The Antioxidants Changes in Ornamental Flowers during Development and Senescence
Abstract
:1. Introduction
2. Antioxidants in Ornamental Flowers
| Edible flower | Total phenolics | Antioxidant capacity (DPPH) | Antioxidant capacity (FRAP) | Ref. | ||
|---|---|---|---|---|---|---|
| mg/g DW | mg/g FW | g AsA equiv./kg FW | % inhibition | μmol Fe2+/g DW | ||
| Antigonon leptopus | 72.1–177.2 | 89.4 | 282.9–619.7 | [4] | ||
| Antirrhinum majus | 3.49 | 5.06 | - | [5] | ||
| Begonia boliviensis | 4.92 | 6.80 | - | [5] | ||
| Bougainvillea glabra | 138.2 | 307.1 | [4] | |||
| Bougainvillea hybrid | 50.0 | 91.4 | 588.0 | [4] | ||
| Centaurea cyanus | 4.76 | 6.81 | - | [5] | ||
| Chrysanthemum frutescens | 2.53 | 4.24 | - | [5] | ||
| Chrysanthemum parthenium | 2.72 | 4.21 | - | [5] | ||
| Clitoria ternatea | 59.0 | - | 73.0 | [4] | ||
| Cassia siamea | 88.5 | 97.6 | 163.7 | [6] | ||
| Cosmos sulphureus | 86.8–102.5 | 87.0 | 99.9–538.6 | [4] | ||
| Dianthus caryophyllus | 5.28 | 6.96 | [5] | |||
| Fuchsia x hybrid | 3.45 | 5.20 | [5] | |||
| Helianthus annuus L. | 47.1 | [7] | ||||
| Hemerocallis spp. | 69–160 | [8] | ||||
| Impatiens walleriana | 4.85 | 6.89 | [5] | |||
| Ixora chinensis | 82.4 | [4] | ||||
| Malvaviscus arboreus | 59.0 | 31.4 | 271.3 | [4] | ||
| Nelumbo nucifera | 60.0 | 96.9 | 585.4 | [4] | ||
| Plumeria obtusa | 37.0 | 69.6 | 260.3 | [4] | ||
| Prunus mume | 150 | [4] | ||||
| Rosa odorata | 5.02 | 6.85 | [5] | |||
| Rosa hydrida | 8.5 | [9] | ||||
| Sophora viciifolia | 143.8 | 20.7 | 3160 | [10] | ||
| Tagetes erecta | 98.0–212.9 | 94.3 | 329.4–609.2 | [4] | ||
| Tagetes patula | 4.58 | 6.70 | [5] | |||
| Telosma minor | 29.0 | 34.1 | 162.6 | [4] | ||
| Tropaeolum majus | 3.31 | 5.12 | [5] | |||
| Viola x wittrockiana | 5.11 | 6.65 | [5] | |||
| Malus domestica | 100–200 | 1.2–6.5 | 83 | 4.2–6.3 | [11] | |
| Lactuca sativa | 0.2–0.3 | 74–82 | 1.8–5.3 | [12] | ||
| Species | Apigenin | Catechin | Chlorogenic acid | Kaempferol | Myricetin | Quercetin | Rutin | Ref. |
|---|---|---|---|---|---|---|---|---|
| Antigonon leptopus | 0.83 | 75.9 | 47.5 | 5.7–21.9 | [15] | |||
| Bougainvillea glabra | 8.9 | 87.2 | 61.5 | 1.3 | [15] | |||
| Bougainvillea hybrida | - | 3.54 | 5.6 | 51.5 | [4] | |||
| Cassia siamea | - | - | - | 3.21 | 4.56 | 61.9 | 64 | [4] |
| Cosmos sulphureus | 7 | 25.6 | 60 | 19.7 | [15] | |||
| Hemerocallis spp. | - | 111.5 | 7.2 | 9 | 14.6 | [16] | ||
| Ixora chinensis | 0.64 | - | - | 3.77 | 5.18 | 102.4 | 139 | [4] |
| Leucaena leucocephalade | - | - | - | 4.23 | 5.72 | 67.1 | 16.2 | [4] |
| Malvaviscus arboreus | - | - | - | 3.18 | 5.05 | 33.6 | 27.7 | [4] |
| Nelumbo nucifera | 0.62 | - | - | 3.79 | 5 | 237.8 | 23.1 | [4] |
| Plumeria obtuse | - | - | - | 3.58 | 5.06 | 193.6 | 500.3 | [4] |
| Tagetes erecta | 8.4 | 83.4 | 54.8 | 5.1 | [15] | |||
| Malus x domestica | - | 38.8–99.3 | 75.1 | 3.1 | 30.9 | 7.7–13.20 | 82 | [17,18,19,20,21] |
| Lactuca sativa | <4 | nd | 47 | 2.9 | <1 | 42.9 | nd | [22,23] |
2.1. The Changes of Antioxidant Compounds Is Often Related to the Senescence Processes
2.1.1. Ascorbic Acid (AsA)
2.1.2. Tocopherol
2.1.3. Phenolic Compounds
2.1.4. Carotenoids
3. Biochemical Pathways Involved in the Antioxidant Responses during Flower Senescence
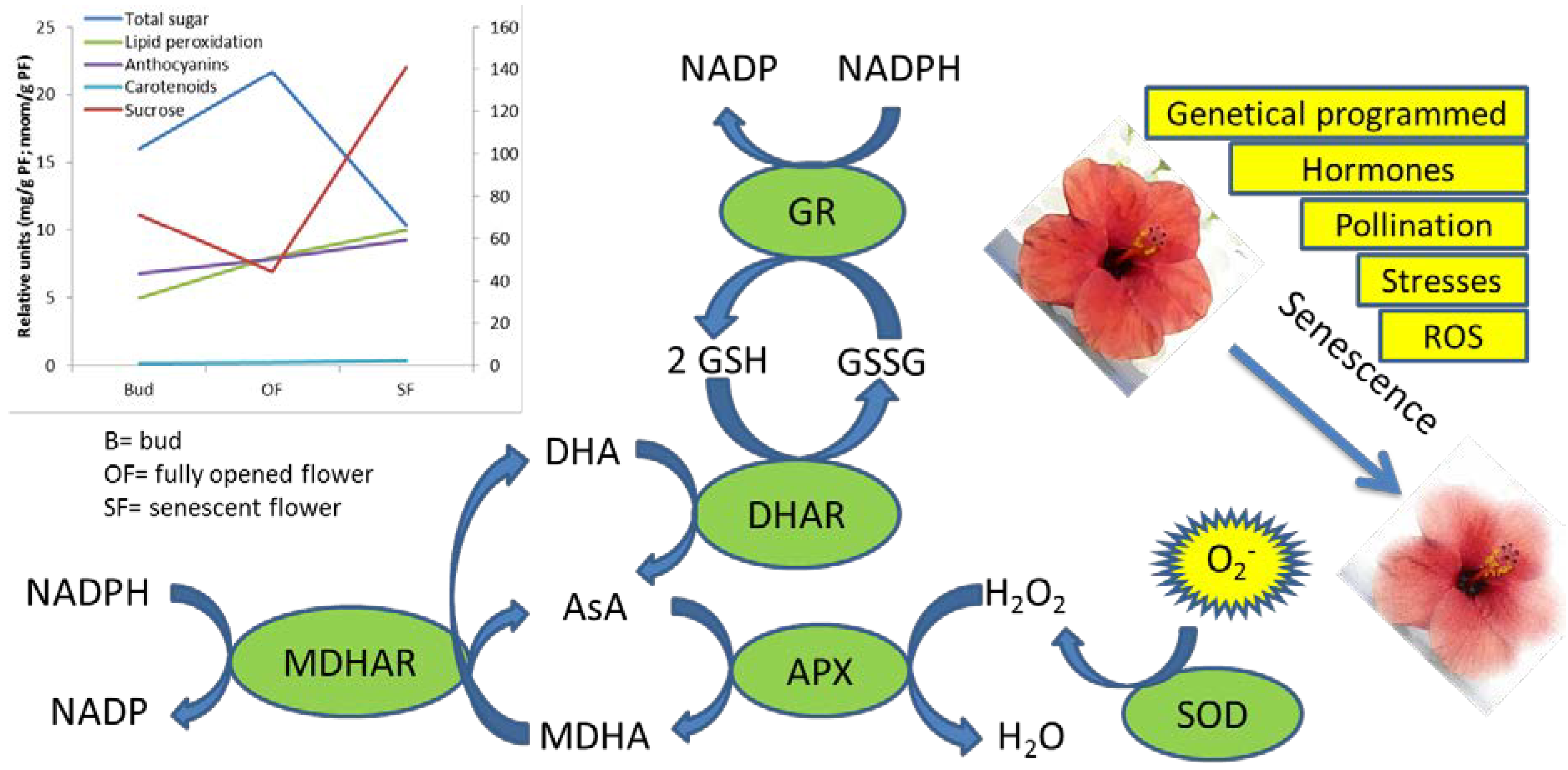
4. The Molecular Regulatory Networks of Flower Development and Senescence
4.1. Gene Regulation of Flower Development
4.2. Senescence Associated Genes (SAGs)
4.3. Transduction and Gene Regulation: Transcription Factors and Small Non Coding RNAs
4.4. Antioxidant Genes
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Kelley, K.M.; Bridget, K.; Behe, B.K.; Biernbaum, J.A.; Poff, K.L. Consumer preference for edible flower color, container size, and price. HortScience 2001, 36, 801–804. [Google Scholar]
- Mlcek, J.; Rop, O. Fresh edible flowers of ornamental plants—A new source of nutraceutical foods. Trends Food Sci. Technol. 2011, 22, 561–569. [Google Scholar] [CrossRef]
- Haslam, E. Practical Polyphenolics: From Structure to Molecular Recognition and Physiological Action; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Kaisoon, O.; Siriamornpun, S.; Weerapreeyakul, N.; Meeso, N. Phenolic compounds and antioxidant activities of edible flowers from Thailand. J. Funct. Foods 2011, 3, 88–99. [Google Scholar] [CrossRef]
- Kaur, G.; Alamb, M.S.; Jabbar, Z.; Javed, K.; Athar, M. Evaluation of antioxidant activity of Cassia siamea flowers. J. Ethnopharmacol. 2006, 108, 340–348. [Google Scholar] [CrossRef]
