Hormetic Property of Ginseng Steroids on Anti-Oxidant Status against Exercise Challenge in Rat Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
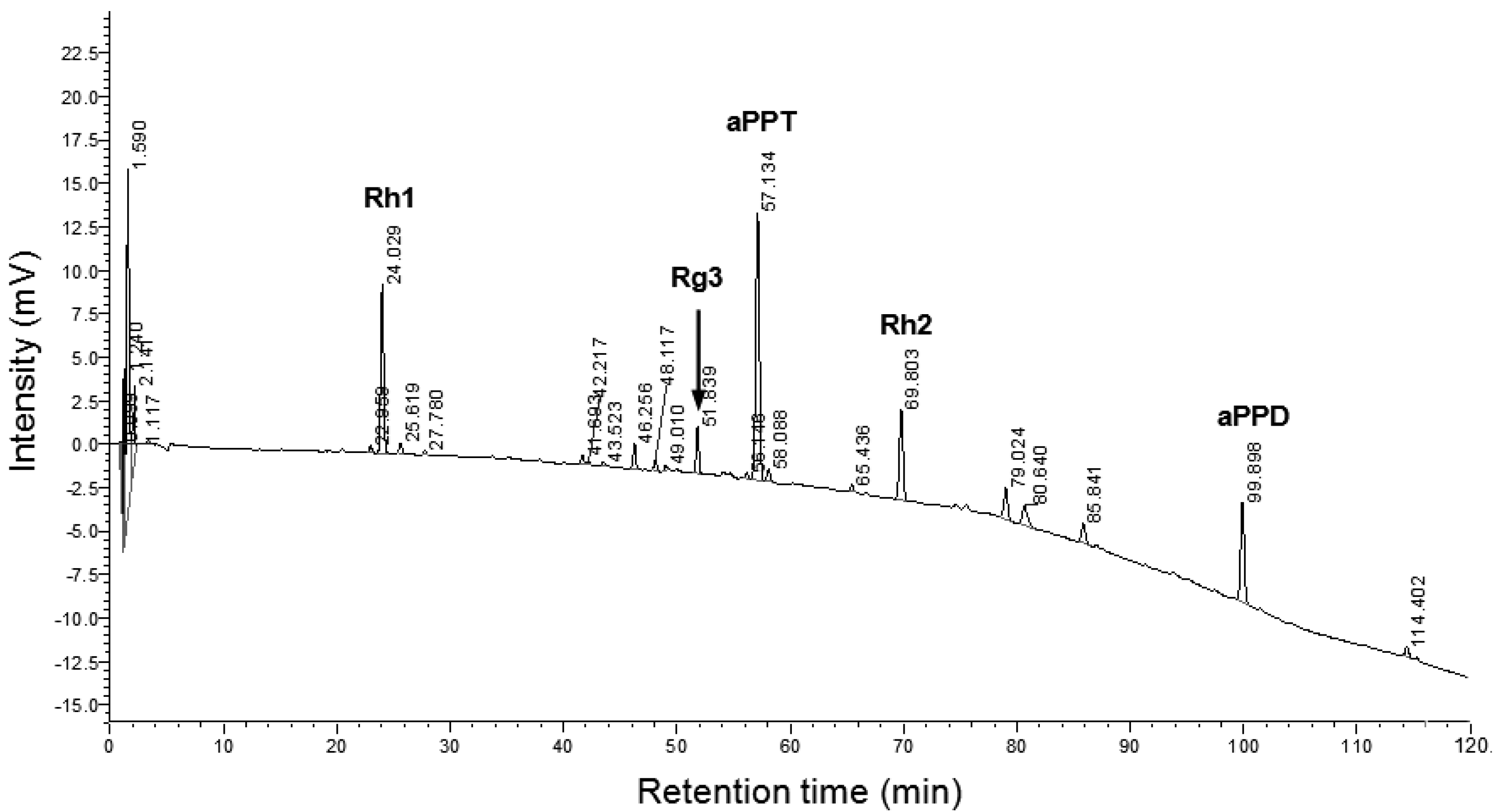
2.2. Ginseng Steroids
2.3. Experimental Design
2.4. Exercise Protocol
2.5. Tissue Isolation
2.6. Lipid Peroxidation
2.7. Reduced and Oxidized Glutathione
2.8. Anti-Oxidant Enzyme
2.9. Xanthine Oxidase
2.10. Citrate Synthase
2.11. Statistical Analysis
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Feng, Z.; Bai, L.; Yan, J.; Li, Y.; Shen, W.; Wang, Y.; Wertz, K.; Weber, P.; Zhang, Y.; Chen, Y.; et al. Mitochondrial dynamic remodeling in strenuous exercise-induced muscle and mitochondrial dysfunction: Regulatory effects of hydroxytyrosol. Free Radic. Biol. Med. 2011, 50, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Ji, L.L.; Stratman, F.W.; Lardy, H.A. Enzymatic down regulation with exercise in rat skeletal muscle. Arch. Biochem. Biophys. 1988, 263, 137–149. [Google Scholar] [CrossRef]
- Wu, J.Q.; Kosten, T.R.; Zhang, X.Y. Free radicals, antioxidant defense systems, and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 46, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Viña, J.; Ji, L.L. Interplay of oxidants and antioxidants during exercise: Implications for muscle health. Phys. Sportsmed. 2009, 37, 116–123. [Google Scholar] [CrossRef] [PubMed]
