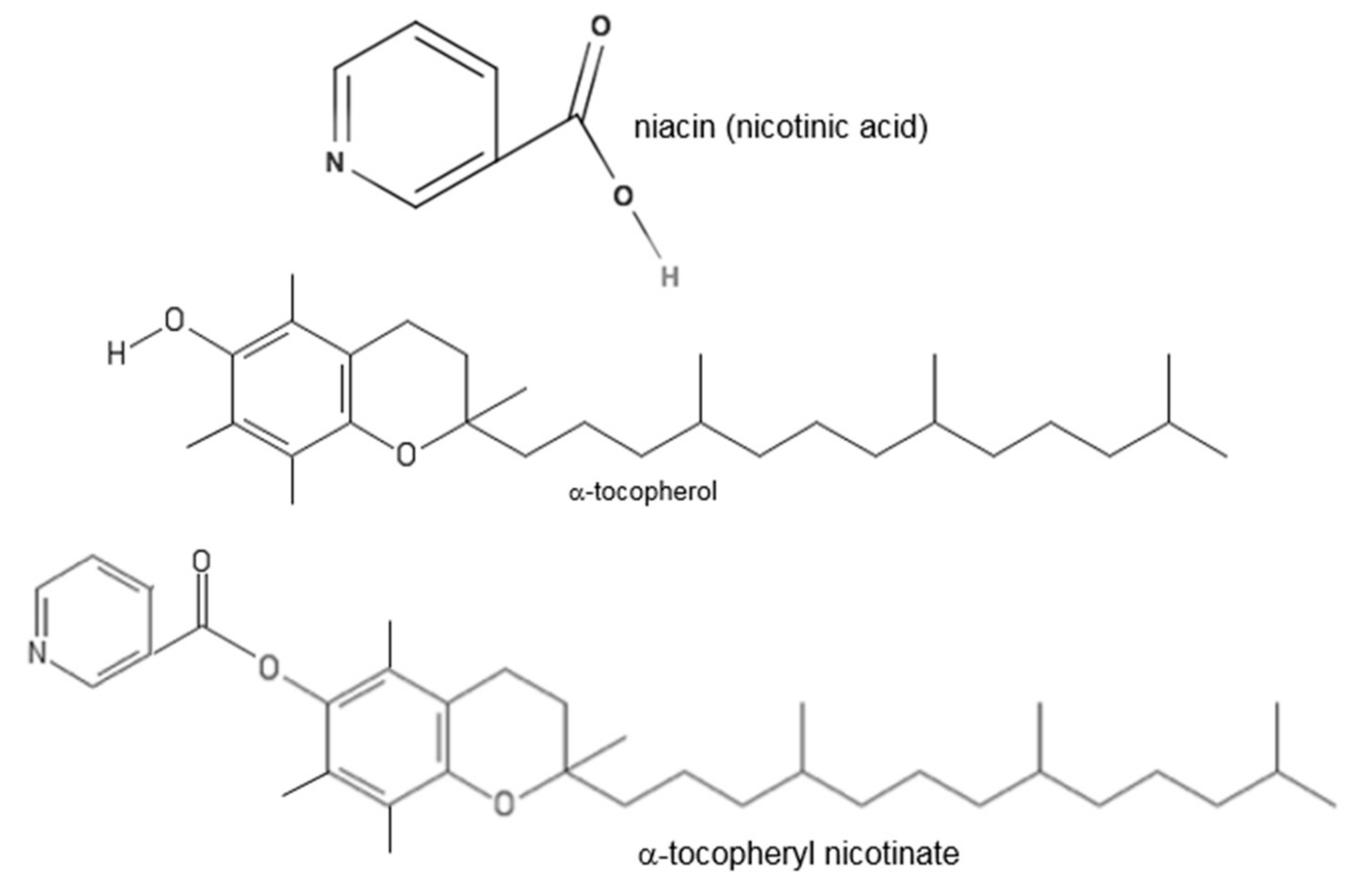
Vitamin E Nicotinate
Abstract
:1. Introduction to Vitamin E
2. Rationale and Purpose of This Review
3. Cosmetic Applications
4. Trademarks
5. Intake and Metabolism
6. Rheology
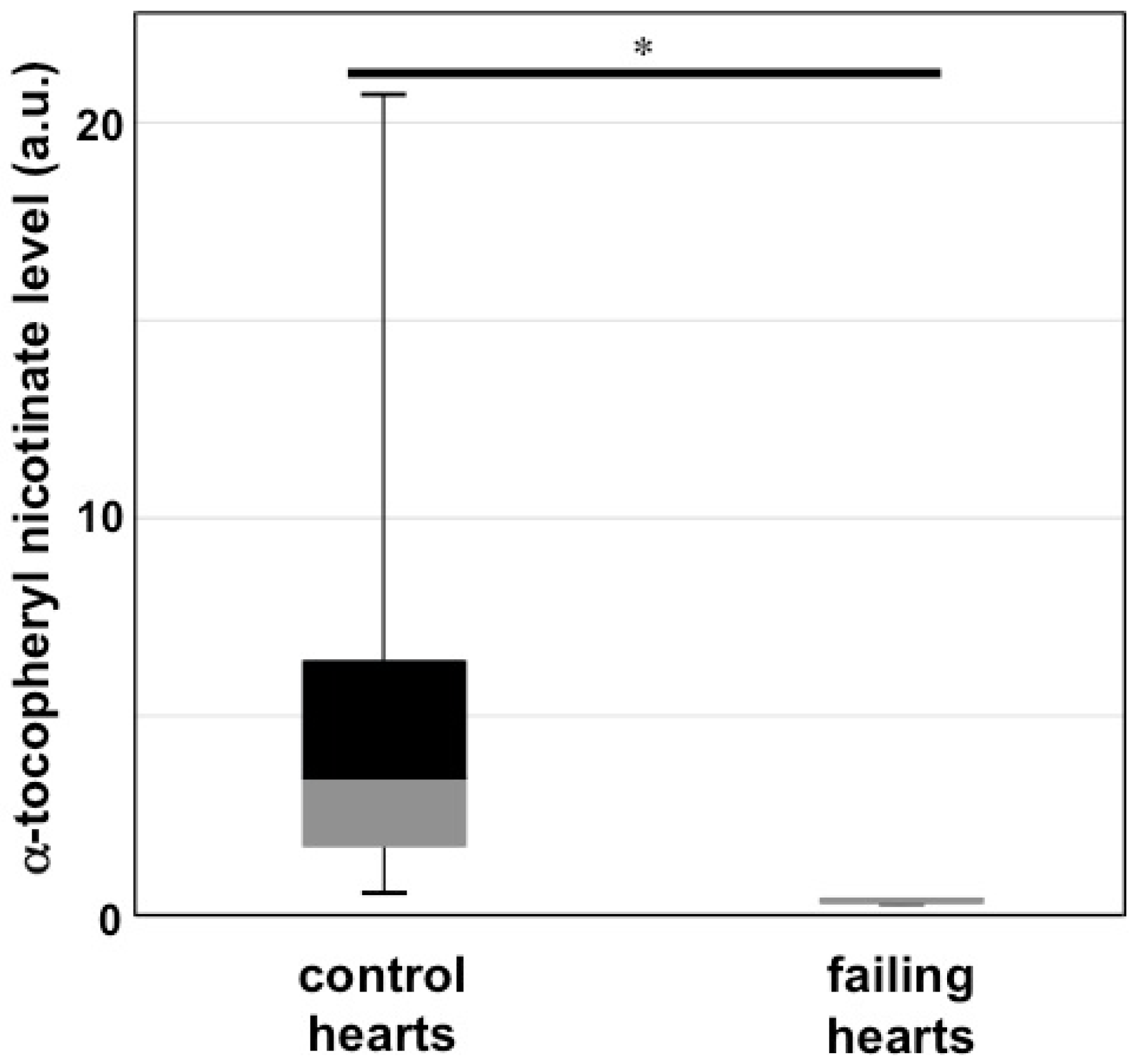
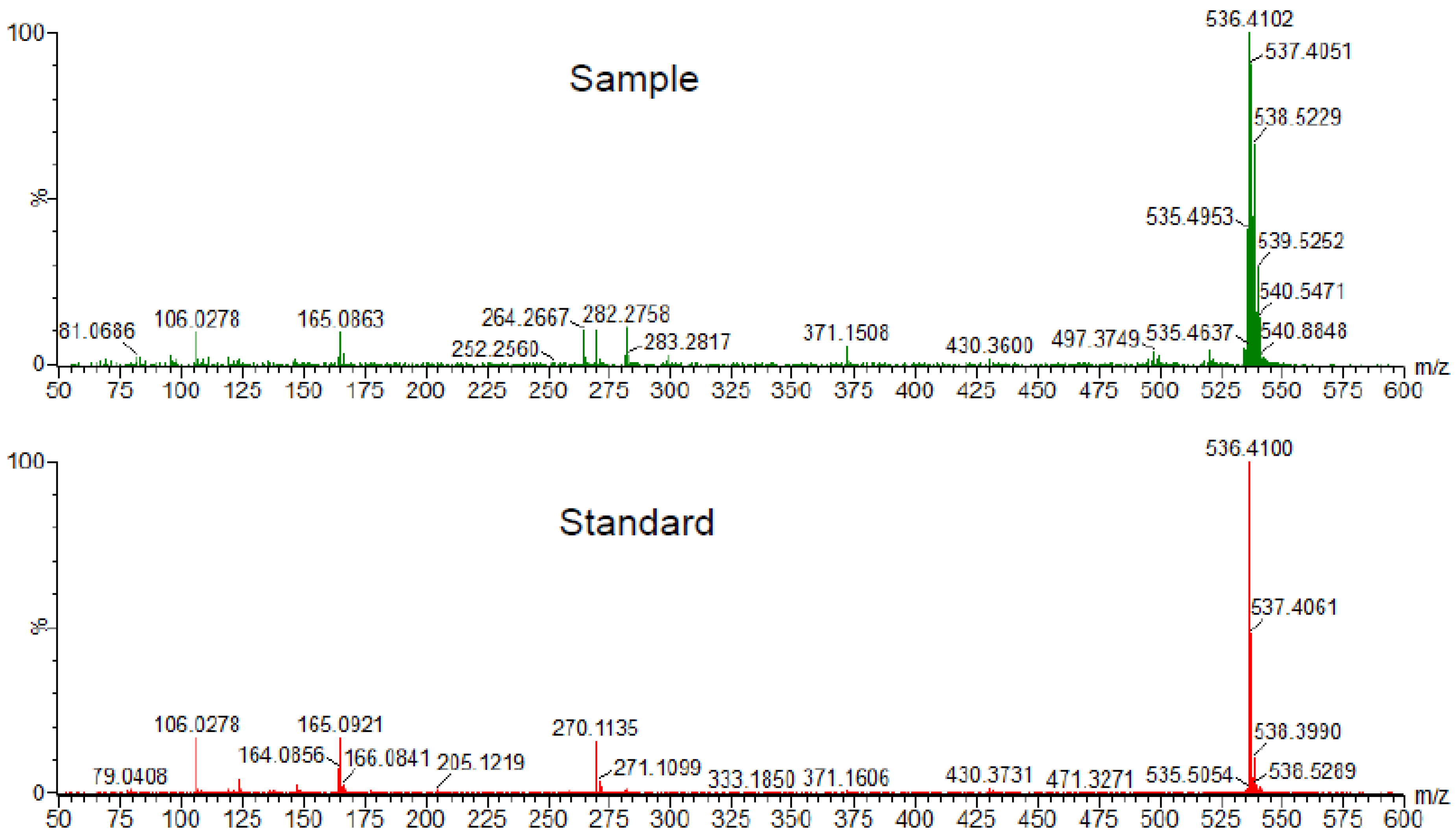
7. Cardiovascular
8. Immune Function
9. Cancer
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brigelius-Flohe, R.; Traber, M.G. Vitamin E: Function and metabolism. FASEB J. 1999, 13, 1145–1155. [Google Scholar] [PubMed]
- Bieri, J.G.; Corash, L.; Hubbard, V.S. Medical uses of vitamin E. N. Engl. J. Med. 1983, 308, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Zingg, J.-M. Vitamin E: An overview of major research directions. Mol. Aspects Med. 2007, 28, 400–422. [Google Scholar] [CrossRef] [PubMed]
- Evans, H.M.; Emerson, O.H.; Emerson, G.A. The isolation from wheat germ oil of an alcohol, α-tocopherol, having the properties of vitamin E. J. Biol. Chem. 1936, 113, 319–332. [Google Scholar] [CrossRef]
- Karrer, P.; Fritzsche, H.; Ringier, B.H.; Salomon, H. Synthesis of α-tocopherol (vitamin E). Nature 1938, 141, 1057. [Google Scholar] [CrossRef]
- Vogelsang, A.; Shute, E.V. Effects of vitamin E in coronary heart disease. Nature 1946, 157, 772. [Google Scholar] [CrossRef] [PubMed]
- Binder, H.J.; Solitare, G.B.; Spiro, H.M. Neuromuscular disease in patients with steatorrhoea. Gut 1967, 8, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.R., III; Pastor-Burriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Abner, E.L.; Schmitt, F.A.; Mendiondo, M.S.; Marcum, J.L.; Kryscio, R.J. Vitamin E and All-Cause Mortality: A Meta-Analysis. Curr. Aging Sci. 2011, 4, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Azzi, A.; Gysin, R.; Kempná, P.; Ricciarelli, R.; Villacorta, L.; Visarius, T.; Zingg, J.M. The role of alpha-tocopherol in preventing disease: From epidemiology to molecular events. Mol. Aspects Med. 2003, 24, 325–336. [Google Scholar] [CrossRef]
- Bjørneboe, A.; Bjørneboe, G.E.; Drevon, C.A. Absorption, transport and distribution of vitamin E. J. Nutr. 1990, 120, 233–242. [Google Scholar] [PubMed]
