Fate and Prediction of Phenolic Secoiridoid Compounds throughout the Different Stages of the Virgin Olive Oil Making Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Olive Fruit Sampling and VOO Processing
2.2. Analytical Determinations in Olive Fruit and Paste
2.3. Analytical Determinations in Virgin Olive Oil
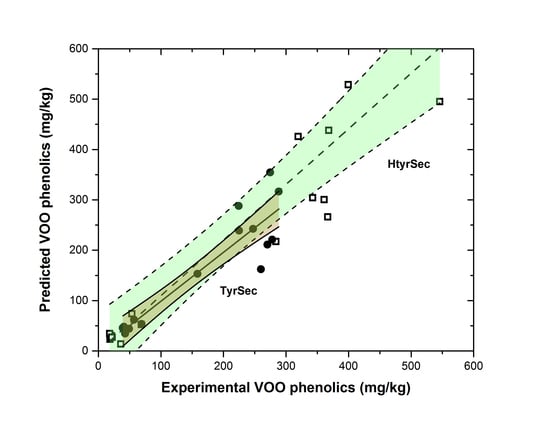
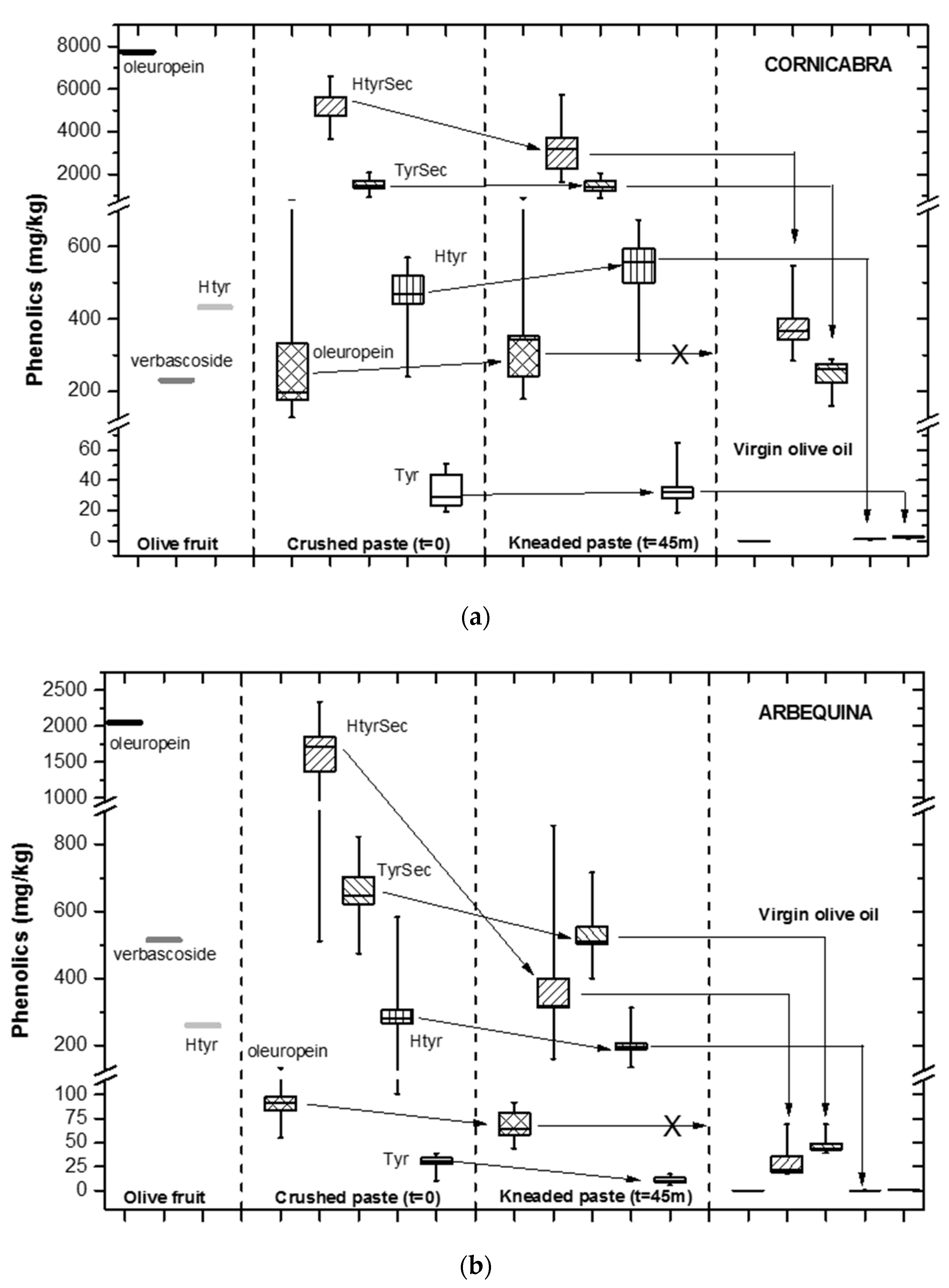
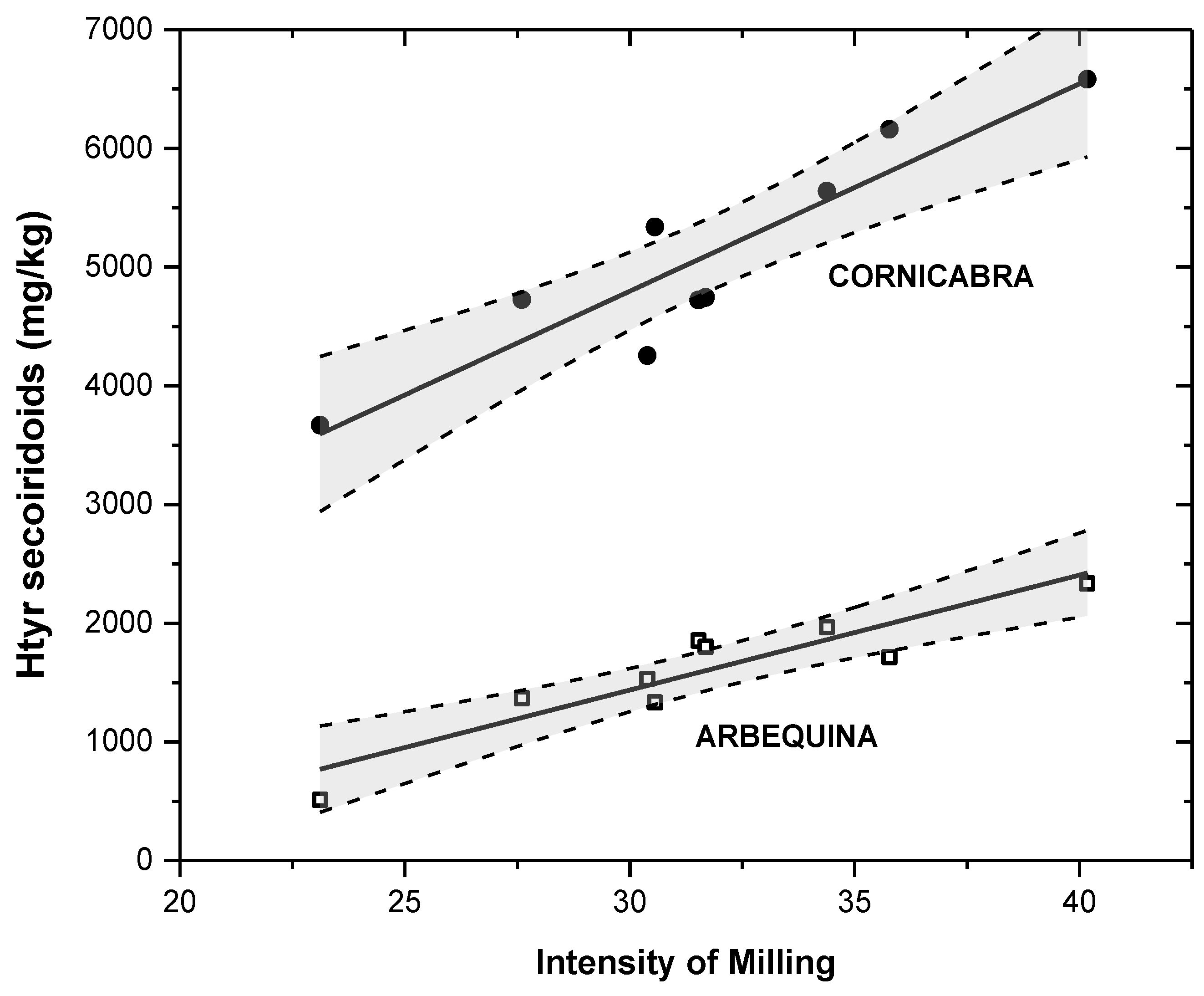
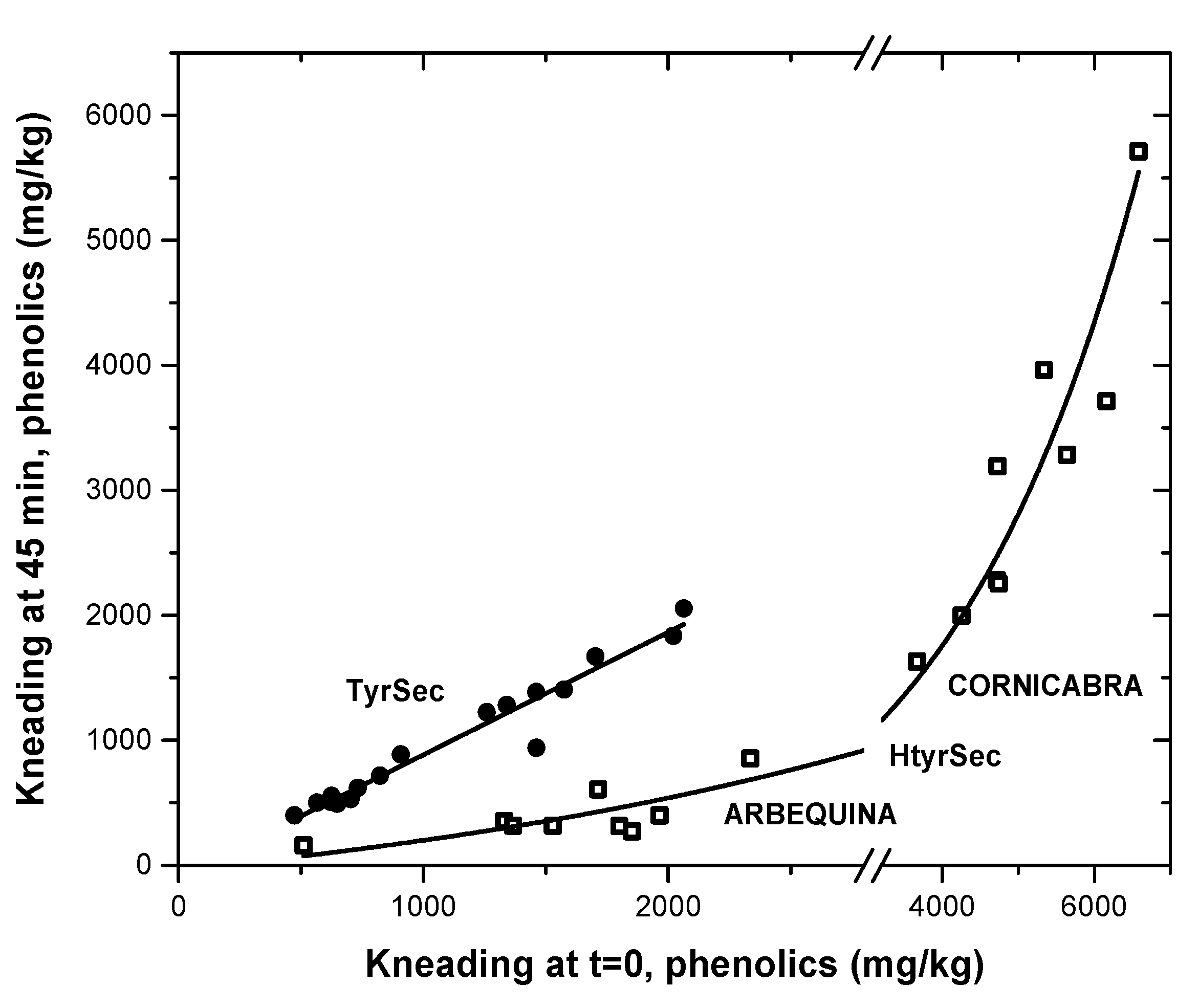
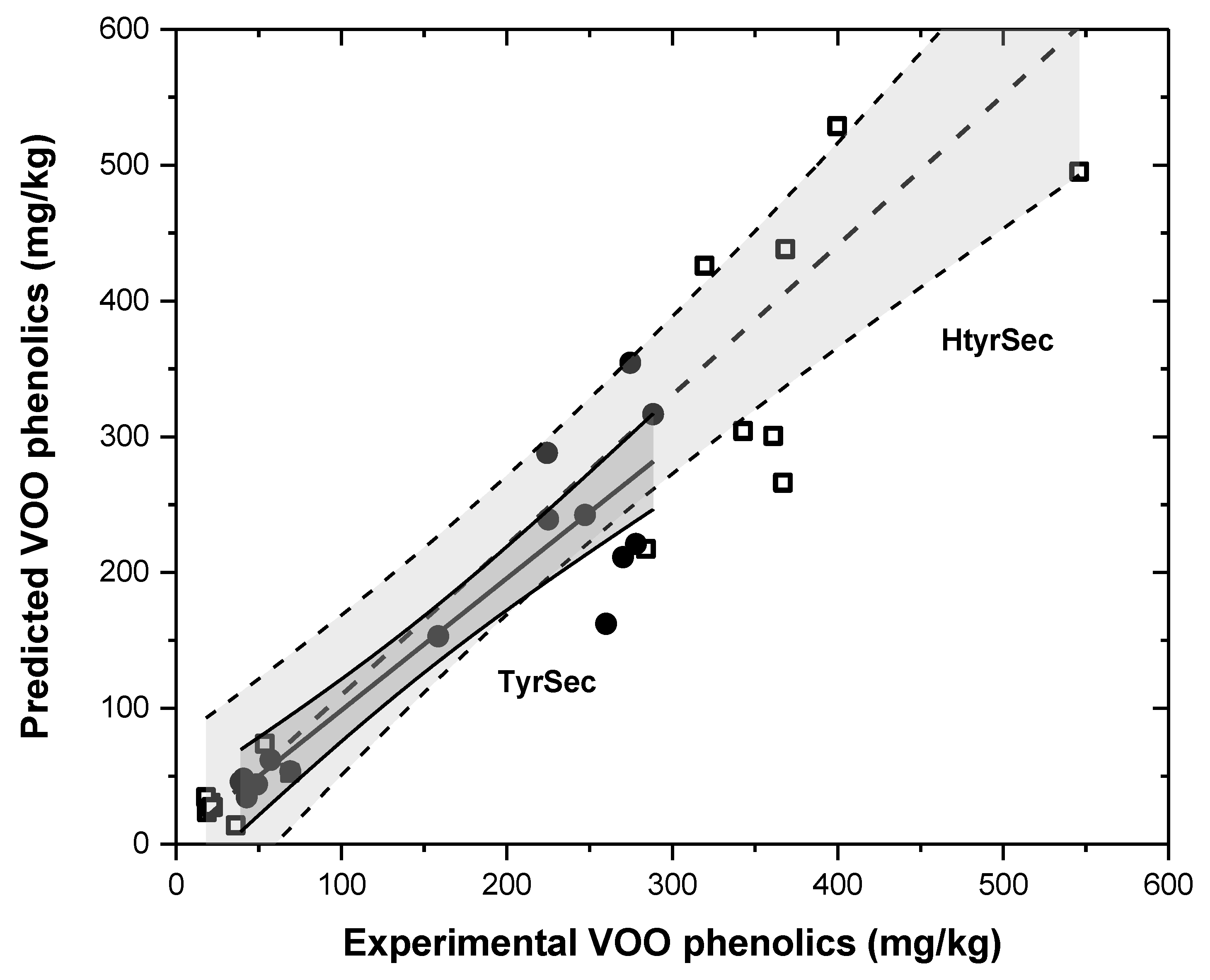
3. Results and Discussion
3.1. Effect of Crushing
3.2. Evolution during Malaxation
3.3. Transfer to the Oily Phase
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Clodoveo, M.L. New advances in the development of innovative virgin olive oil extraction plants: Looking back to see the future. Food Res. Int. 2013, 54, 726–729. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Hachicha, R.; Kotti, F.; Scarascia, G.; Gargouri, M. Mechanical strategies to increase nutritional and sensory quality of virgin olive oil by modulating the endogenous enzyme activities. Compr. Rev. Food Sci. 2014, 13, 135–154. [Google Scholar] [CrossRef]
- Caponio, F.; Alloggio, V.; Gomes, T. Phenolic compounds of virgin olive oil: Influence of paste preparation techniques. Food Chem. 1999, 64, 203–209. [Google Scholar] [CrossRef]
- Di Giovachino, L.; Sestili, S.; di Vincenzo, D. Influence of olive processing on virgin olive oil quality. Eur. J. Lipid Sci. Technol. 2002, 104, 587–601. [Google Scholar] [CrossRef]