- VinÑa, J.; Gomez-Cabrera, M.C.; Lloret, A.; Marquez, R.; Miñana, J.B.; Pallardó, F.V.; Sastre, J. Free radicals in exhaustive physical exercise: Mechanism of production, and protection by antioxidants. IUBMB Life 2000, 50, 271–277. [Google Scholar] [CrossRef]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Viña, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [PubMed]
- Ramesh, T.; Kim, S.W.; Sung, J.H.; Hwang, S.Y.; Sohn, S.H.; Yoo, S.K.; Kim, S.K. Effect of fermented panax ginseng extract (ginst) on oxidative stress and antioxidant activities in major organs of aged rats. Exp. Gerontol. 2012, 47, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lee, S.M. The effects of ginseng total saponin, panaxadiol and panaxatriol on ischemia/reperfusion injury in isolated rat heart. Food Chem. Toxicol. 2010, 48, 1516–1520. [Google Scholar] [CrossRef] [PubMed]
- Dickman, J.R.; Koenig, R.T.; Ji, L.L. American ginseng supplementation induces an oxidative stress in postmenopausal women. J. Am. Coll. Nutr. 2009, 28, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Arnason, J.T.; Leiter, L.A.; Vuksan, V. Decreasing, null and increasing effects of eight popular types of ginseng on acute postprandial glycemic indices in healthy humans: The role of ginsenosides. J. Am. Coll. Nutr. 2004, 23, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Sievenpiper, J.L.; Arnason, J.T.; Leiter, L.A.; Vuksan, V. Variable