- Rop, O.; Mlcek, J.; Jurikova, T.; Neugebauerova, J.; Vabkova, J. Edible flowers—A new promising source of mineral elements in human nutrition. Molecules 2012, 17, 6672–6683. [Google Scholar] [CrossRef]
- Liang, Q.; Cui, J.; Li, H.; Liu, J.; Zhao, G. Florets of Sunflower (Helianthus annuus L.): Potential new sources of dietary fiber and phenolic acids. J. Agric. Food Chem. 2013, 61, 3435–3442. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential health enhancing properties of edible flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Osterc, G.; Stampar, F. Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J. Am. Soc. Hortic.Sci. 2009, 134, 491–496. [Google Scholar]
- Mao, L.C.; Pan, X.; Que, F.; Fang, X.H. Antioxidant properties of water and ethanol extracts from hot air-dried and freeze-dried daylily flowers. Eur. Food Res. Technol. 2006, 222, 236–241. [Google Scholar] [CrossRef]
- Tai, Z.; Cai, L.; Dai, L.; Dong, L.; Wang, M.; Yang, Y.; Cao, Q.; Ding, Z. Antioxidant activity and chemical constituents of edible flower of Sophora viciifolia. Food Chem. 2011, 126, 1648–1654. [Google Scholar] [CrossRef]
- Szeto, Y.T.; Tomlinson, B.; Benzie, I.F.F. Total antioxidant and ascorbic acid content of fresh fruits and vegetables: Implications for dietary planning and food preservation. Br. J. Nutr. 2002, 87, 55–59. [Google Scholar] [CrossRef]
- Alía, M.; Ramos, S.; Mateos, R.; Granado-Serrano, A.B.; Bravo, L.; Goya, L. Quercetin protects human hepatoma HepG2 against oxidative stress induced by tert-butyl hydroperoxide. Toxicol. Appl. Pharmacol. 2006, 212, 110–118. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. Review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Liu, X.; Ardo, S.; Bunning, M.; Parry, J.; Zhou, K.; Stushnoff, C.; Stoniker, F.; Yu, L.; Kendall, P. Total phenolic content and DPPH• radical scavenging activity of lettuce (Lactuca sativa L.) grown in Colorado. LWT 2007, 40, 552–557. [Google Scholar] [CrossRef]
- Der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef]
- Justesen, U.; Knuthsen, P.; Leth, T. Quantitative analysis of flavonols, flavones, and flavanones in fruits, vegetables and beverages by high-performance liquid chromatography with photo-diode array and mass spectrometric detection. J. Chromatogr. A 1998, 799, 101–110. [Google Scholar] [CrossRef]
- Dragović-Uzelac, V.; Pospišil, J.; Levaj, B.; Delonga, K.; Đaković, S. Phenolic profiles of apricot, apple and pumpkin purees in evaluation of apricot nectars and jams authenticity. Food Chem. 2005, 91, 373–383. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F. Flavonols (kaempeferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Łata, B.; Trampczynska, A.; Paczesna, J. Cultivar variation in apple peel and whole fruit phenolic composition. Sci. Hort. 2009, 121, 176–181. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Bilyk, A.; Sapers, G.M. Distribution of quercetin and kaempferol in lettuce, kale, chive, garlic chive, leek, horseradish, red radish, and red cabbage tissues. J. Agric. Food Chem. 1985, 33, 226–228. [Google Scholar] [CrossRef]
- Hertog, M.G.; Hollman, P.C.; Venema, D.P. Optimization of a quantitative HPLC determination of potentially anticarcinogenic flavonoids in vegetables and fruits. J. Agric. Food Chem. 1992, 40, 1591–1598. [Google Scholar] [CrossRef]
- Trivellini, A.; Ferrante, A.; Vernieri, P.; Serra, G. Effects of abscisic acid on ethylene biosynthesis and perception in Hibiscus rosa-sinensis L. flower development. J. Exp. Bot. 2011, 62, 5437–5452. [Google Scholar] [CrossRef]
- Davey, M.W.; van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.J.J.; Strain, J.J.; Favell, D.; Fletcher, J. Plant l-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Conklin, P.L. Recent advances in the role and biosynthesis of ascorbic acid in plants. Plant Cell Environ. 2001, 24, 383–394. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. Hopes, disillusions and more hopes from vitamin C. Cell. Mol. Life Sci. 2004, 61, 209–219. [Google Scholar] [CrossRef]
- Smirnoff, N. The function and metabolism of ascorbic acid in plants. Ann. Bot. 1996, 78, 661–669. [Google Scholar] [CrossRef]
- Fry, S.C. Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem. J. 1998, 332, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Barth, C.; Moeder, W.; Klessig, D.F.; Conklin, P.L. The timing of senescence and response to pathogens is altered in the ascorbate-deficient Arabidopsis mutant vitamin c-1. Plant Physiol. 2004, 134, 1784–1792. [Google Scholar] [CrossRef]
