Development of a Rapid Method for the Determination of Caffeine in Coffee Grains by GC-FID—A Fully Validated Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instrumentation
2.2. Analytical Procedure
2.3. Method Validation
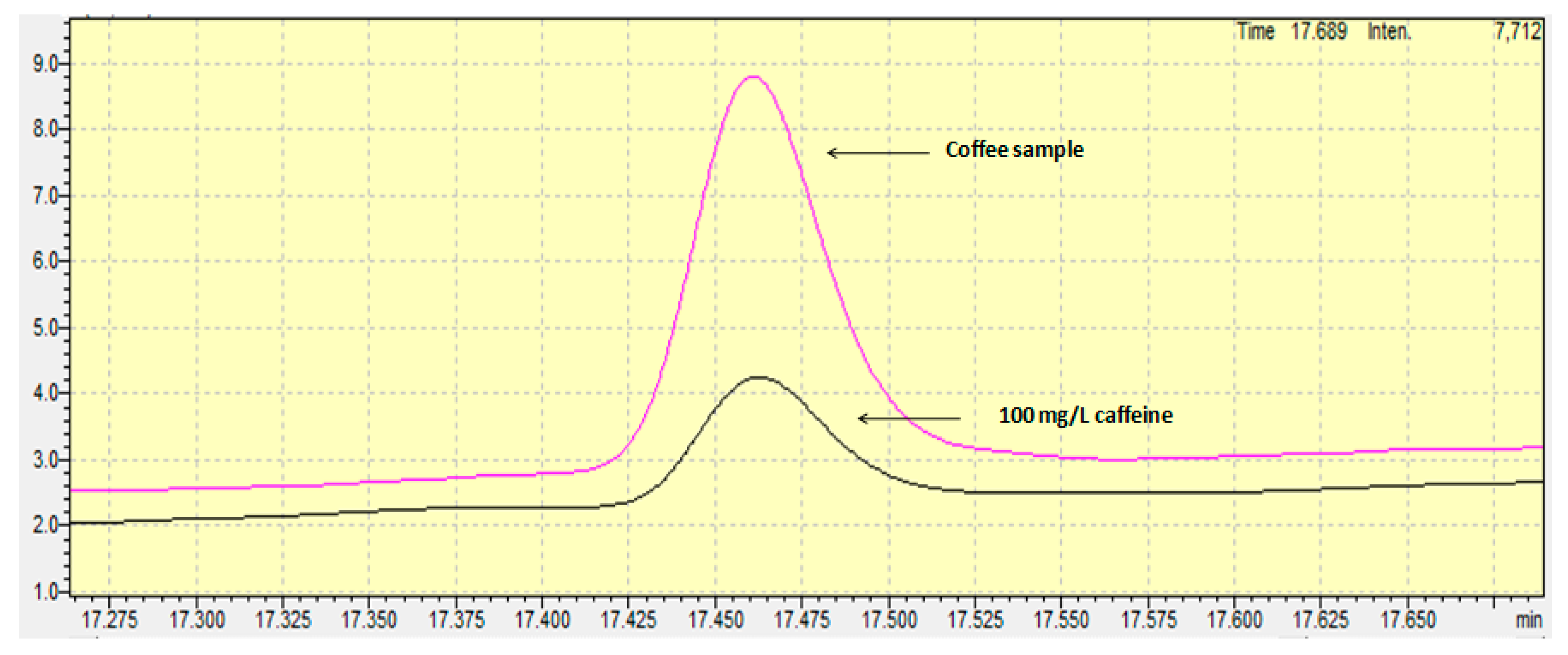
3. Results and Discussion
3.1. Results of Method Validation
3.2. Health Claim
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Dziki, D.; Gawlik-Dziki, U.; Pecio, Ł.; Rózyło, R.; Krzykowski, M.S.A.; Rudy, S. Ground green coffee beans as a functional food supplement-Preliminary study. LWT Food Sci. Technol. 2015, 63, 691–699. [Google Scholar] [CrossRef]
- Richards, G.; Smith, A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef] [Green Version]
- Jeszka-Skowron, M.; Sentkowska, A.; Pyrzynska, K.; De Peña, M.P. Chlorogenic acids, caffeine content and antioxidant properties of green coffee extracts: Influence of green coffee bean preparation. Eur. Food Res. Technol. 2016, 242, 1403–1409. [Google Scholar] [CrossRef]
- Zou, J.; Li, N. Simple and environmental friendly procedure for the gas chromatographic–mass spectrometric determination of caffeine in beverages. J. Chromatogr. A 2006, 1136, 106–110. [Google Scholar] [CrossRef] [PubMed]
- ISO/IEC 17025:2005. General Requirements for the Competence of Testing and Calibration Laboratories. Available online: https://www.iso.org/standard/39883.html (accessed on 26 July 2017).