- Gómez-Rico, A.; Inarejos-García, A.; Salvador, M.D.; Fregapane, G. Effect of malaxation conditions on phenol and volatile profiles in olive paste and the corresponding virgin olive oils (Olea europaea L. cv. Cornicabra). J. Agric. Food Chem. 2009, 57, 3587–3595. [Google Scholar] [CrossRef] [PubMed]
- Inarejos-García, A.M.; Gómez-Rico, A.; Salvador, M.D.; Fregapane, G. Influence of malaxation conditions on virgin olive oil yield, overall quality and composition. Eur. Food Res. Technol. 2009, 228, 671–677. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Salvador, M.D.; Fregapane, G. Effect of crushing on olive paste and virgin olive oil minor components. Eur. Food Res. Technol. 2011, 232, 441–451. [Google Scholar] [CrossRef]
- Fregapane, G.; Salvador, M.D. Production of superior extra virgin olive oil modulating the content and profile of its minor components. Food Res. Int. 2013, 54, 1907–1914. [Google Scholar] [CrossRef]
- Aparicio, R.; Morales, M.T.; Alonso, M.V. Relationship between volatile compounds and sensory attributes of olive oil by the sensory wheel. J. Am. Oil Chem. Soc. 1996, 73, 1253–1264. [Google Scholar] [CrossRef]
- Angerosa, F. Influence of volatile compounds on virgin olive oil quality evaluated by analytical approaches and sensor panels. J. Lipid Sci. Technol. 2002, 104, 639–660. [Google Scholar] [CrossRef]
- Cerretani, L.; Salvador, M.D.; Bendini, A.; Lercker, G.; Fregapane, G. Relationship between sensory evaluation performed by Italian and Spanish official panels and volatile and phenolic profiles of virgin olive oils. Chemoses. Percept. 2008, 1, 258–267. [Google Scholar] [CrossRef]
- Inarejos-García, A.M.; Santacatterina, M.; Gómez-Alonso, S.; Salvador, M.D.; Fregapane, G. PDO virgin olive oils quality. Minor components profiles and organoleptic evaluation. Food Res. Int. 2010, 43, 2138–2146. [Google Scholar] [CrossRef]