- Panavas, T.; Rubinstein, B. Oxidative events during programmed cell death of daylily (Hemerocallis hybrid) petals. Plant Sci. 1998, 133, 125–138. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Montaldi, E.R.; Puntarulo, S. Oxidants and antioxidants during aging of Chrysanthemum petals. Plant Sci. 1997, 129, 157–165. [Google Scholar] [CrossRef]
- Burton, G.W.; Ingold, K.U. Vitamin E: Application of the principles of physical organic chemistry to the exploration of its structure and function. Acc. Chem. Res. 1986, 19, 194–201. [Google Scholar] [CrossRef]
- Liebler, D.C. Antioxidant reactions of carotenoids. Ann. N. Y. Acad. Sci. 1993, 691, 20–31. [Google Scholar] [CrossRef]
- Munné-Bosch, S.; Alegre, L. The function of tocopherols and tocotrienols in plants. Crit. Rev. Plant Sci. 2002, 21, 31–57. [Google Scholar] [CrossRef]
- Kunert, K.J.; Ederer, M. Leaf aging and lipid peroxidation: The role of the antioxidants vitamin C and E. Physiol. Plant. 1985, 65, 85–88. [Google Scholar] [CrossRef]
- Bohm, B.A. Introduction to Flavonoids; Hardwood Academic Publishers: Reading, UK, 1998. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Weisshaar, B.; Jenkins, G.I. Phenylpropanoid biosynthesis and its regulation. Curr. Opin. Plant Biol. 1998, 1, 251–257. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Evidence for enzyme complexes in the phenylpropanoid and flavonoid pathways. Physiol. Plant. 1999, 107, 142–149. [Google Scholar] [CrossRef]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Noda, N.; Kanno, Y.; Kato, N.; Kazuma, K.; Suzuki, M. Regulation of gene expression involved in flavonol and anthocyanin biosynthesis during petal development in lisianthus (Eustoma grandiflorum). Physiol. Plant. 2004, 122, 305–313. [Google Scholar] [CrossRef]
- Kumar, N.; Bhandari, P.; Singh, B.; Gupta, A.P.; Kaul, V.K. Reversed phase-HPLC for rapid determination of polyphenols in flowers of rose species. J. Sep. Sci. 2008, 31, 262–267. [Google Scholar] [CrossRef]
- Nielsen, K.; Deroles, S.C.; Markham, K.R.; Bradley, M.J.; Podivinsky, E.; Manson, D. Antisense flavonol synthase alters copigmentation and flower color in Lisianthus. Mol. Breed. 2002, 9, 217–229. [Google Scholar] [CrossRef]
- Takahama, U.; Oniki, T. A peroxidase/phenolics/ascorbate system can scavenge hydrogen peroxide in plant cells. Physiol. Plant. 1997, 101, 845–852. [Google Scholar] [CrossRef]
- Trivellini, A.; Vernieri, P.; Ferrante, A.; Serra, G. Physiological characterization of flower senescence in long life and ephemeral hibiscus (Hibiscus rosa-sinensis L.). Acta Hortic. 2007, 755, 457–464. [Google Scholar]
- Ferrante, A.; Vernieri, P.; Tognoni, F.; Serra, G. Changes in abscisic acid and flower pigments during flower senescence of Petunia. Biol. Plant. 2006, 50, 581–585. [Google Scholar] [CrossRef]
- Chadwick, A.V.; Hogan, N.M.; Arditti, J. Postpollination phenomena in orchid flowers. IX. Induction and inhibition of ethylene evolution, anthocyanin synthesis, and perianth senescence. Bot. Gaz. 1980, 141, 422–427. [Google Scholar] [CrossRef]
- Macnish, A.J.; Jiang, C.Z.; Reid, M.S. Treatment with thidiazuron improves opening and vase life of iris flowers. Postharvest Biol. Technol. 2010, 56, 77–84. [Google Scholar] [CrossRef]
- Rogers, H.J. Is there an important role for reactive oxygen species and redox regulation during floral senescence? Plant Cell Environ. 2012, 35, 217–233. [Google Scholar] [CrossRef]
- Kumar, N.; Srivastava, G.C.; Dixit, K. Flower bud opening and senescence in roses (Rosa hybrida L.). Plant Growth Regul. 2008, 55, 81–99. [Google Scholar] [CrossRef]
- Chakrabarty, D.; Verma, A.K.; Datta, S.K. Oxidative stress and antioxidant activity as the basis of senescence in Hemerocallis (day lily) flowers. J. Agric. For. 2009, 1, 113–119. [Google Scholar]
- Droillard, M.J.; Paulin, A. Isozymes of superoxide dismutase in mitochondria and peroxisomes isolated from petals of carnation (Dianthus caryophyllus) during senescence. Plant Physiol. 1990, 94, 1187–1192. [Google Scholar] [CrossRef]
- Bailly, C.; Corbineau, F.; van Doorn, W.G. Free radical scavenging and senescence in Iris tepals. Plant Physiol. Biochem. 2001, 39, 649–656. [Google Scholar] [CrossRef]
- Yamane, K.; Kawabata, S.; Fujishige, N. Changes in activities of superoxide dismutase, catalase and peroxidase during senescence of Gladiolus florets. J. Jpn. Soc. Hortic. Sci. 1999, 68, 798–802. [Google Scholar] [CrossRef]