- Azzi, A.; Ricciarelli, R.; Zingg, J.M. Non-antioxidant molecular functions of alpha-tocopherol (vitamin E). FEBS Lett. 2002, 519, 8–10. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Packer, L. Inhibition of NF-κB activation by vitamin E derivatives. Biochem. Biophys. Res. Commun. 1993, 193, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Zondlo Fiume, M. Final report on the safety assessment of tocopherol, tocopheryl acetate, tocopheryl linoleate, tocopheryl linoleate/oleate, tocopheryl nicotinate, tocopheryl succinate, dioleyl tocopheryl methylsilanol, potassium ascorbyl tocopheryl phosphate, and tocophersolan. Int. J. Toxicol. 2002, 21, 51–116. [Google Scholar] [PubMed]
- Mizuno, S.; Farkas, L.; Al Husseini, A.; Farkas, D.; Gomez-Arroyo, J.; Kraskauskas, D.; Nicolls, M.R.; Cool, C.D.; Bogaard, H.J.; Voelkel, N.F. Severe pulmonary arterial hypertension induced by SU5416 and ovalbumin immunization. Am. J. Respir. Cell Mol. Biol. 2012, 47, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ibrahim, Y.F.; Das, D.; Zungu-Edmondson, M.; Shults, N.V.; Suzuki, Y.J. Carfilzomib reverses pulmonary arterial hypertension. Cardiovasc. Res. 2016, 110, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Schlieper, P.; Tawfik, H. Antiarrhythmic activity of alpha-tocopheryl nicotinate and related compounds and their physico-chemical properties. Arzneimittelforschung 1987, 37, 920–923. [Google Scholar] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.; Friedel, A.; Roos, F.R.; Wyss, A.; Eggersdorfer, M.; Hoffmann, K.; Weber, P. A Systematic Review of Global Alpha-tocopherol Status as Assessed by Nutritional Intake Levels and Blood Serum Concentrations. Int. J. Vitam. Nutr. Res. 2016, 14, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Gallo-Torres, H.E. Obligatory role of bile for the intestinal absorption of vitamin E. Lipids 1970, 5, 379–384. [Google Scholar] [CrossRef]
- Gallo-Torres, H.E. Intestinal absorption and lymphatic transport of d,1-3,4-3H2-α-tocopheryl nicotinate in the rat. Int. J. Vitam. Nutr. Res. 1970, 40, 505–514. [Google Scholar]