- Sanchez, J.; Harwood, J.L. Byosinthesis of triacylglycerols and volatiles in olives. Eur. J. Lipid Sci. Technol. 2002, 104, 564–573. [Google Scholar] [CrossRef]
- Angerosa, F.; Mostallino, R.; Basti, C.; Vito, R. Virgin olive oil odour notes: Their relationship with volatile compounds from the lipoxygenase pathway and secoiridoid compounds. Food Chem. 2000, 68, 283–287. [Google Scholar] [CrossRef]
- Cicerale, S.; Conlan, X.A.; Sinclair, A.J.; Keast, R.S.J. Chemistry and Health of Olive Oil Phenolics. Crit. Rev. Food Sci. Nutr. 2009, 49, 218–236. [Google Scholar] [CrossRef] [PubMed]
- Cicerale, S.; Lucas, L.J.; Keast, R.S.J. Antimicrobial, antioxidant and anti-inflammatory phenolic activities in extra virgin olive oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef] [PubMed]
- European Community Regulation No 432/2012. European Community Regulation Establishing a List of 199 Permitted Health Claims Made on Foods, Other Than Those Referring to the 200 Reduction of Disease Risk and to Children’s Development and Health. Available online: http://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A32012R0432 (assessed on 1 June 2017).
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; León-Camacho, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones and lignans in virgin olive oil by solid phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Pancorbo, A.; Cerretani, L.; Bendini, A.; Segura-Carretero, A.; Gallina-Toschi, T.; Fernandez-Gutierez, A. Analytical determination of polyphenols in olive oils. J. Sep. Sci. 2005, 28, 837–858. [Google