- Shu, Z.; Shi, Y.; Qian, H.; Tao, Y.; Tang, D. Distinct respiration and physiological changes during flower development and senescence in two Freesia cultivars. HortScience 2010, 45, 1088–1092. [Google Scholar]
- Dietz, K.J.; Jacob, S.; Oelze, M.L.; Laxa, M.; Tognetti, V.; Nunes de Miranda, S.M.; Baier, M.; Finkemeier, I. The function of peroxiredoxins in plant organelle redox metabolism. J. Exp. Bot. 2006, 57, 1697–1709. [Google Scholar] [CrossRef]
- Eshdat, Y.; Holland, D.; Faltin, Z.; Ben-Hayyim, G. Plant glutathione peroxidases. Physiol. Plant. 1997, 100, 234–240. [Google Scholar] [CrossRef]
- Welinder, K.G.; Justesen, A.F.; Kjærsga, I.V.H.; Jensen, R.B.; Rasmussen, S.K.; Jespersen, H.M.; Duroux, L. Structural diversity and transcription of class III peroxidases from Arabidopsis thaliana. Eur. J. Biochem. 2002, 269, 6063–6081. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, W.; Chen, S.; Han, L.; Li, Z. The role of N-lauroylethanolamine in the regulation of senescence of cut carnations (Dianthus caryophyllus). J. Plant Physiol. 2007, 164, 993–1001. [Google Scholar] [CrossRef]
- Desikan, R.; Reynolds, A.; Hancock, J.T.; Neill, S.J. Harpin and hydrogen peroxide both initiate programmed cell death but have differential effects on defence gene expression in Arabidopsis suspension cultures. Biochem. J. 1998, 330, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hossain, Z.; Mandal, A.K.A.; Datta, S.K.; Biswas, A.K. Decline in ascorbate peroxidase activity—A prerequisite factor for tepal senescence in Gladiolus. J. Plant Physiol. 2006, 163, 186–194. [Google Scholar] [CrossRef]
- Bartoli, C.G.; Simontacchi, M.; Guiamet, J.; Montaldi, E.R.; Puntarulo, S. Antioxidant enzymes and lipid peroxidation during aging of Chrysanthemum morifolium RAM petals. Plant Sci. 1995, 104, 161–168. [Google Scholar] [CrossRef]
- Del Rio, L.A.; Palma, J.M.; Sandalio, L.M.; Corpas, F.J.; Pastori, G.M.; Bueno, P.; Lopez-Huertas, E. Peroxisomes as a source of superoxide and hydrogen peroxide in stressed plants. Biochem. Soc. Trans. 1996, 24, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Ezhilmathi, K.; Singh, V.P.; Arora, A.; Sairam, R.K. Effect of 5-sulfosalicylic acid on antioxidant activity in relation to vase life of Gladiolus cut flowers. Plant Growth Regul. 2007, 51, 99–108. [Google Scholar] [CrossRef]
- Krizek, B.A.; Fletcher, J.C. Molecular mechanisms of flower development: An armchair guide. Nat. Rev. Genet. 2005, 6, 688–698. [Google Scholar] [CrossRef]
- Lohmann, J.U.; Weigel, D. Building beauty: The genetic control of floral patterning. Dev. Cell 2002, 2, 135–142. [Google Scholar] [CrossRef]
- Michaels, S.D.; Amasino, R.M. FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 1999, 11, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Alvarez, J.; Smyth, D.R.; Yanofsky, M.F.; Meyerowitz, E.M. LEAFY controls floral meristem identity in Arabidopsis. Cell 1992, 69, 843–859. [Google Scholar] [CrossRef]
- Parcy, F.; Nilsson, O.; Busch, M.A.; Lee, I.; Weigel, D. A genetic framework for floral patterning. Nature 1998, 395, 561–566. [Google Scholar] [CrossRef]
- Liljegren, S.J.; Gustafson-Brown, C.; Pinyopich, A.; Ditta, G.S.; Yanofsky, M.F. Interactions among APETALA1, LEAFY, and TERMINAL FLOWER1 specify meristem fate. Plant Cell 1999, 11, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- William, D.A.; Su, Y.; Smith, M.R.; Lu, M.; Baldwin, D.A.; Wagner, D. Genomic identification of direct target genes of LEAFY. Proc. Natl. Acad. Sci. USA 2004, 101, 1775–1780. [Google Scholar] [CrossRef]
- Coen, E.S.; Meyerowitz, E.M. The war of the whorls: Genetic interactions controlling flower development. Nature 1991, 353, 31–37. [Google Scholar] [CrossRef]
- Jack, T. Molecular and genetic mechanisms of floral control. Plant Cell 2004, 16, S1–S17. [Google Scholar] [CrossRef]
- Pelaz, S.; Ditta, G.S.; Baumann, E.; Wisman, E.; Yanofsky, M.F. B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 2000, 405, 200–203. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Tuteja, N. Integrated signaling in flower senescence: An overview. Plant Signal. Behav. 2007, 2, 437–445. [Google Scholar] [CrossRef]
- Lawton, K.A.; Raghothama, K.G.; Goldsbrough, P.B.; Woodson, W.R. Regulation of senescence-related gene expression in carnation flower petals by ethylene. Plant Physiol. 1990, 93, 1370–1375. [Google Scholar] [CrossRef]