- Gallo-Torres, H.E.; Miller, O.N.; Hamilton, J.G.; Tratnyek, C. Distribution and metabolism of two orally administered esters of tocopherol. Lipids 1971, 6, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Aoyama, Y.; Fujita, T.; Katsui, G. Studies on tocopherol derivatives: V. Intestinal absorption of several d,1-3,4-3H2-alpha-tocopheryl esters in the rat. Lipids 1975, 10, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, J.; Tomono, Y.; Fujita, T.; Sugiyama, K.; Hamamura, K. The effect of food on the absorption of alpha-tocopheryl nicotinate in beagle dogs and human volunteers. Int. J. Clin. Pharmacol. Ther. Toxicol. 1981, 19, 216–219. [Google Scholar] [PubMed]
- Suzuki, N.; Nakamura, T. Metabolism of the nicotinic acid moiety of d,l-alpha-tocopheryl nicotinate. J. Nutr. Sci. Vitaminol. 1983, 29, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, A.; Kimura, T.; Shinozaki, H.; Ibayashi, H. Comparisons between absorption of vitamin E in patients with chronic pancreatitis and healthy controls: The bioavailability of vitamin E. Tohoku J. Exp. Med. 1986, 148, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Araiso, T. Effects of alpha-tocopherol-nicotinate administration on the microdynamics of phospholipids of erythrocyte membranes in human subjects. J. Nutr. Sci. Vitaminol. 1988, 34, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Chung, T.W.; Chen, T.Z.; Yu, J.J.H.; Lin, S.Y.; Chen, S.C. Effects of α-tocopheryl nicotinate on hemorheology and retinal capillary blood flow in female NIDDM with retinopathy. Clin. Hemorheol. Microcirc. 1995, 15, 775–782. [Google Scholar]
- Chung, T.W.; Yu, J.J.; Liu, D.Z. Reducing lipid peroxidation stress of erythrocyte membrane by alpha-tocopherol nicotinate plays an important role in improving blood rheological properties in type 2 diabetic patients with retinopathy. Diabet. Med. 1998, 15, 380–385. [Google Scholar] [CrossRef]
- Kamimura, M. Comparative studies of the effects of alpha-tocopheryl nicotinate and the combination alpha-tocopheryl acetate and nicotinic acid. J. Nutr. Sci. Vitaminol. 1973, 19, 375–381. [Google Scholar] [CrossRef]
- Kamimura, M. Comparison of alpha-tocopheryl nicotinate and acetate on skin microcirculation. Am. J. Clin. Nutr. 1974, 27, 1110–1116. [Google Scholar]
- Gey, K.F.; Puska, P. Plasma vitamins E and A inversely correlated to mortality from ischemic heart disease in cross-cultural epidemiology. Ann. N. Y. Acad. Sci. 1989, 570, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Stephens, N.G.; Parsons, A.; Schofield, P.M.; Kelly, F.; Cheeseman, K.; Mitchinson, M.J. Randomised controlled trial of vitamin E in patients with coronary disease: Cambridge Heart Antioxidant Study (CHAOS). Lancet 1996, 347, 781–786. [Google Scholar] [CrossRef]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar]