effects of american ginseng: A batch of american ginseng (panax quinquefolius l.) with a depressed ginsenoside profile does not affect postprandial glycemia. Eur. J. Clin. Nutr. 2003, 57, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Chung, H.S.; Kim, M.S.; Na, H.J.; Kwon, H.J.; Kim, J.J.; Lee, K.M.; Lee, S.J.; Lim, J.P.; Do, K.R. Molecular authentication of panax ginseng species by rapd analysis and pcr-rflp. Biol. Pharm. Bull. 2001, 24, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Tawab, M.A.; Bahr, U.; Karas, M.; Wurglics, M.; Schubert-Zsilavecz, M. Degradation of ginsenosides in humans after oral administration. Drug Metab. Dispos. 2003, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Dong, T.T.; Cui, X.M.; Song, Z.H.; Zhao, K.J.; Ji, Z.N.; Lo, C.K.; Tsim, K.W. Chemical assessment of roots of panax notoginseng in china: Regional and seasonal variations in its active constituents. J. Agric. Food Chem. 2003, 51, 4617–4623. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Voces, J.; Cabral de Oliveira, A.C.; Prieto, J.G.; Vila, L.; Perez, A.C.; Duarte, I.D.G.; Alvarez, A.I. Ginseng administration protects skeletal muscle from oxidative stress induced by acute exercise in rats. Braz. J. Med. Biol. Res. 2004, 37, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Bejma, J.; Ji, L.L. Aging and acute exercise enhance free radical generation in rat skeletal muscle. J. Appl. Physiol. 1999, 87, 465–470. [Google Scholar] [PubMed]