- European Commission. Decision of 12 August 2002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results, L 221/8–L 221/36. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32002D0657 (accessed on 12 August 2002).
- Thompson, M. Recent trends in inter-laboratory precision at ppb and sub-ppb concentrations in relation to fitness for purpose criteria in proficiency testing. Analyst 2000, 125, 385–386. [Google Scholar] [CrossRef]
- Pasias, I.N.; Kiriakou, I.K.; Proestos, C. HMF and diastase activity in honeys: A fully validated approach and a chemometric analysis for identification of honey freshness and adulteration. Food Chem. 2017, 229, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Psoma, A.K.; Pasias, I.N.; Rousis, N.I.; Barkonikos, K.A.; Thomaidis, N.S. Development, validation and accreditation of a method for the determination of Pb, Cd, Cu and As in seafood and fish feed samples. Food Chem. 2014, 151, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Raptopoulou, K.G.; Pasias, I.N.; Thomaidis, N.S.; Proestos, C. Study of the migration phenomena of specific metals in canned tomato paste before and after opening. Validation of a new quality indicator for opened cans. Food Chem. Toxicol. 2014, 69, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Pasias, I.N.; Papageorgiou, V.; Thomaidis, N.S.; Proestos, C. Development and Validation of an ETAAS Method for the Determination of Tin in Canned Tomato Paste Samples. Food Anal. Methods 2012, 5, 835–840. [Google Scholar] [CrossRef]
- Taverniers, I.; de Loose, M.; van Bockstaele, E. Trends in quality in the analytical laboratory. II. Analytical method validation and quality assurance. Trends Anal. Chem. 2004, 23, 535–552. [Google Scholar] [CrossRef]
- Eurachem Citac Guide CG4 (2012). Quantifying Uncertainty in Analytical Measurement. Available online: https://www.researchgate.net/profile/Ales_Fajgelj/publication/236884917_Quantifying_Uncertainty_in_Analytical_Measurement_QUAM/links/02e7e519e53565dfa9000000.pdf (accessed on 26 July 2017).
- Hewavitharana, A.K. Internal Standard—Friend or Foe? Crit. Rev. Anal. Chem. 2009, 39, 272–275. [Google Scholar] [CrossRef]
- Fernández, P.L.; Martín, M.J.; González, A.G.; Pablos, F. HPLC determination of catechins and caffeine in tea. Differentiation of green, black and instant teas. Analyst 2002, 125, 421–425. [Google Scholar] [CrossRef]
- Alpdogan, G.; Karabina, K.; Sungur, S. Derivative spectrophotometric determination of caffeine in some beverages. Turk. J. Chem. 2002, 26, 295–302. [Google Scholar]
- Shrivas, K.; Wu, H. Rapid determination of caffeine in one drop of beverages and foods using drop-to-drop solvent microextraction with gas chromatography/mass spectrometry. J. Chromatogr. A 2007, 1170, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Ashley, D.L. Sample purification for the analysis of caffeine in tobacco by gas chromatography-mass spectrometry. J. Chromatogr. A 1998, 814, 171–180. [Google Scholar] [CrossRef]
- Frizzarin, R.M.; Maya, F.; Estela, J.M.; Cerdà, V. Fully-automated in-syringe dispersive liquid-liquid microextraction for the determination of caffeine in coffee beverages. Food Chem. 2016, 212, 759–767. [Google Scholar] [CrossRef] [PubMed]
| Fortification Level | % RSDr | % RSDR |
|---|---|---|
| 0.5 | 4.1 | 4.5 |
| 1.0 | 2.5 | 3.4 |
| 2.0 | 2.0 | 2.2 |
| Fortification Level | % Recovery | Standard Deviation |
|---|---|---|
| 0.5 | 93.5 | 4.3 |
| 1.0 | 96.7 | 3.3 |
| 2.0 | 102.0 | 2.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasias, I.N.; Kiriakou, I.; Proestos, C. Development of a Rapid Method for the Determination of Caffeine in Coffee Grains by GC-FID—A Fully Validated Approach. Antioxidants 2017, 6, 67. https://doi.org/10.3390/antiox6030067
Pasias IN, Kiriakou I, Proestos C. Development of a Rapid Method for the Determination of Caffeine in Coffee Grains by GC-FID—A Fully Validated Approach. Antioxidants. 2017; 6(3):67. https://doi.org/10.3390/antiox6030067
Chicago/Turabian StylePasias, Ioannis N., I. Kiriakou, and Charalampos Proestos. 2017. "Development of a Rapid Method for the Determination of Caffeine in Coffee Grains by GC-FID—A Fully Validated Approach" Antioxidants 6, no. 3: 67. https://doi.org/10.3390/antiox6030067