- Salonen, R.M.; Nyyssönen, K.; Kaikkonen, J.; Porkkala-Sarataho, E.; Voutilainen, S.; Rissanen, T.H.; Tuomainen, T.P.; Valkonen, V.P.; Ristonmaa, U.; Lakka, H.M.; et al. Six-year effect of combined vitamin C and E supplementation on atherosclerotic progression: The Antioxidant Supplementation in Atherosclerosis Prevention (ASAP) Study. Circulation 2003, 107, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Nakajima, Y.; Kobayashi, M.; Ohtake, S. Anti-hypertensive action of dl-alpha-tocopheryl esters in rats. Clin. Sci. Mol. Med. Suppl. 1976, 3, 163s–164s. [Google Scholar] [PubMed]
- Igarashi, T.; Nakajima, Y.; Kobayashi, M.; Ohtake, S. Antihypertensive action of d,l-alpha-tocopheryl nicotinate in rats. J. Nutr. Sci. Vitaminol. 1979, 25, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Iino, K.; Abe, K.; Kariya, S.; Kimura, H.; Kusaba, T. A controlled, double-blind study of dl-alpha-tocopheryl nicotinate (Juvela-Nicotinate) for treatment of symptoms in hypertension and cerebral arteriosclerosis. Jpn. Heart J. 1977, 18, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Hidiroglou, N.; Wolynetz, M.S.; McDowell, L.R.; Papas, A.M.; Antapli, M.; Wilkinson, N.S. Serum total cholesterol, high-density lipoprotein-cholesterol and triglyceride concentrations in lambs following supplementation with various forms of tocopherol. Reprod. Nutr. Dev. 1993, 33, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Higashi, O.; Kikuchi, Y. Effects of vitamin E on the aggregation and the lipid peroxidation of platelets exposed to hydrogen peroxide. Tohoku J. Exp. Med. 1974, 112, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Svensson, J.; Oki, T. Inhibition of platelet aggregation by alpha-tocopherol and its nicotinate and acetate esters. Int. J. Vitam. Nutr. Res. 1978, 48, 250–254. [Google Scholar] [PubMed]
- Noma, A.; Maeda, S.; Okuno, M.; Abe, A.; Muto, Y. Reduction of serum lipoprotein(a) levels in hyperlipidaemic patients with α-tocopheryl nicontinate. Atherosclerosis 1990, 84, 213–217. [Google Scholar] [CrossRef]
