced SWFs
3.2. Activation of HKL on Antioxidant Capacity and SIRT3/AMPK Pathway in the H2O2-Induced SWFs
3.3. The Suppression of SIRT3 Inhibitor on Antioxidant Effect of HKL
3.4. The Suppression of AMPK Inhibitor on Antioxidant Effect of HKL
3.5. Effects of HKL on Egg Production and Follicle Development of Aging Chickens
3.6. Effects of HKL on Serum Biochemical Indicators of Aging Chickens
3.7. Activation of SIRT3/AMPK Pathway by HKL Feeding in SWFs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Telfer, E.E.; Grosbois, J.; Odey, Y.L.; Rosario, R.; Anderson, R.A. Making a good egg: Human oocyte health, aging, and in vitro development. Physiol. Rev. 2023, 103, 2623–2677. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, A.J.; Kawamura, K.; Cheng, Y.; Fauser, B.C. Intraovarian control of early folliculogenesis. Endocr. Rev. 2015, 36, 1–24. [Google Scholar] [CrossRef]
- Zhou, S.; Zhao, A.; Wu, Y.; Bao, T.; Mi, Y.; Zhang, C. Protective effect of follicle-stimulating hormone on DNA damage of chicken follicular granulosa cells by inhibiting CHK2/p53. Cells 2022, 11, 1291. [Google Scholar] [CrossRef]
- Li, Q.; Du, X.; Liu, L.; Liu, H.; Pan, Z.; Li, Q. Upregulation of miR-146b promotes porcine ovarian granulosa cell apoptosis by attenuating CYP19A1. Domest. Anim. Endocrinol. 2021, 74, 106509. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.; Zhou, S.; Bao, T.; Mi, Y.; Zeng, W.; Li, J.; Zhang, C. Metformin prevents follicular atresia in aging laying chickens through activation of PI3K/AKT and calcium signaling pathways. Oxid. Med. Cell Longev. 2020, 2020, 3648040. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ma, Y.; Lin, X.; Zhou, S.; Mi, Y.; Zhang, C. The attenuating effect of the intraovarian bone morphogenetic protein 4 on age-related endoplasmic reticulum stress in chicken follicular cells. Oxidative Med. Cell. Longev. 2020, 2020, 4175613. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Gorgoulis, V.; Adams, P.D.; Alimonti, A.; Bennett, D.C.; Bischof, O.; Bishop, C.; Campisi, J.; Collado, M.; Evangelou, K.; Ferbeyre, G.; et al. Cellular senescence: Defining a path forward. Cell 2019, 179, 813–827. [Google Scholar] [CrossRef]
- Tanaka, Y.; Takahashi, A. Senescence-associated extracellular vesicle release plays a role in senescence-associated secretory phenotype (SASP) in age-associated diseases. J. Biochem. 2021, 169, 147–153. [Google Scholar] [CrossRef]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell 2018, 17, e12734. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef]
- Kaplon, J.; Zheng, L.; Meissl, K.; Chaneton, B.; Selivanov, V.A.; Mackay, G.; van der Burg, S.H.; Verdegaal, E.M.; Cascante, M.; Shlomi, T.; et al. A key role for mitochondrial gatekeeper pyruvate dehydrogenase in oncogene-induced senescence. Nature 2013, 498, 109–112. [Google Scholar] [CrossRef]
- Birch, J.; Barnes, P.J.; Passos, J.F. Mitochondria, telomeres and cell senescence: Implications for lung ageing and disease. Pharmacol. Ther. 2018, 183, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Gouspillou, G.; Bourdel-Marchasson, I.; Rouland, R.; Calmettes, G.; Biran, M.; Deschodt-Arsac, V.; Miraux, S.; Thiaudiere, E.; Pasdois, P.; Detaille, D.; et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging Cell 2014, 13, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Green, D.R.; Galluzzi, L.; Kroemer, G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science 2011, 333, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in ovarian aging and reproductive longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, H.; Hamatani, T.; Kamijo, S.; Iwai, M.; Kobanawa, M.; Ogawa, S.; Miyado, K.; Tanaka, M. Impact of oxidative stress on age-associated decline in oocyte developmental competence. Front. Endocrinol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Ye, X.; Liu, K.; Huang, J.; Wang, L.; Ji, G.; Liu, N.; Tang, X.; Baltz, J.M.; et al. Delay in oocyte aging in mice by the antioxidant N-acetyl-L-cysteine (NAC). Hum. Reprod. 2012, 27, 1411–1420. [Google Scholar] [CrossRef]