Scholar] [CrossRef] [PubMed]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: A survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tovar, M.J.; Romero, M.P.; Motilva, M.J. Changes in the phenolic composition of olive oil from young trees (Olea europaea L. cv. Arbequina) grown under linear irrigation strategies. J. Agric. Food Chem. 2001, 49, 5502–5508. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Salvador, M.D.; La Greca, M.; Fregapane, G. Phenolic and volatile compounds of extra virgin olive oil (Olea europea L. cv. Cornicabra) with regards to fruit ripening and irrigation management. J. Agric. Food Chem. 2006, 54, 7130–7136. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rico, A.; Fregapane, G.; Salvador, M.D. Effect of cultivar and ripening on minor components in Spanish olive fruits and their corresponding virgin olive oils. Food Res. Int. 2008, 41, 433–440. [Google Scholar] [CrossRef]
- El Riachy, M.; Priego-Capote, F.; Leon, L.; Rallo, L.; Luque de Castro, M.D. Hydrophilic antioxidants of virgin olive oil. Part 2: Biosynthesis and biotransformation of phenolic compounds in virgin olive oil as affected by agronomic and processing factors. Eur. J. Lipid Sci. Technol. 2011, 113, 692–707. [Google Scholar] [CrossRef]
- Servili, M.; Selvaggini, R.; Taticchi, A.; Esposto, S.; Montedoro, G.F. Volatile compounds and phenolic composition of virgin olive oil: Optimization of temperature and time of exposure of olive pastes to air contact during the mechanical extraction process. J. Agric. Food Chem. 2003, 51, 7980–7988. [Google Scholar] [CrossRef] [PubMed]