- Huang, C.C.; Tsai, S.C.; Lin, W.T. Potential ergogenic effects of l-arginine against oxidative and inflammatory stress induced by acute exercise in aging rats. Exp. Gerontol. 2008, 43, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Popovic, L.M.; Mitic, N.R.; Radic, I.; Miric, D.; Kisic, B.; Krdzic, B.; Djokic, T. The effect of exhaustive exercise on oxidative stress generation and antioxidant defense in guinea pigs. Adv. Clin. Exp. Med. 2012, 21, 313–320. [Google Scholar] [PubMed]
- Wadley, G.D.; Nicolas, M.A.; Hiam, D.; McConell, G.K. Xanthine oxidase inhibition attenuates skeletal muscle signaling following acute exercise but does not impair mitochondrial adaptations to endurance training. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E853–E862. [Google Scholar] [CrossRef] [PubMed]
- Cabral de Oliveira, A.C.; Perez, A.C.; Prieto, J.G.; Duarte, I.D.; Alvarez, A.I. Protection of panax ginseng in injured muscles after eccentric exercise. J. Ethnopharmacol. 2005, 97, 211–214. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.M.; Li, N.; Zhang, H.; Wu, C.F.; Piao, H.R.; Zhao, Y.Q. Novel dammarane-type sapogenins from panax ginseng berry and their biological activities. Bioorg. Med. Chem. Lett. 