- Chen, Z.G.; Luo, L.L.; Xu, J.J.; Zhuang, X.L.; Kong, X.X.; Fu, Y.C. Effects of plant polyphenols on ovarian follicular reserve in aging rats. Biochem. Cell Biol. 2010, 88, 737–745. [Google Scholar] [CrossRef]
- Tarín, J.J.; Pérez-Albalá, S.; Pertusa, J.F.; Cano, A. Oral administration of pharmacological doses of vitamins C and E reduces reproductive fitness and impairs the ovarian and uterine functions of female mice. Theriogenology 2002, 57, 1539–1550. [Google Scholar] [CrossRef]
- Evans, J.R.; Lawrenson, J.G. Antioxidant vitamin and mineral supplements for slowing the progression of age-related macular degeneration. Cochrane Database Syst. Rev. 2017, 7, Cd000254. [Google Scholar] [CrossRef] [PubMed]
- Pillai, V.B.; Samant, S.; Sundaresan, N.R.; Raghuraman, H.; Kim, G.; Bonner, M.Y.; Arbiser, J.L.; Walker, D.I.; Jones, D.P.; Gius, D.; et al. Honokiol blocks and reverses cardiac hypertrophy in mice by activating mitochondrial Sirt3. Nat. Commun. 2015, 6, 6656. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br. J. Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Yao, J.; Zhou, S.; Ma, Y.; Dong, J.; Zhang, C.; Mi, Y. Naringin prevents follicular atresia by inhibiting oxidative stress in the aging chicken. Poult. Sci. 2022, 101, 101891. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Wang, J.; Zhang, H.; Wu, S.; Qi, G. Uterine microbial communities and their potential role in the regulation of epithelium cell cycle and apoptosis in aged hens. Microbiome 2023, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Liu, C.; Sun, M.; Liu, J.; Lin, P.; Wang, A.; Jin, Y. Oxidative stress induces bovine endometrial epithelial cell damage through mitochondria-dependent pathways. Animals 2022, 12, 2444. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Marzetti, E. Advancements in anti-aging treatment development. Int. J. Mol. Sci. 2023, 24, 8515. [Google Scholar] [CrossRef]
- Di Micco, R.; Krizhanovsky, V.; Baker, D.; D’Adda di Fagagna, F. Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nat. Rev. Mol. Cell Biol. 2021, 22, 75–95. [Google Scholar] [CrossRef]
- Wang, H.; Cong, X.; Qin, K.; Yan, M.; Xu, X.; Liu, M.; Xu, X.; Zhang, Y.; Gao, Q.; Cheng, S.; et al. Se-enriched Cardamine violifolia improves laying performance and regulates ovarian antioxidative function in aging laying hens. Antioxidants 2023, 12, 450. [Google Scholar] [CrossRef]
- Youle, R.J.; Strasser, A. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span—From yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Toyama, E.Q.; Herzig, S.; Courchet, J.; Lewis, T.L., Jr.; Losón, O.C.; Hellberg, K.; Young, N.P.; Chen, H.; Polleux, F.; Chan, D.C.; et al. Metabolism. AMP-activated protein kinase mediates mitochondrial fission in response to energy stress. Science 2016, 351, 275–281. [Google Scholar] [CrossRef]
- Wu, Q.J.; Zhang, T.N.; Chen, H.H.; Yu, X.F.; Lv, J.L.; Liu, Y.Y.; Liu, Y.S.; Zheng, G.; Zhao, J.Q.; Wei, Y.F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef]
