Antioxidant Activity of Grapevine Leaf Extracts against Oxidative Stress Induced by Carbon Tetrachloride in Cerebral Cortex, Hippocampus and Cerebellum of Rats
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents
2.2. Grapevine Leaf Extracts
2.3. Phenolic Compounds
2.4. Animals
2.5. Treatment
2.6. Parameters of Oxidative Stress
2.7. Determination of Antioxidant Enzymes
2.8. Protein Determination
2.9. Statistical Analysis
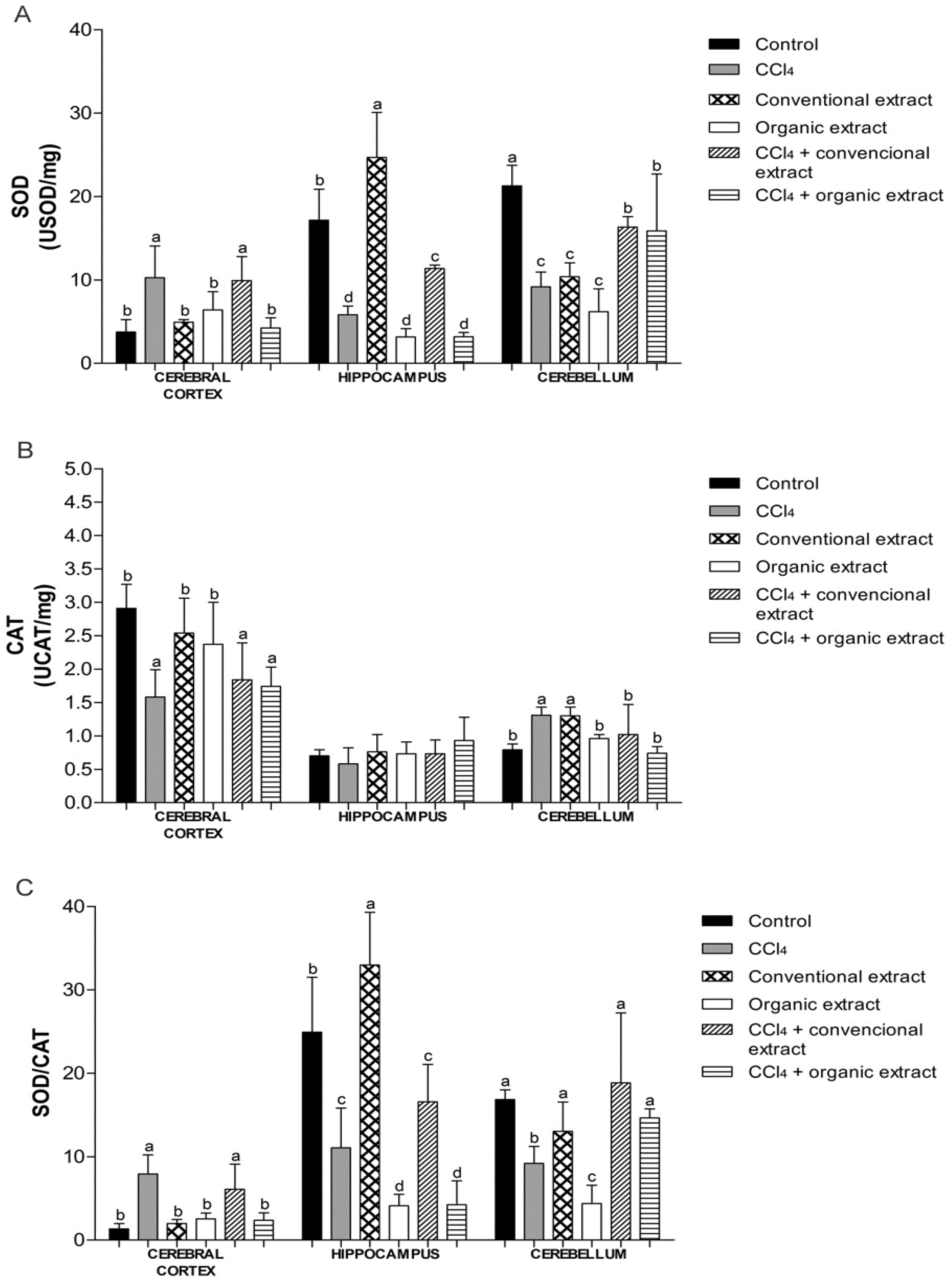
3. Results and Discussion
| EXTRACT | Total Polyphenols (mg/100 g) | Catechin (mg/100 g) | Resveratrol (mg/100 g) |
|---|---|---|---|
| CONVENTIONAL | 19.83 ± 0.76 | 211.82 ± 5.05 | 0.01 ± 0.003 |
| ORGANIC | 81.79 ± 2.68* | 161.10 ± 0.97 * | 0.04 ± 0.004 * |

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Dani, C.; Oliboni, L.S.; Bonatto, D.; Vanderlinde, R.; Salvador, M.; Henriques, J.A. Phenolic content and antioxidant activities of white and purple juices manufactured with organically-produced grapes. Food Chem. Toxicol. 2007, 45, 2574–2580. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.; Barrett, D.M.; Mitchell, A. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1247. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Xia, E.; Deng, G.; Guo, Y.; Li, H. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef]
- Belguendouz, F.L.; Gozzelino, M.T. Interaction of transresveratrol with plasma lipoproteins. Biochem. Pharmacol. 1998, 15, 811–816. [Google Scholar] [CrossRef]
- Frémont, L.; Belquendouz, L.; Depal, S. Antioxidant activity of resveratrol and alcohol-free wine polyphenols related to LDL oxidation and polysaturated fatty acids. Life Sci. 1999, 64, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Oliboni, L.; Dani, C.; Funchal, C.; Henriques, J.A.; Salvador, M. Hepatoprotective, cardioprotective, and renal-protective effects of organic and conventional grapevine leaf extracts (Vitis labrusca var. Bordo) on rats tissues. An. Acad. Bras. Ciênc. 2011, 83, 1403–1411. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. In Vitro 2010, 24, 148–153. [Google Scholar] [CrossRef]
- Markesberry, W.R. The role of oxidative stress in Alzheimer disease. Arch. Neurol. 1999, 56, 1449–1452. [Google Scholar] [CrossRef]
- Pietta, P. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Pari, L.; Suresh, A. Effect of grape (Vitis vinifera L.) leaf extract on alcohol induced oxidative stress in rats. Food Chem. Toxicol. 2008, 46, 1627–1634. [Google Scholar] [CrossRef]
- Haliwell, B. Role of free radicals in the neurodegenerative diseases: Therapeutic implications for antioxidant treatment. Drug Aging 2001, 18, 685–716. [Google Scholar] [CrossRef]
- Singh, R.P.; Sharad, S.; Kapur, S. Free radicals and oxidative stress in neurodegenerative diseases: Relevance of dietary antioxidants. J. Indian Acad. Clin. Med. 2004, 5, 218–225. [Google Scholar]
- Devi, S.A.; Chandrasekar, B.K.; Manjula, K.R.; Ishi, N. Grape seed proanthocyanidin lowers brain oxidative stress in adult and middle-aged rats. Exp. Gerontol. 2011, 46, 958–964. [Google Scholar] [CrossRef]