- Servili, M.; Taticchi, A.; Esposto, S.; Urbani, S.; Selvaggini, R.; Montedoro, G.F. Influence of the decrease in oxygen during malaxation of olive paste on the composition of volatiles and phenolic compounds in virgin olive oil. J. Agric. Food Chem. 2008, 56, 10048–10055. [Google Scholar] [CrossRef] [PubMed]
- Amirante, P.; Clodoveo, M.L.; Dugo, G. New mixer equipped with control atmosphere system: Influence of malaxation on the shelf life of extra virgin olive oil Italian. J. Food Sci. 2007, 19, 215–220. [Google Scholar]
- Leone, A.; Romaniello, R.; Zagaria, R.; Tamborrino, A. Development of a prototype malaxer to investigate the influence of oxygen in extra-virgin olive oil quality and yield to define a new desing of machine. Biosyst. Eng. 2014, 118, 95–104. [Google Scholar] [CrossRef]
- Tamborrino, A.; Pari, S.; Romaniello, R.; Quinto, M.; Zagaria, R.; Leone, A. Desing and implementation of an automatically controlled malaxer pilot plant equipped with an in line oxigen injection systems into the olive paste. J. Food Eng. 2014, 141, 1–12. [Google Scholar] [CrossRef]
- Sanchez-Ortiz, A.; Bejaoui, M.A.; Aguilera, M.P.; Jimenez, A.; Beltran, G. Application of oxygen during olive fruit crushing impacts on the characteristics and sensory profile of the virgin olive oil. Eur. J. Lipid Sci. Technol. 2016, 118, 1018–1029. [Google Scholar] [CrossRef]
- Clodoveo, M.L.; Durante, V.; La Notte, D.; Punzi, R.; Gambacorta, G. Ultrasound-assisted extraction of virgin olive oil to improve the process efficiency. Eur. J. Lipid Sci. Technol. 2013, 115, 1062–1069. [Google Scholar] [CrossRef]
- Abenoza, M.; Benito, M.; Saldana, G. Effects of Pulsed Electric Field on Yield Extraction and Quality of Olive Oil. Food Bioprocess Technol. 2013, 6, 1367–1373. [Google Scholar] [CrossRef]