- Valpuesta, V.; Lange, N.E.; Guerrero, C.; Reid, M.S. Up-regulation of a cysteine protease accompanies the ethylene-insensitive senescence of daylily (Hemerocallis) flowers. Plant Mol. Biol. 1995, 28, 575–582. [Google Scholar] [CrossRef]
- Rubinstein, B. Regulation of cell death in flower petals. Plant Mol. Biol. 2000, 44, 303–318. [Google Scholar] [CrossRef]
- Hunter, D.A.; Ferrante, A.; Vernieri, P.; Reid, M.S. Role of abscisic acid in perianth senescence of daffodil (Narcissus pseudonarcissus “Dutch Master”). Physiol. Plant. 2004, 121, 313–321. [Google Scholar] [CrossRef]
- Breeze, E.; Wagstaff, C.; Harrison, E.; Bramke, I.; Rogers, H.; Stead, A.; Thomas, B.; Buchanan-Wollaston, V. Gene expression patterns to define stages of post-harvest senescence in Alstroemeria petals. Plant Biotechnol. J. 2004, 2, 155–168. [Google Scholar] [CrossRef]
- Van Doorn, W.G.; Balk, P.A.; van Houwelingen, A.M.; Hoeberichts, F.A.; Hall, R.D.; Vorst, O.; van Wordragen, M.F. Gene expression during anthesis and senescence in Iris flowers. Plant Mol. Biol. 2003, 53, 845–863. [Google Scholar] [CrossRef]
- Channelière, S.; Rivière, S.; Scalliet, G.; Szecsi, J.; Jullien, F.; Dolle, C.; Vergnea, P.; Dumasa, C.; Bendahmanea, M.; Hugueneya, P.; et al. Analysis of gene expression in rose petals using expressed sequence tags. FEBS Lett. 2002, 515, 35–38. [Google Scholar] [CrossRef]
- Xu, X.; Gookin, T.; Jiang, C.Z.; Reid, M.S. Genes associated with opening and senescence of Mirabilis jalapa flowers. J. Exp. Bot. 2007, 58, 2193–2201. [Google Scholar] [CrossRef]
- Wang, H.; Brandt, A.S.; Woodson, W.R. A flower senescence-related mRNA from carnation encodes a novel protein related to enzymes involved in phosphonate biosynthesis. Plant Mol. Biol. 1993, 22, 719–724. [Google Scholar] [CrossRef]
- Park, K.Y.; Drory, A.; Woodson, W.R. Molecular cloning of an 1-aminocyclopropane-1-carboxylate synthase from senescing carnation flower petals. Plant Mol. Biol. 1992, 18, 377–386. [Google Scholar] [CrossRef]
- Panavas, T.; Pikula, A.; Reid, P.D.; Rubinstein, B.; Walker, E.L. Identification of senescence-associated genes from daylily petals. Plant Mol. Biol. 1999, 40, 237–248. [Google Scholar] [CrossRef]
- Woodson, W.R.; Park, K.Y.; Drory, A.; Larsen, P.B.; Wang, H. Expression of ethylene biosynthetic pathway transcripts in senescing carnation flowers. Plant Physiol. 1992, 99, 526–532. [Google Scholar] [CrossRef]
- Meyer, R.C.; Goldsbrough, P.B.; Woodson, W.R. An ethylene-responsive flower senescence-related gene from carnation encodes a protein homologous to glutathione S-transferases. Plant Mol. Biol. 1999, 17, 277–281. [Google Scholar]
- Michael, M.Z.; Savin, K.W.; Baudinette, S.C.; Graham, M.W.; Chandler, S.F.; Lu, C.Y.; Caesar, C.; Gautrais, I.; Young, R.; Nugent, C.D.; et al. Cloning of Ethylene Biosynthetic Genes Involved in Petal Senescence of Carnation and Petunia, and Their Antisense Expression in Transgenic Plants. In Cellular and Molecular Aspects of the Plant Hormone Ethylene; Pech, J.C., Latche, A., Balogue, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1993; pp. 298–303. [Google Scholar]
- Xu, Y.; Ishida, H.; Reisen, D.; Hanson, M.R. Upregulation of a tonoplast-localized cytochrome P450 during petal senescence in Petunia inflata. BMC Plant Biol. 2006, 6, 8. [Google Scholar] [CrossRef]
- Fukuchi-Mizutani, M.; Savin, K.; Cornish, E.; Tanaka, Y.; Ashikari, T.; Kusumi, T.; Murata, N. Senescence-induced expression of a homologue of Δ9 desaturase in rose petals. Plant Mol. Biol. 1995, 29, 627–635. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, C.Z.; Donnelly, L.; Reid, M.S. Functional analysis of a RING domain ankyrin repeat protein that is highly expressed during flower senescence. J. Exp. Bot. 2007, 58, 3623–3630. [Google Scholar] [CrossRef]
- McClung, C.R. Circadian rhythms in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 139–162. [Google Scholar] [CrossRef]
- Courtney, S.E.; Rider, C.C.; Stead, A.D. Changes in protein ubiquitination and the expression of ubiquitin-encoding transcripts in daylily petals during floral development and senescence. Physiol. Plant. 1994, 91, 196–204. [Google Scholar] [CrossRef]
- Hunter, D.A.; Steele, B.C.; Reid, M.S. Identification of genes associated with perianth senescence in Daffodil (Narcissus pseudonarcissus L. “Dutch Master”). Plant Sci. 2002, 163, 13–21. [Google Scholar] [CrossRef]
- Jones, M.L.; Chaffin, G.S.; Eason, J.R.; Clark, D.G. Ethylene-sensitivity regulates proteolytic activity and cysteine protease gene expression in petunia corollas. J. Exp. Bot. 2005, 56, 2733–2774. [Google Scholar] [CrossRef]