- Rodrigues, A.D.; Scheffel, T.B.; Scola, G.; Santos, M.T.; Fank, B.; de Freitas, S.C.V.; Dani, C.; Vanderlinde, R.; Henriques, J.A.P.; Coitinho, A.S.; et al. Neuroprotective and anticonvulsant effects of organic and conventional purple grape juices on seizures in Wistar rats induced by pentylenetetrazole. Neurochem. Int. 2012, 60, 799–805. [Google Scholar] [CrossRef]
- Scola, G.; Scheffel, T.; Gambato, G.; Freitas, S.; Dani, C.; Funchal, C.; Gomez, R.; Coitinho, A.; Salvador, M. Flavan-3-ol compounds prevent pentylenetetrazol-induced oxidative damage in rats without producing mutations and genotoxicity. Neurosci. Lett. 2013, 534, 145–149. [Google Scholar] [CrossRef]
- Jayakumar, T.; Sakthivel, M.; Thomas, P.A.; Geraldine, P. Pleutorus ostreatus, an oyester mushroom, decreases the oxidative stress induced by carbon tetrachloride in rat kidneys, heart and brain. Chem. Biol. Interact. 2008, 176, 108–120. [Google Scholar] [CrossRef]
- Ozturk, F.; Ucar, M.; Ozturk, I.C.; Vardi, N.; Batcioglu, K. Carbon tetrachloride induced nephotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology 2003, 62, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Simile, M.M.; Banni, S.; Angioni, E.; Carta, G.; de Miglio, M.R.; Muroni, M.R.; Calvisi, D.F.; Carru, A.; Pascale, R.M.; Feo, F. 5-Methyl thioadenosine administration prevents lipid peroxidation and fibrogenesis induced in rat liver by carbon tetrachloride intoxication. J. Hepatol. 2001, 34, 386–394. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin–Ciocalteau Reagent. In Methods in Enzymology; Packer, L., Ed.; Academic Press: San Diego, CA, USA, 1999; pp. 159–178. [Google Scholar]
- Saucier, C.; Mirabel, M.; Daviud, F.; Longieras, A.; Glories, T. Rapid fractionation of grape seeds proanthocyanidins. J. Agric. Food Chem. 2001, 49, 5732–5735. [Google Scholar] [CrossRef]
- Fillip, R.; López, P.; Gilberti, J.; Coussio, J.; Ferraro, G. Phenolic compounds in seven South American Ilex species. Fitoterapia 2001, 72, 774–778. [Google Scholar] [CrossRef]
- Ector, B.J.; Magee, J.B.; Hegwood, C.P.; Coign, M.J. Resveratrol concentration in muscadine berries, juice, pomace, purees, seeds, and wines. Am. J. Enol. Vitic. 1996, 47, 57–62. [Google Scholar]
- Dani, C.; Pasquali, M.A.; Oliveira, M.R.; Umezu, F.M.; Salvador, M.; Henriques, J.A.; Moreira, J.C.F. Protective effects of purple grape juice on carbon tetrachloride-induced oxidative stress in brains of adult wistar rats. J. Med. Food 2008, 11, 55–61. [Google Scholar] [CrossRef]
- Wills, E.D. Mechanism of lipid peroxide formation in animal tissues. Biochem. J. 1996, 99, 667–676. [Google Scholar]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Ahn, B.W. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [CrossRef]
- Bannister, J.V.; Calabrese, L. Assays for SOD. Method Biochem. Anal. 1987, 32, 279–312. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrouh, N.J.; Lewis-Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- IFOAM—International Foundation for Organic Agriculture. Norms for Organic Production and Processing; IFOAM: Bonn, Germany, 2005. [Google Scholar]
- Orhan, D.D.; Orhan, N.; Ergun, E.; Ergun, F. Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. J. Ethnopharmacol. 2007, 112, 145–151. [Google Scholar] [CrossRef]