- Tamborrino, A.; Romaniello, R.; Zagaria, R. Microwave-assisted treatment for continuous olive paste conditioning: Impact on olive oil quality and yield. Biosyst. Eng. 2014, 127, 92–102. [Google Scholar] [CrossRef]
- Puertolas, E.; Martinez de Maranon, I. Olive oil pilot-production assisted by pulsed electric field: Impact on extraction yield, chemical parameters and sensory properties. Food Chem. 2015, 167, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Bejaoui, M.A.; Beltran, G.; Sanchez-Ortiz, A.; Sanchez, S.; Jimenez, A. Continuous high power ultrasound treatment before malaxation, a laboratory scale approach: Effect on virgin olive oil quality criteria and yield. Eur. J. Lipid Sci. Technol. 2016, 118, 332–336. [Google Scholar] [CrossRef]
- Fiori, F.; di Lecce, G.; Boselli, E.; Pieralisi, G.; Frega, N.G. Effects of olive paste fast preheating on the quality of extra virgin olive oil during storage. LWT-Food Sci. Technol. 2014, 58, 511–518. [Google Scholar] [CrossRef]
- Veneziani, G.; Esposto, S.; Taticchi, A.; Urbani, S.; Selvaggini, R.; di Maio, I.; Sordini, B.; Servili, M. Cooling treatment of olive paste during the oil processing: Impact on the yield and extra virgin olive oil quality. Food Chem. 2017, 221, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Caponio, F.; Gomes, T.; Summo, C.; Pasqualone, A. Influence of the type of olive-crusher used on the quality of extra virgin olive oils. Eur. J. Lipid Sci. Technol. 2003, 105, 201–206. [Google Scholar] [CrossRef]
- Catalano, P.; Caponio, F. Machines for olive paste preparation producing quality virgin olive oil. Eur. J. Lipid Sci. Technol. 1996, 98, 408–412. [Google Scholar] [CrossRef]