- Eason, J.R.; Ryan, D.J.; Pinkney, T.T.; O’Donoghue, E.M. Programmed cell death during flower senescence: Isolation and characterization of cysteine proteases from Sandersonia aurantiaca. Funct. Plant Biol. 2002, 29, 1055–1064. [Google Scholar] [CrossRef]
- Wagstaff, C.; Leverentz, M.K.; Griffiths, G.; Thomas, B.; Chanasut, U.; Stead, A.D.; Rogers, H.J. Cysteine protease gene expression and proteolytic activity during senescence of Alstroemeria petals. J. Exp. Bot. 2002, 53, 233–240. [Google Scholar] [CrossRef]
- Guerrero, C.; de la Calle, M.; Reid, M.S.; Valpuesta, V. Analysis of the expression of two thiolprotease genes from daylily (Hemerocallis spp.) during flower senescence. Plant Mol. Biol. 1998, 36, 565–571. [Google Scholar] [CrossRef]
- Jones, M.L.; Larsen, P.B.; Woodson, W.R. Ethylene-regulated expression of a carnation cysteine proteinase during flower petal senescence. Plant Mol. Biol. 1995, 28, 505–512. [Google Scholar] [CrossRef]
- Sugawara, H.; Shibuya, K.; Yoshioka, T.; Hashiba, T.; Satoh, S. Is a cysteine proteinase inhibitor involved in the regulation of petal wilting in senescing carnation (Dianthus caryophyllus L.) flowers? J. Exp. Bot. 2002, 53, 407–413. [Google Scholar] [CrossRef]
- Xu, Y.; Hanson, M.R. Programmed cell death during pollination-induced petal senescence in petunia. Plant Physiol. 2000, 122, 1323–1233. [Google Scholar] [CrossRef]
- Taylor, C.B.; Bariola, P.A.; Raines, R.T.; Green, P.J. RNS2: A senescence-associated RNase of Arabidopsis that diverged from the S-RNases before speciation. Proc. Natl. Acad. Sci. USA 1993, 90, 5118–5122. [Google Scholar] [CrossRef]
- Lers, A.; Khalchitski, A.; Lomaniec, E.; Burd, S.; Green, P.J. Senescence-induced RNases in tomato. Plant Mol. Biol. 1998, 36, 439–449. [Google Scholar] [CrossRef]
- Papini, A.; Mosti, S.; Brighigna, L. Programmed-cell death events during tapetum development of angiosperms. Protoplasma 1999, 207, 213–221. [Google Scholar] [CrossRef]
- Wang, M.; Hoekstra, S.; van Bergen, S.; Lamers, G.E.M.; Oppedijk, B.J.; van der Heijden, M.W. Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol. Biol. 1999, 39, 489–501. [Google Scholar] [CrossRef]
- Balk, J.; Leaver, C.J. The PET1-CMS mitochondrial mutation in sunflower is associated with premature programmed cell death and cytochrome c release. Plant Cell 2010, 13, 1803–1818. [Google Scholar]
- Thomas, S.G.; Franklin-Tong, V.E. Self-incompatibility triggers programmed cell death in Papaver pollen. Nature 2004, 429, 305–309. [Google Scholar] [CrossRef]
- Müller, R.; Stummann, B.M. Genetic regulation of ethylene perception and signal transduction related to flower senescence. J. Food Agric. Environ. 2003, 1, 87–94. [Google Scholar]
- Borochov, A.; Cho, M.H.; Boss, W.F. Plasma membrane lipid metabolism of Petunia petals during senescence. Physiol. Plant. 1994, 90, 279–284. [Google Scholar] [CrossRef]
- Porat, R.; Borochov, A.; Halevy, A.H. Pollination induced senescence in Phalaenopsis petals: Relationship of ethylene sensitivity to activity of GTP-binding proteins and protein phosphorylation. Physiol. Plant. 1994, 90, 679–684. [Google Scholar] [CrossRef]
- Kakkar, R.J.; Rai, V.K. Plant polyamines in flowering and fruit ripening. Phytochemistry 1993, 33, 1281–1288. [Google Scholar] [CrossRef]
- Bagni, N.; Tassoni, A. The Role of Polyamines in Relation to Flower Senescence. In Floriculture, Ornamental and Plant Biotechnology; Global Science Books, Ltd.: London, UK, Ikenobe, Japan, 2006; pp. 88–95. [Google Scholar]
- Upfold, S.J.; van Staden, J. Polyamines and carnation flower senescence: Endogenous levels and the effect of applied polyamines on senescence. Plant Growth Regul. 1991, 10, 355–362. [Google Scholar] [CrossRef]
- Lee, M.; Lee, S.H.; Park, K.Y. Effects of spermine on ethylene biosynthesis in cut carnation (Dianthus caryophyllus L.) flowers during senescence. J. Plant Physiol. 1997, 151, 68–73. [Google Scholar] [CrossRef]
- Hoeberichts, F.A.; van Doorn, W.G.; Vorst, O.; Hall, R.D.; van Wordragen, M.F. Sucrose prevents up-regulation of senescence-associated genes in carnation petals. J. Exp. Bot. 2007, 58, 2873–2885. [Google Scholar] [CrossRef]
- Oh, S.A.; Park, J.H.; Lee, G.I.; Paek, K.H.; Park, S.K.; Nam, H.G. Identification of three genetic loci controlling leaf senescence in Arabidopsis thaliana. Plant J. 1997, 12, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Ellis, C.M.; Nagpal, P.; Young, J.C.; Hagen, G.; Guilfoyle, T.J.; Reed, J.W. AUXIN RESPONSE FACTOR1 and AUXIN RESPONSE FACTOR2 regulate senescence and floral organ abscission in Arabidopsis thaliana. Development 2005, 132, 4563–4574. [Google Scholar] [CrossRef]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef]