- Beharka, A.; Redican, S.; Leka, L.; Meydani, S.N. Vitamin E Status and Immune Function. Methods Enzymol. 1997, 282, 247–263. [Google Scholar] [PubMed]
- Serafini, M. Dietary vitamin E and T cell-mediated function in the elderly: Effectiveness and mechanism of action. Int. J. Dev. Neurosci. 2000, 18, 401–410. [Google Scholar] [CrossRef]
- Moriguchi, S. The role of vitamin E in T-cell differentiation and the decrease of cellular immunity with aging. Biofactors 1998, 7, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, S.; Itoh, T. Vitamin E enhances T cell differentiation through increased epithelial cell function in rat thymus. Nutr. Res. 1997, 17, 873–883. [Google Scholar] [CrossRef]
- Inagaki, N.; Nagai, H.; Koda, A. Effect of vitamin E on IgE antibody formation in mice. J. Pharmacobiodyn. 1984, 7, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, J.; Fujiwara, H.; Torisu, M. Vitamin E and immune response. I. Enhancement of helper T cell activity by dietary supplementation of vitamin E in mice. Immunology 1979, 38, 727–734. [Google Scholar] [PubMed]
- Prasad, K.N.; Kumar, B.; Yan, X.D.; Hanson, A.J.; Cole, W.C. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: A review. J. Am. Coll. Nutr. 2003, 22, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.N.; Edwards-Prasad, J.; Prasad, K.N. Alpha tocopheryl succinate inhibits melanocyte-stimulating hormone (MSH)-sensitive adenylate cyclase activity in melanoma cells. J. Cell Physiol. 1987, 133, 585–589. [Google Scholar] [CrossRef] [PubMed]
| Ranking | Vitamin E Derivative | Frequency of Use |
|---|---|---|
| 1 | Tocopheryl acetate | 1322 |
| 2 | Tocopherol | 1072 |
| 3 | Tocopheryl linoleate | 279 |
| 4 | Potassium ascorbyl tocopheryl phosphate | 15 |
| 5 | Dioleyl tocopheryl methylsilanol | 12 |
| 6 | Tocopheryl succinate | 4 |
| 7 | Tocopheryl nicotinate | 3 |
| 8 | Tocophersolan | 2 |
| Reference | Test | Object | Subjects | αTN Dose | Duration | Design | Control | Method | Conclusion |
|---|---|---|---|---|---|---|---|---|---|
| Koyama & Araiso [28] | Rheological properties | Erythrocytes | 7 healthy human patients | 400 mg/day | 1 month | Paired | Untreated baseline | Oral at mealtime | Decrease in membrane viscosity |