- Cea, M.; Soncini, D.; Fruscione, F.; Raffaghello, L.; Garuti, A.; Emionite, L.; Moran, E.; Magnone, M.; Zoppoli, G.; Reverberi, D.; et al. Synergistic interactions between HDAC and sirtuin inhibitors in human leukemia cells. PLoS ONE 2011, 6, e22739. [Google Scholar] [CrossRef]
- Cui, X.X.; Li, X.; Dong, S.Y.; Guo, Y.J.; Liu, T.; Wu, Y.C. SIRT3 deacetylated and increased citrate synthase activity in PD model. Biochem. Biophys. Res. Commun. 2017, 484, 767–773. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, J.; Liang, L.-F.; Shen, S.-Y.; Li, W.; Chen, X.-R.; Li, B.; Zhang, Y.-Q.; Yu, J. Sirtuin 3 plays a critical role in the antidepressant- and anxiolytic-like effects of Kaempferol. Antioxidants 2022, 11, 1886. [Google Scholar] [CrossRef] [PubMed]
- Price, N.L.; Gomes, A.P.; Ling, A.J.; Duarte, F.V.; Martin-Montalvo, A.; North, B.J.; Agarwal, B.; Ye, L.; Ramadori, G.; Teodoro, J.S.; et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012, 15, 675–690. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Y.Y.; Si, H.B.; Lu, Y.R.; Shen, B. Mechanistic insights into AMPK-SIRT3 positive feedback loop-mediated chondrocyte mitochondrial quality control in osteoarthritis pathogenesis. Pharmacol. Res. 2021, 166, 105497. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, J.; Wang, Y.; Liu, H.; Sun, X.; Li, A.; Cui, P.; Yu, L.; Yan, X.; He, Z. Rapidly inhibiting the inflammatory cytokine storms and restoring cellular homeostasis to alleviate sepsis by blocking pyroptosis and mitochondrial apoptosis pathways. Adv. Sci. 2023, 10, e2207448. [Google Scholar] [CrossRef]
- Ravera, S.; Podestà, M.; Sabatini, F.; Dagnino, M.; Cilloni, D.; Fiorini, S.; Barla, A.; Frassoni, F. Discrete changes in glucose metabolism define aging. Sci. Rep. 2019, 9, 10347. [Google Scholar] [CrossRef] [PubMed]
- Moshtaghion, S.M.; Caballano-Infantes, E.; Plaza Reyes, Á.; Valdés-Sánchez, L.; Fernández, P.G.; de la Cerda, B.; Riga, M.S.; Álvarez-Dolado, M.; Peñalver, P.; Morales, J.C.; et al. Piceid octanoate protects retinal cells against oxidative damage by regulating the Sirtuin 1/poly-ADP-ribose polymerase 1 Axis In Vitro and in rd10 Mice. Antioxidants 2024, 13, 201. [Google Scholar] [CrossRef]
- Rajman, L.; Chwalek, K.; Sinclair, D.A. Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metab. 2018, 27, 529–547. [Google Scholar] [CrossRef]
- Hong, W.; Mo, F.; Zhang, Z.; Huang, M.; Wei, X. Nicotinamide mononucleotide: A promising molecule for therapy of diverse diseases by targeting NAD+ metabolism. Front. Cell Dev. Biol. 2020, 8, 246. [Google Scholar] [CrossRef]
- Trammell, S.A.; Weidemann, B.J.; Chadda, A.; Yorek, M.S.; Holmes, A.; Coppey, L.J.; Obrosov, A.; Kardon, R.H.; Yorek, M.A.; Brenner, C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 2016, 6, 26933. [Google Scholar] [CrossRef]
- Yang, Q.; Cong, L.; Wang, Y.; Luo, X.; Li, H.; Wang, H.; Zhu, J.; Dai, S.; Jin, H.; Yao, G.; et al. Increasing ovarian NAD(+) levels improve mitochondrial functions and reverse ovarian aging. Free Radic. Biol. Med. 2020, 156, 1–10. [Google Scholar] [CrossRef]
- Bertoldo, M.J.; Listijono, D.R.; Ho, W.J.; Riepsamen, A.H.; Goss, D.M.; Richani, D.; Jin, X.L.; Mahbub, S.; Campbell, J.M.; Habibalahi, A.; et al. NAD+ Repletion rescues female fertility during reproductive aging. Cell Rep. 2020, 30, 1670–1681.e1677. [Google Scholar] [CrossRef]
- Camacho-Pereira, J.; Tarragó, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A.; et al. CD38 Dictates age-related NAD decline and mitochondrial dysfunction through an SIRT3-dependent mechanism. Cell Metab. 2016, 23, 1127–1139. [Google Scholar] [CrossRef]