- Miao, Y.; Laun, T.; Zimmermann, P.; Zentgraf, U. Targets of the WRKY53 transcription factor and its role during leaf senescence in Arabidopsis. Plant Mol. Biol. 2004, 55, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Bejerano, P.; Urbez, C.; Carbonell, J.; Granell, A.; Perez-Amador, M.A. A fertilization-independent developmental program triggers partial fruit development and senescence processes in pistils of Arabidopsis. Plant Physiol. 2010, 154, 163–172. [Google Scholar] [CrossRef]
- Fang, S.C.; Fernandez, D.E. Effect of regulated overexpression of the MADS domain factor AGL15 on flower senescence and fruit maturation. Plant Physiol. 2002, 130, 78–89. [Google Scholar] [CrossRef]
- Yu, H.; Goh, C.J. Identification and characterization of three orchid MADS-box genes of the AP1/AGL9 subfamily during floral transition. Plant Physiol. 2000, 123, 1325–1336. [Google Scholar] [CrossRef]
- Fernandez, D.E.; Heck, G.R.; Perry, S.E.; Patterson, S.E.; Bleecker, A.B.; Fang, S.C. The embryo MADS domain factor AGL15 acts postembryonically. Inhibition of perianth senescence and abscission via constitutive expression. Plant Cell 2000, 12, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Zhang, L.; Zhou, C.; Yu, C.W.; Chaikam, V. HDA6 is required for jasmonate response, senescence and flowering in Arabidopsis. J. Exp. Bot. 2008, 59, 225–234. [Google Scholar] [CrossRef]
- Laufs, P.; Peaucelle, A.; Morin, H.; Traas, J. MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 2004, 131, 4311–4322. [Google Scholar] [CrossRef]
- Aukerman, M.J.; Sakai, H. Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 2003, 15, 2730–2741. [Google Scholar] [CrossRef]
- Rubio-Somoza, I.; Weigel, D. MicroRNA networks and developmental plasticity in plants. Trends Plant Sci. 2011, 16, 258–264. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.H.; Lim, C.J.; Lim, J.Y.; Ryu, J.Y.; Lee, B.W.; Choi, J.P.; Kim, W.B.; Lee, H.Y.; Choi, Y.; et al. Small RNA and transcriptome deep sequencing proffers insight into floral gene regulation in Rosa cultivars. BMC Genomics 2012, 13, 657. [Google Scholar] [CrossRef]
- Pei, H.; Ma, N.; Chen, J.; Zheng, Y.; Tian, J.; Li, J.; Zhang, S.; Fei, Z.; Gao, J. Integrative analysis of miRNA and mRNA profiles in response to ethylene in rose petals during flower opening. PLoS One 2013, 8, e64290. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.Y.; Felippes, F.F.; Liu, C.J.; Weigel, D.; Wang, J.W. Negative regulation of anthocyanin biosynthesis in Arabidopsis by a miR156-targeted SPL transcription factor. Plant Cell 2011, 23, 1512–1522. [Google Scholar] [CrossRef]
- Wetzel, C.M.; Jiang, C.Z.; Meehan, L.J.; Voytas, D.F.; Rodermel, S.R. Nuclear-organelle interactions: The immutans variegation mutant of Arabidopsis is plastid autonomous and impaired in carotenoid biosynthesis. Plant J. 1994, 6, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Mylona, P.V.; Polidoros, A.N. ROS Regulation of Antioxidant Genes. In Reactive Oxygen Species and Antioxidants in Higher Plants; Dutta Gupta, S., Ed.; Science Publishers: Enfield, New Hampshire, UK, 2010; pp. 101–127. [Google Scholar]
- Wagstaff, C.; Yang, T.J.; Stead, A.D.; Buchanan-Wollaston, V.; Roberts, J.A. A molecular and structural characterization of senescing Arabidopsis siliques and comparison of transcriptional profiles with senescing petals and leaves. Plant J. 2009, 57, 690–705. [Google Scholar] [CrossRef]
- Price, A.M.; Aros Orellana, D.F.; Salleh, F.M.; Stevens, R.; Acock, R.; Buchanan-Wollaston, V.; Stead, A.D.; Rogers, H.J. A comparison of leaf and petal senescence in wallflower reveals common and distinct patterns of gene expression and physiology. Plant Physiol. 2008, 147, 1898–1912. [Google Scholar] [CrossRef]
- Wagstaff, C.; Bramke, I.; Breeze, E.; Thornber, S.; Harrison, E.; Thomas, B.; Rogers, H. A specific group of genes respond to cold dehydration stress in cut Alstroemeria flowers whereas ambient dehydration stress accelerates developmental senescence expression patterns. J. Exp. Bot. 2010, 61, 2905–2921. [Google Scholar] [CrossRef]
- Itzhaki, H.; Maxson, J.M.; Woodson, W.R. An ethylene-responsive enhancer element is involved in the senescence-related expression of the carnation glutathione-S-transferase (GST1) gene. Proc. Natl. Acad. Sci. USA 1994, 91, 8925–8929. [Google Scholar] [CrossRef]