- Di Giovacchino, L.; Surricchio, G. La preparazione delle paste de olive in oleificio: Influenza sulla qualitá dell´olio. Frutticolt 2000, 20, 55–59. [Google Scholar]
- Artajo, L.S.; Romero, M.P.; Suarez, L.; Motilva, M.J. Partition of phenolics compounds during the virgin olive oil industry extraction process. Eur. Food Res. Technol. 2007, 225, 617–625. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Mozetic Vodopivec, B. The fate of olive fruit phenols during commercial olive oil processing: Traditional press versus continuous two-and three-phase centrifuge. LWT-Food Sci. Technol. 2012, 49, 267–274. [Google Scholar] [CrossRef]
- Jerman Klen, T.; Wondra, A.G.; Vrhovšek, U.; Sivilotti, P.; Mozetic Vodopivec, B. Olive fruit phenols transfer, transformation, and partition trail during laboratory-scale olive oil processing. J. Agric. Food Chem. 2015, 63, 4570–4579. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; de la Rosa, L.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From Olive Fruits to Olive Oil: Phenolic Compound Transfer in Six Different Olive Cultivars Grown under the Same Agronomical Conditions. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Martínez Suárez, J.M.; Muñoz Aranda, E.; Alba Mendoza, J.; Lanzón Rey, A. Informe sobre la utilización del Analizador de rendimientos Abencor. Grasas Aceites 1975, 26, 379–385. [Google Scholar]
- AENOR (1973). Spanish Standard Method UNE 55032:1973 Asociación Española de Normalización y Certificación (AENOR). Available online: http://www.aenor.es/aenor/normas/normas/fichanorma.asp?tipo=N&codigo=N0005672#.WYKmH1ERWUm (assessed on 1 June 2017).