| Chung et al. [29] | Retinal blood flow | Blood viscosity, composition | 7 female diabetes patients | 900 mg/day | 3 months | Paired | Untreated baseline | Oral at mealtime | Improved red blood cell deformity |
| Chung et al. [30] | Rheological properties | Erythrocytes | 13 type II diabetic patients w/ retinopathy | 900 mg/day | 3 months | Paired | Untreated baseline | Oral at mealtime | Reduction in blood viscosity & red blood cell oxidation |
| Kamimura [32] | Microcirculation | Mean rewarming time (MRT) | 36 microcirculatory deficiency patients | 400 mg/day | 2 weeks | Paired, cross administration | αTA & nicotinic acid | Oral at mealtime | αTN superior to αTA in reducing MRT |
| Kamimura [31] | Microcirculation | Mean rewarming time (MRT) | 10 microcirculatory deficiency patients | 400 mg/day | 2 weeks | Paired, cross administration | αTA & nicotinic acid | Oral at mealtime | αTN superior to αTA in reducing MRT |
| Igarishi et al. [38] | Hypertension | Blood pressure, animal weight | SHR and DOCA-salt hypertensive rats | 100 mg/kg/day | 4 weeks | Treated vs. controls | Gum arabic solution | Oral gavage once daily | Systolic blood pressure reduced by 15% compared to controls |
| Iino et al. [39] | Hypertension | Subjective symptoms | 89 patients with hypertension or arteriosclerosis | 600 mg/day | 4–6 weeks | Treated vs. controls | Placebo | Oral at mealtime | Symptoms improved with αTN |
| Hidiroglou et al. [40] | Cholesterol, HDL | Blood concentrations | 40 wether lambs | 300 mg/day | 8 weeks | Treated vs. controls | Placebo | Mixed with commercial diet | No significant effects on cholesterol or HDL levels |
| Higashi & Kikuchi [41] | Platelet aggregation | Platelet-rich plasma | in vitro | 0.1–0.25 mM | 1 h | Treated vs. controls | αTA | 3uL in vitro | αTN superior to αTA in reducing hydrogen peroxide-induced platelet aggregation |
| Svensson & Oki [42] | Platelet aggregation | Platelet-rich plasma | in vitro | 200 μg/mL | 2–3 h | treated vs. controls | α-Tocopherol and αTA | in Vitro bath | αTN 18x more potent than αT and 5x more potent than αTA at inhibiting platelet aggregation due to arachidonic acid |