2011, 21, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Ji, L.; Leeuwenburgh, C. Exercise training-induced alterations in skeletal muscle antioxidant capacity: A brief review. Med. Sci. Sports Exerc. 1999, 31, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Fu, R. Responses of glutathione system and antioxidant enzymes to exhaustive exercise and hydroperoxide. J. Appl. Physiol. 1992, 72, 549–554. [Google Scholar] [PubMed]
- Voces, J.; Alvarez, A.; Vila, L.; Ferrando, A.; De Oliveira, C.C.; Prieto, J. Effects of administration of the standardized panax ginseng extract g115 on hepatic antioxidant function after exhaustive exercise. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 123, 175–184. [Google Scholar] [CrossRef]
- Gum, S.I.; Jo, S.J.; Ahn, S.H.; Kim, S.G.; Kim, J.T.; Shin, H.M.; Cho, M.K. The potent protective effect of wild ginseng (panax ginseng ca meyer) against benzo [α] pyrene-induced toxicity through metabolic regulation of cyp1a1 and gsts. J. Ethnopharmacol. 2007, 112, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Oberley, T.; Oberley, L.; Slattery, A.; Lauchner, L.J.; Elwell, J. Immunohistochemical localization of antioxidant enzymes in adult syrian hamster tissues and during kidney development. Am. J. Pathol. 1990, 137, 199. [Google Scholar] [PubMed]