- Dong, L.; Teh, D.B.L.; Kennedy, B.K.; Huang, Z. Unraveling female reproductive senescence to enhance healthy longevity. Cell Res. 2023, 33, 11–29. [Google Scholar] [CrossRef]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The role of estrogen in brain and cognitive aging. Neurotherapeutics 2019, 16, 649–665. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; El-Etr, M. Progesterone and related progestins: Potential new health benefits. Climacteric 2013, 16 (Suppl. S1), 69–78. [Google Scholar] [CrossRef]
- Cui, Z.; Amevor, F.K.; Feng, Q.; Kang, X.; Song, W.; Zhu, Q.; Wang, Y.; Li, D.; Zhao, X. Sexual maturity promotes yolk precursor synthesis and follicle development in hens via liver-blood-ovary signal axis. Animals 2020, 10, 2348. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, Y.; Liu, Z.; Guo, X.; Sun, Y.; Kang, L.; Jiang, Y. Transcriptomic and proteomic analyses of ovarian follicles reveal the role of VLDLR in chicken follicle selection. BMC Genom. 2020, 21, 486. [Google Scholar] [CrossRef]









| Gene Name | Accession No. | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|---|
| PCNA | NM_204170.2 | F: GGGCGTCAACCTAAACAGCA R: AGCCAACGTATCCGCATTGT | 97 |
| CDK2 | NM_001199857.1 | F: TCCGTATCTTCCGCACGTTG R: GCTTGTTGGGATCGTAGTGC | 183 |
| CCND1 | NM_205381.1 | F: CCTCAAGAAAAGCCGGTTGC R: CTGCGGTCAGAGGAATCGTT | 86 |
| BCL-2 | NM_205339.2 | F: ATCGTCGCCTTCTTCGAGTT R: ATCCCATCCTCCGTTGTCCT | 150 |
| Bax | XM_015290060.2 | F: GGATGACAGGAAAGTACGGCA R: TCACCAGGAAGACAGCGTAT | 173 |
| Caspase-3 | NM_204725.1 | F: CAGCTGAAGGCTCCTGGTTT R: GCCACTCTGCGATTTACACG | 98 |
| SIRT1 | NM_001004767.2 | F: CCCCGCAGCCCGATAAC R: ATACGTGGTCTTGGGGTCCA | 127 |
| SIRT2 | NM_001017414.2 | F: GTGACGCCCCGTCCTATC R: TTCCGCAGCAGCTCCATATC | 104 |
| SIRT3 | NM_001199493.2 | F: AGACCCAACTACGCCCACTA R: GAGGGATCCCAGCAACTCG | 109 |
| SIRT4 | XM_025155741.3 | F: GATTTTGTGCACCAACGCCT R: GCCAGTGCAAACCTGCATAG | 75 |
| SIRT5 | NM_001276364.2 | F: TCGATGCACCAACTGTGGAA R: CTGGATCTGGAGCCCCTTTC | 86 |
| SIRT6 | NM_001039320.2 | F: ACAACAATGAGCTCTCCGGT R: GTGGATCGAAAATCTCGGGGA | 113 |
| SIRT7 | NM_001291971.1 | F: CGGCAGGAGGAGGTATGTGA R: CGGTAGTCTGGGATCGAAGC | 140 |
| ApoB | NM_001044633.2 | F: GCGGTACAAGCAGAAGGTGT R: AGACGTCGCTGGTCAGAATC | 88 |
| ApoVLDLII | NM_205483.3 | F: CACCACTGTCCCTGAAGTGC R: CATCAGGGATGACCAGCCAG | 115 |
| VTGII | NM_001031276.2 | F: CTTACCTCCTCAAGGTCCGC R: CCGGGTGAAACACGATGAGA | 116 |
| VLDLR | NM_205229.2 | F: TCTGAGATGTGGAGGATTCAAC R: GAAGAACAGCCCAAGCTCCT | 83 |
| LPL | NM_205282.2 | F: ACTGAAACTTTTTCGCCGCTG R: TTCATCTCAGCTTCGGGATCG | 127 |
| OCLN | NM_205128.1 | F: GGCGGAGGGCCACCA R: GTCGTCCACGTAGTAGGAGC | 137 |
| β-actin | NM_205518 | F: ACACCCACACCCCTGTGATGAA R: TGCTGCTGACACCTTCACCATTC | 136 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, Z.; Bai, J.; Wang, X.; Yuan, Q.; Mi, Y.; Zhang, C. Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway. Antioxidants 2024, 13, 377. https://doi.org/10.3390/antiox13030377
Chen Y, Yang Z, Bai J, Wang X, Yuan Q, Mi Y, Zhang C. Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway. Antioxidants. 2024; 13(3):377. https://doi.org/10.3390/antiox13030377
Chicago/Turabian StyleChen, Yiqiu, Zhaoyu Yang, Jingchun Bai, Xinyu Wang, Qiongyu Yuan, Yuling Mi, and Caiqiao Zhang. 2024. "Bioactive Lignan Honokiol Alleviates Ovarian Oxidative Stress in Aging Laying Chickens by Regulating SIRT3/AMPK Pathway" Antioxidants 13, no. 3: 377. https://doi.org/10.3390/antiox13030377