- Wollgast, J.; Anklam, E. Polyphenols in chocolate: Is there a contribution to human health? Food Res. Int. 2000, 22, 449–459. [Google Scholar] [CrossRef]
- Altas, S.; Kızıl, G.; Kızıl, M.; Ketani, A.; Haris, P.I. Protective effect of Diyarbakır watermelon juice on carbon tetrachloride-induced toxicity in rats. Food Chem. Toxicol. 2011, 49, 2433–2438. [Google Scholar] [CrossRef]
- De Groot, H. Reactive oxygen species in tissue injury. Hepatogastroenterology 1994, 41, 328–332. [Google Scholar] [PubMed]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef]
- Gama, C.S.; Berk, M.; Andreazza, A.C.; Kapczinski, F.; Belmonte-de-Abreu, P. Serum levels of brain-derived neurotrophic factor and thiobarbituric acid reative substances in chronically medicated schizophrenic patients: A positive correlation. Rev. Bras. Psiquiatr. 2008, 30, 337–340. [Google Scholar] [PubMed]
- Takayama, F.; Nakamoto, K.; Kawasaki, H.; Mankura, M.; Egashira, T.; Ueki, K.; Hasegawa, A.; Okada, S.; Mori, A. Beneficial effects of Vitis coignetiae Pulliat leaves on nonalcoholic steatohepatitis in a rat model. Acta Med. 2009, 63, 105–111. [Google Scholar]
- Dal-Pizzol, F.; Klamt, F.; Benfato, M.S.; Bernard, E.A.; Moreira, J.C. Retinol supplementation induces oxidative stress and modulates antioxidant enzyme activities in rat Sertoli cells. Free Radic. Res. 2001, 34, 395–404. [Google Scholar] [CrossRef]
- Hota, S.K.; Barhwal, K.; Singh, S.B.; Ilavazhagan, G. Differential temporal response of hippocampus, cortex and cerebellum to hypobaric hypoxia: A biochemical approach. Neurochem. Int. 2007, 51, 384–390. [Google Scholar] [CrossRef]
- Zupan, G.; Pilipović, K.; Hrelja, A.; Peternel, S. Oxidative stress parameters in different rat brain structures after electroconvulsive shock-induced seizures. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2008, 32, 771–777. [Google Scholar] [CrossRef]
- Erecinska, M.; Silver, I.A. Calcium Handling by hippocampal Neurons under Physiologic and Pathologic Condition. In Advances in Neurology: Cellular and Molecular Mechanism of Ischemic Brain Damage; Seisjo, B.K., Wieloch, T., Eds.; Lippincott Raven Publishers: Philadelphia, PA, USA, 1996; pp. 119–131. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wohlenberg, M.; Almeida, D.; Bokowski, L.; Medeiros, N.; Agostini, F.; Funchal, C.; Dani, C. Antioxidant Activity of Grapevine Leaf Extracts against Oxidative Stress Induced by Carbon Tetrachloride in Cerebral Cortex, Hippocampus and Cerebellum of Rats. Antioxidants 2014, 3, 200-211. https://doi.org/10.3390/antiox3020200
Wohlenberg M, Almeida D, Bokowski L, Medeiros N, Agostini F, Funchal C, Dani C. Antioxidant Activity of Grapevine Leaf Extracts against Oxidative Stress Induced by Carbon Tetrachloride in Cerebral Cortex, Hippocampus and Cerebellum of Rats. Antioxidants. 2014; 3(2):200-211. https://doi.org/10.3390/antiox3020200
Chicago/Turabian StyleWohlenberg, Mariane, Daniela Almeida, Liane Bokowski, Niara Medeiros, Fabiana Agostini, Cláudia Funchal, and Caroline Dani. 2014. "Antioxidant Activity of Grapevine Leaf Extracts against Oxidative Stress Induced by Carbon Tetrachloride in Cerebral Cortex, Hippocampus and Cerebellum of Rats" Antioxidants 3, no. 2: 200-211. https://doi.org/10.3390/antiox3020200