| Noma et al. [43] | Atherogenesis | Serum lipoprotein(a) | 28 Hyperlipidemic patients | 600 mg/day | 2 months | Paired | Untreated baseline | Oral at mealtime | Lipoprotein(a) concentrations declined significantly in patients with initial lipoprotein(a) concentrations >18 mg/dL |
| Schlieper & Tawfil [18] | Arrhythmias | Inotropic action of glycosides | Guinea pigs atria | 100 μM | 1 h | Treated vs. controls | Ethanol, dodecanoic acid, α-tocopherol | in vitro bath | αTN more potent than α-tocopherol and dodecanoic acid in supressing inotropic effect of digoxin but not ouabain and also results in >90% decrease in arrhythmic activity of glycosides |
| Moriguchi & Itoh [47] | Immune system | T-cell differentiation | Male Fischer rats | 585 mg/kg/day | 7 weeks | High vs. low αTN | Low-αTN diet rats | Mixed with commercial diet | Interleukin 2 production increased and PGE2 production decreased in thymocytes and CD4+ cells increased in rats fed high αTN diet |
| Inagaki et al. [48] | Immune system | IgE antibody generation | Female BALB/c mice, male Wistar rats | 226 mg/kg food | 4 weeks | Treated vs. controls | Low vitamin E diets, αTA | Mixed with commercial diet | αTN more potent than αTA in suppressing IgE production and stimulating non-IgE antibody in antigen challenge studies |
| Tanaka et al. [49] | Immune system | Humoral immune response | Female SL and DDD mice | 226 mg/kg food | 50 days | Treated vs. controls | Low vitamin E diets, αTA | Mixed with commercial diet | αTA diet more potent than αTN diet in enhancing humoral immune response to antigen challenge; neither αTA nor αTN produced a significant effect |
| Prasad et al. [50] | Cancer | Melanoma cell | murine melanoma (B-16) and fibroblast (L-cells) cells | 1–100 μg/mL | 2 days | Treated vs. controls | Free alcohol, αTA, αTS | in vitro bath | αTS inhibiting melanoma cell proliferation; αTN and αTA not suppressing melanoma proliferation |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duncan, K.R.; Suzuki, Y.J. Vitamin E Nicotinate. Antioxidants 2017, 6, 20. https://doi.org/10.3390/antiox6010020
Duncan KR, Suzuki YJ. Vitamin E Nicotinate. Antioxidants. 2017; 6(1):20. https://doi.org/10.3390/antiox6010020
Chicago/Turabian StyleDuncan, Kimbell R., and Yuichiro J. Suzuki. 2017. "Vitamin E Nicotinate" Antioxidants 6, no. 1: 20. https://doi.org/10.3390/antiox6010020