- Allen, R.; Balin, A.K. Oxidative influence on development and differentiation: An overview of a free radical theory of development. Free. Radic. Biol. Med. 1989, 6, 631–661. [Google Scholar] [CrossRef]
- Zhou, L.Z.; Johnson, A.P.; Rando, T.A. Nfκb and ap-1 mediate transcriptional responses to oxidative stress in skeletal muscle cells. Free Radic. Biol. Med. 2001, 31, 1405–1416. [Google Scholar] [CrossRef]
- Fernström, M.; Tonkonogi, M.; Sahlin, K. Effects of acute and chronic endurance exercise on mitochondrial uncoupling in human skeletal muscle. J. Physiol. 2004, 554, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Rosa, E.F.; Ribeiro, R.F.; Pereira, F.M.; Freymüller, E.; Aboulafia, J.; Nouailhetas, V.L. Vitamin C and E supplementation prevents mitochondrial damage of ileum myocytes caused by intense and exhaustive exercise training. J. Appl. Physiol. 2009, 107, 1532–1538. [Google Scholar] [CrossRef] [PubMed]
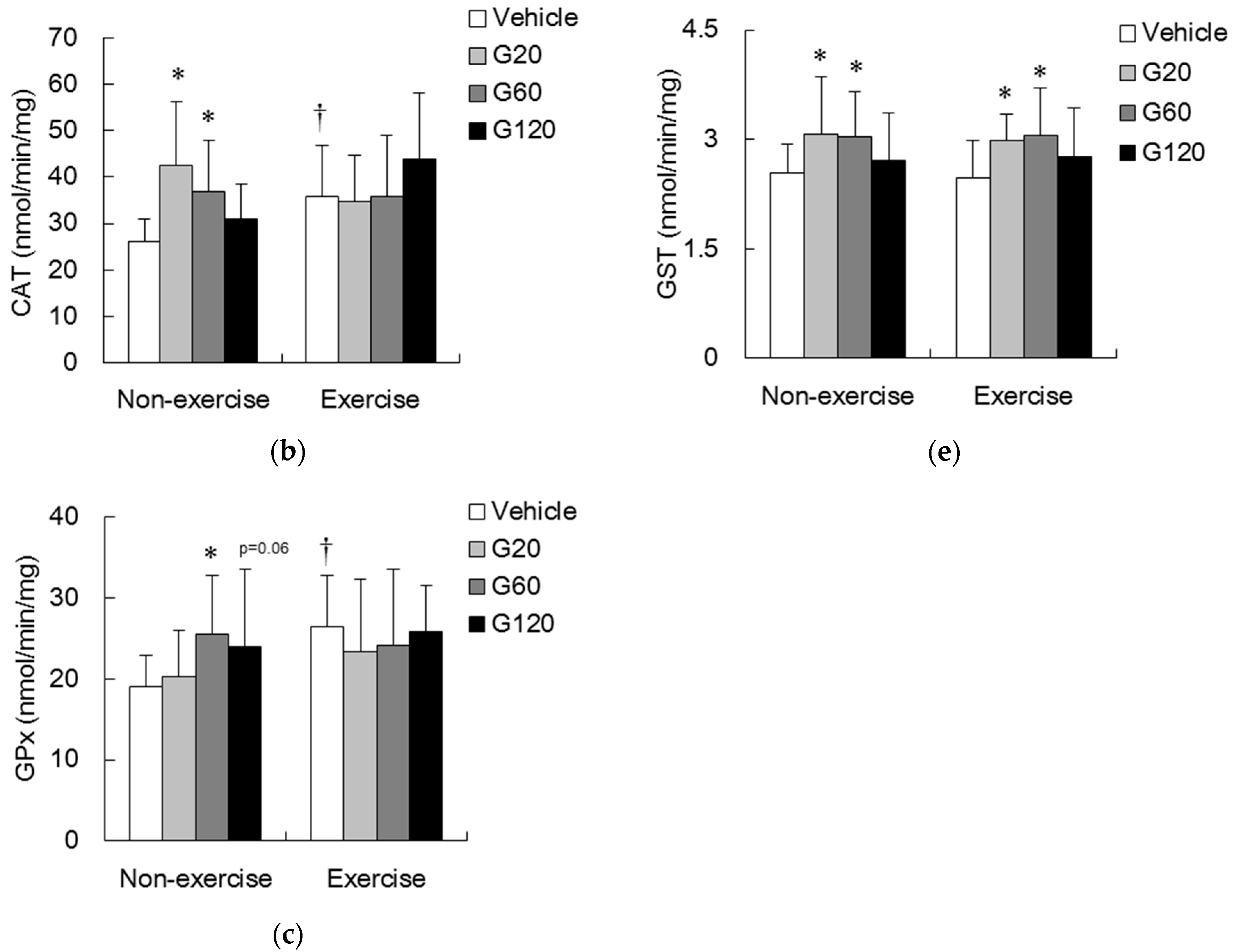
 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.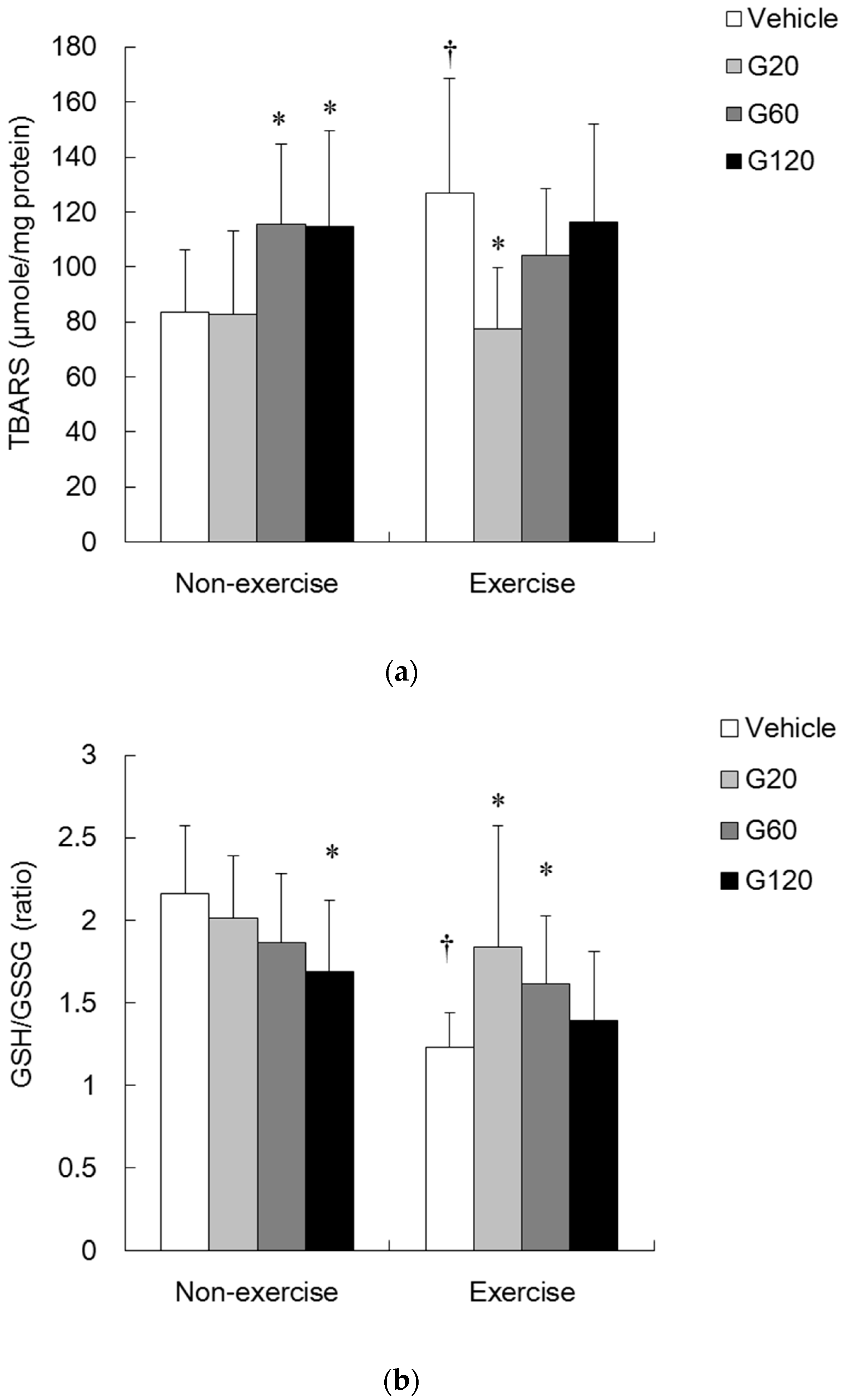
 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.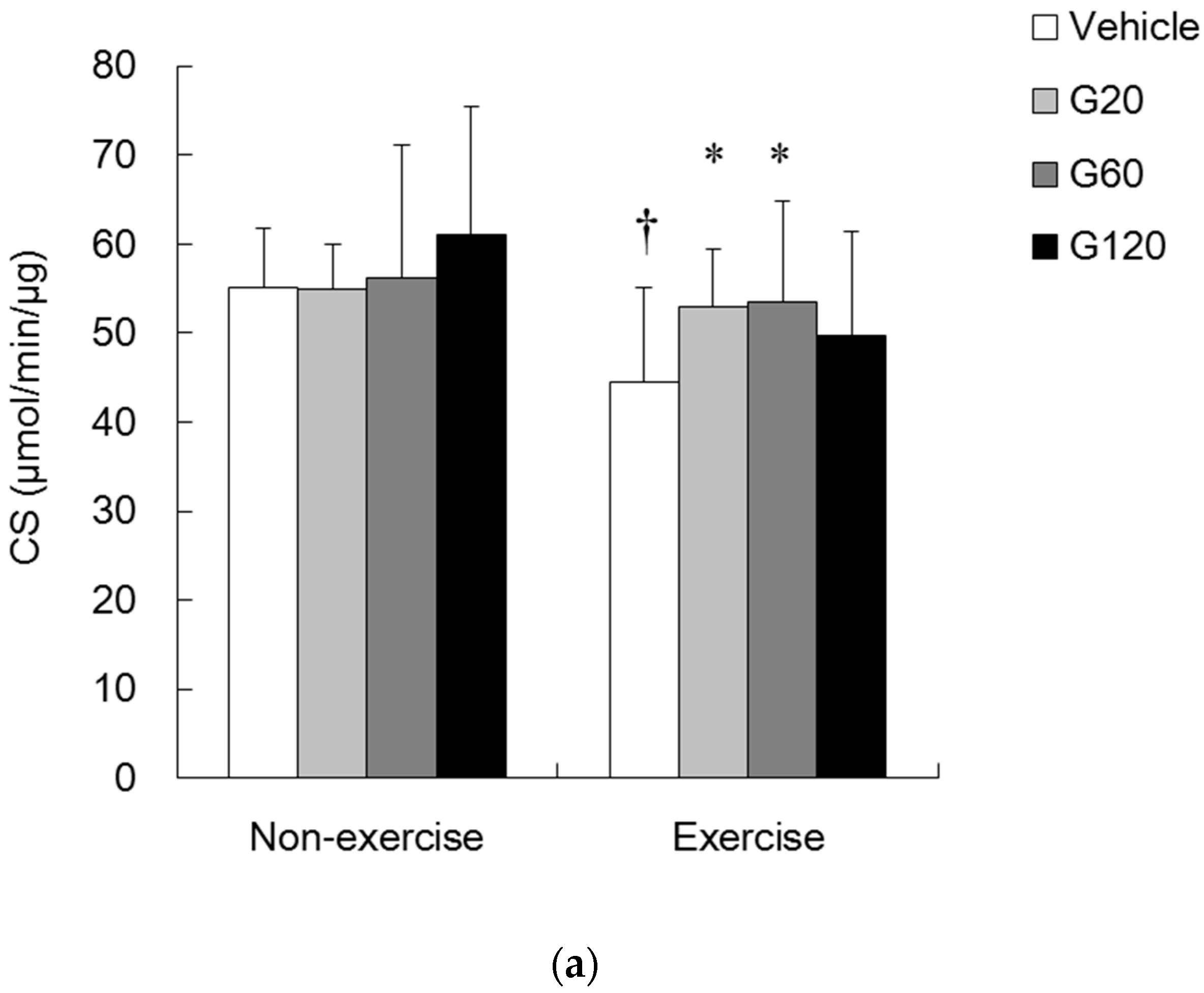

 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
 denotes significant difference against non-exercise vehicle (p < 0.05).
denotes significant difference against non-exercise vehicle (p < 0.05).  denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.