- Wagner, U.; Edwards, R.; Dixon, D.P.; Mauch, F. Probing the diversity of the Arabidopsis glutathione S-transferase gene family. Plant Mol. Biol. 2002, 49, 515–532. [Google Scholar] [CrossRef]
- Larsen, E.S.; Alfenito, M.R.; Briggs, W.R.; Walbot, V. A carnation anthocyanin mutant is complemented by the glutathione S-transferases encoded by maize Bz2 and Petunia An9. Plant Cell Rep. 2003, 21, 900–904. [Google Scholar] [PubMed]
- Gibbings, J.G.; Cook, B.P.; Dufault, M.R.; Madden, S.L.; Khuri, S.; Turnbull, C.J.; Dunwell, J.M. Global transcript analysis of rice leaf and seed using SAGE technology. Plant Biotechnol. J. 2003, 1, 271–285. [Google Scholar] [CrossRef]
- Aharoni, A.; Keizer, L.C.; Bouwmeester, H.J.; Sun, Z.; Alvarez-Huerta, M.; Verhoeven, H.A.; Jan Blaas, J.; van Houwelingen, A.M.M.L.; de Vos, R.C.H.; van der Voetb, H.; et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell 2000, 12, 647–662. [Google Scholar] [CrossRef] [PubMed]
- Kleber-Janke, T.; Krupinska, K. Isolation of cDNA clones for genes showing enhanced expression in barley leaves during dark-induced senescence as well as during senescence under field conditions. Planta 1997, 203, 332–340. [Google Scholar] [CrossRef]
- Rise, M.; Cojocaru, M.; Gottlieb, H.E.; Goldschmidt, E.E. Accumulation of α-tocopherol in senescing organs as related to chlorophyll degradation. Plant Physiol. 1989, 89, 1028–1030. [Google Scholar] [CrossRef]
- Arrom, L.; Munné-Bosch, S. Tocopherol composition in flower organs of Lilium and its variations during natural and artificial senescence. Plant Sci. 2010, 179, 289–295. [Google Scholar] [CrossRef]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The biosynthetic pathway of vitamin C in higher plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef]
- Wolucka, B.A.; van Montagu, M. GDP-mannose 3′,5′-epimerase forms GDP-l-gulose, a putative intermediate for the de novo biosynthesis of vitamin C in plants. J. Biol. Chem. 2003, 278, 47483–47490. [Google Scholar] [CrossRef]
- Nishikimi, M. Recent advance in the study of ascorbic acid biosynthesis. Seikagaku 1996, 68, 377–380. [Google Scholar] [PubMed]
- Conklin, P.L.; Saracco, S.A.; Norris, S.R.; Last, R.L. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics 2000, 154, 847–856. [Google Scholar] [PubMed]
- Conklin, P.L.; Pallanca, J.E.; Last, R.L.; Smirnoff, N. l-ascorbic acid metabolism in the ascorbate-deficient Arabidopsis mutant vtc1. Plant Physiol. 1997, 115, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Kotchoni, S.O.; Larrimore, K.E.; Mukherjee, M.; Kempinski, C.F.; Barth, C. Alterations in the endogenous ascorbic acid content affect flowering time in Arabidopsis. Plant Physiol. 2009, 149, 803–815. [Google Scholar] [PubMed]
- Mol, J.; Grotewold, E.; Koes, R. How genes paint flowers and seeds. Trends Plant Sci. 1998, 3, 212–217. [Google Scholar] [CrossRef]
- Wigoda, N.; Ben-Nissan, G.; Granot, D.; Schwartz, A.; Weiss, D. The gibberellin-induced, cysteine-rich protein GIP2 from Petunia hybrida exhibits in planta antioxidant activity. Plant J. 2006, 48, 796–805. [Google Scholar] [CrossRef]
- Wingler, A. Interactions between flowering and senescence regulation and the influence of low temperature in Arabidopsis and crop plants. Ann. Appl. Biol. 2011, 159, 320–338. [Google Scholar] [CrossRef]
- Savin, K.W.; Baudinette, S.C.; Graham, M.W.; Michael, M.Z.; Nugent, G.D.; Lu, C.Y.; Chandler, S.F.; Cornish, E.C. Antisense ACC Oxidase RNA delays carnation petal senescence. HortScience 1995, 30, 970–972. [Google Scholar]
- Wilkinson, J.Q.; Lanahan, M.B.; Clark, D.G.; Bleecker, A.B.; Chang, C.; Meyerowitz, E.M.; Klee, H.J. A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat. Biotechnol. 1997, 15, 444–447. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cavaiuolo, M.; Cocetta, G.; Ferrante, A. The Antioxidants Changes in Ornamental Flowers during Development and Senescence. Antioxidants 2013, 2, 132-155. https://doi.org/10.3390/antiox2030132
Cavaiuolo M, Cocetta G, Ferrante A. The Antioxidants Changes in Ornamental Flowers during Development and Senescence. Antioxidants. 2013; 2(3):132-155. https://doi.org/10.3390/antiox2030132
Chicago/Turabian StyleCavaiuolo, Marina, Giacomo Cocetta, and Antonio Ferrante. 2013. "The Antioxidants Changes in Ornamental Flowers during Development and Senescence" Antioxidants 2, no. 3: 132-155. https://doi.org/10.3390/antiox2030132