- European Community Regulation 2568/91. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A01991R2568-20151016 (assessed on 1 June 2017).
- Servili, M.; Selvaggini, R.; Esposto, S.; Taticchi, A.; Montedoro, G.F.; Morozzi, G. Health and sensory properties of virgin olive oil hydrophilic phenols: Agronomic and technological aspects of production that affect their occurrence in the oil. J. Chromatogr. A 2004, 1054, 113–127. [Google Scholar] [CrossRef]
- Brenes, M.; García, A.; García, P.; Ríos, J.J.; Garrido, A. Phenolic compounds in Spanish olive oils. J. Agric. Food Chem. 1999, 47, 3535–3540. [Google Scholar] [CrossRef]
- European Community Regulation 702/2007. Off. J. Eur. Communities 2007, 178, 11–16. Available online: http://eur-lex.europa.eu/eli/reg/2007/702/oj (assessed on 1 June 2017).
- Marsilio, V.; Lanza, B. Characterisation of an oleuropein degrading strain of Lactobacillus plantarum. Combined effects of compounds present in olive fermenting brines (phenols, glucose and NaCl) on bacterial activity. J. Sci. Food Agric. 1998, 76, 5203–5524. [Google Scholar] [CrossRef]
- Romero-Segura, C.; Sanz, C.; Pérez, A.G. Purification and characterization of an olive fruit β-glucosidase involved in the biosynthesis of virgin olive oil phenolics. J. Agric. Food Chem. 2009, 57, 7983–7988. [Google Scholar] [CrossRef] [PubMed]
- Rodis, P.S.; Karathanos, V.T.; Mantzavinou, A. Partitioning of olive oil antioxidants between oil and water phases. J. Agric. Food Chem. 2002, 50, 596–601. [Google Scholar] [CrossRef] [PubMed]
- Romero-Segura, C.; Garcia-Rodriguez, R.; Sanchez-Ortiz, A.; Sanz, C.; Pérez, A.G. The role of olive β-glucosidase in shaping the phenolic profile of virgin olive oil. Food Res. Int. 2012, 45, 191–196. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fregapane, G.; Salvador, M.D. Fate and Prediction of Phenolic Secoiridoid Compounds throughout the Different Stages of the Virgin Olive Oil Making Process. Antioxidants 2017, 6, 61. https://doi.org/10.3390/antiox6030061
Fregapane G, Salvador MD. Fate and Prediction of Phenolic Secoiridoid Compounds throughout the Different Stages of the Virgin Olive Oil Making Process. Antioxidants. 2017; 6(3):61. https://doi.org/10.3390/antiox6030061
Chicago/Turabian StyleFregapane, Giuseppe, and M. Desamparados Salvador. 2017. "Fate and Prediction of Phenolic Secoiridoid Compounds throughout the Different Stages of the Virgin Olive Oil Making Process" Antioxidants 6, no. 3: 61. https://doi.org/10.3390/antiox6030061