denotes significant difference against vehicle (p < 0.05). Vehicle: vehicle-treated in non-exercise (Non-exercise vehicle) or in exercise (Exercise vehicle) group which only received 0.9% saline for 10 weeks. G20: received 20 mg/kg body weight of ginseng steroids for 10 weeks. G60: received 60 mg/kg body weight of ginseng steroids for 10 weeks. G120: received 120 mg/kg body weight of ginseng steroids for 10 weeks.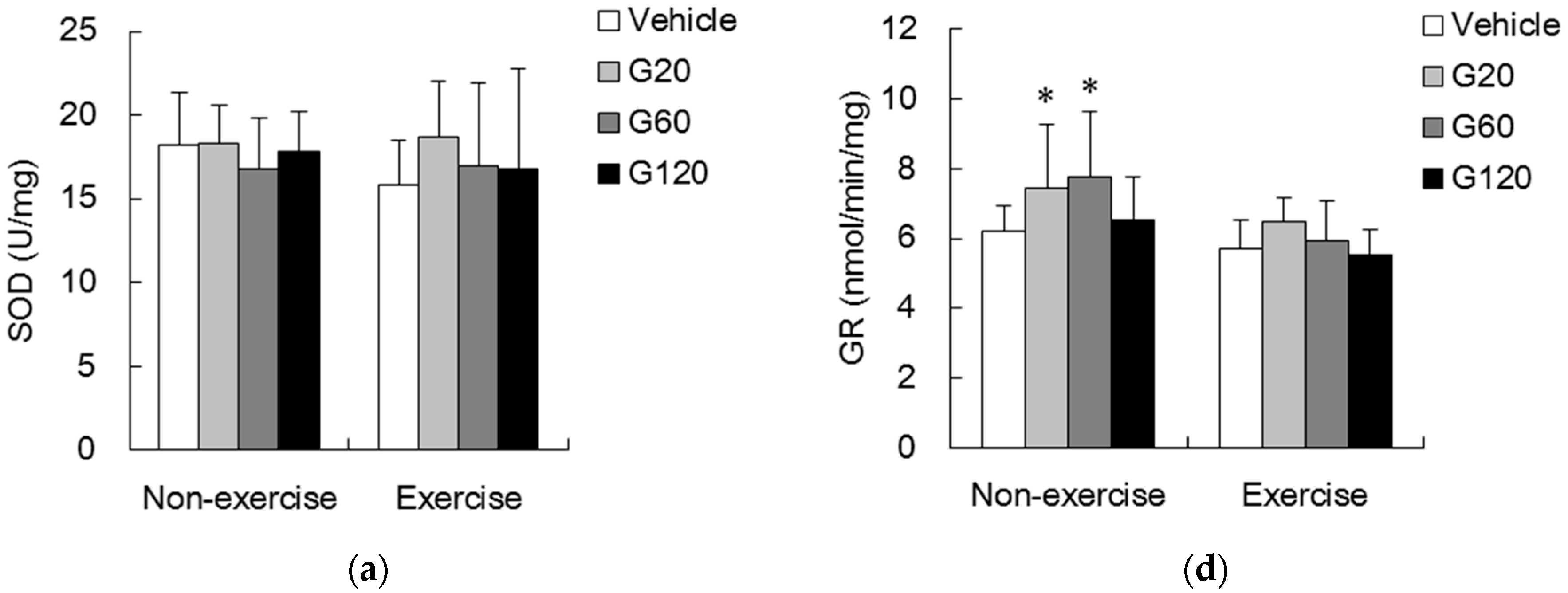
| Vehicle | G20 | G60 | G120 | |
|---|---|---|---|---|
| Non-exercise | N = 10 | N = 10 | N = 10 | N = 10 |
| Exercise | N = 10 | N = 10 | N = 10 | N = 10 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, M.-F.; Yu, S.-H.; Korivi, M.; Jean, W.-H.; Lee, S.-D.; Huang, C.-Y.; Liao, Y.-H.; Lu, J.; Kuo, C.-H. Hormetic Property of Ginseng Steroids on Anti-Oxidant Status against Exercise Challenge in Rat Skeletal Muscle. Antioxidants 2017, 6, 36. https://doi.org/10.3390/antiox6020036
Hsu M-F, Yu S-H, Korivi M, Jean W-H, Lee S-D, Huang C-Y, Liao Y-H, Lu J, Kuo C-H. Hormetic Property of Ginseng Steroids on Anti-Oxidant Status against Exercise Challenge in Rat Skeletal Muscle. Antioxidants. 2017; 6(2):36. https://doi.org/10.3390/antiox6020036
Chicago/Turabian StyleHsu, Ming-Fen, Szu-Hsien Yu, Mallikarjuna Korivi, Wei-Horng Jean, Shin-Da Lee, Chih-Yang Huang, Yi-Hung Liao, Jessica Lu, and Chia-Hua Kuo. 2017. "Hormetic Property of Ginseng Steroids on Anti-Oxidant Status against Exercise Challenge in Rat Skeletal Muscle" Antioxidants 6, no. 2: 36. https://doi.org/10.3390/antiox6020036









