3.1. Extraction Optimization in Batch System
The effects of three independent variables on the yields of TPC and DPPH radical scavenging values of extracts are shown in
Table 1. Phenolic compounds extracted from BSG ranged from 1.59 to 3.57 mg GAE/g, whereas antioxidant activity varied between 1.86 and 11.93% of DPPH radical inhibition. These values are comparable to those reported by other authors [
10,
11], whose results ranged from 1.26 to 7.13 mg GAE/g DW and 12.02 to 16.91% inhibition, for TPC and DPPH assays, respectively. A value of 20 mg GAE/g DW for TPC assay was reported by Moreira [
1] using an optimized microwave-assisted method under alkaline conditions, thus showing an advantage of these novel extraction techniques compared to conventional ones.
For each response, regression equations evaluating the effects of each factor and their interactions were obtained. After subsequent statistical analysis by the ANOVA test (
Table 4), the predictive fitted equation models and significant terms (
p > 0.05) were obtained (
Table 5).
The corresponding coefficients of determination (R2) of the models were 0.9701, and 0.9864 for TPC and antioxidant activity, respectively. These values showed that more than 97.01% of the total variation in the response was explained by the models. Additionally, the very low p-values (<0.0001) in each evaluated response indicated the significance of the model terms. In addition, the non-significant value of lack of fit (p > 0.05) showed that the models could be used to predict the results. Finally, response surface plots were established using the fitted models in order to determine the optimal levels of the evaluated variables on extraction of phenolic compounds and antioxidant activity.
3.2. Effect of Extraction Conditions on TPC Yield
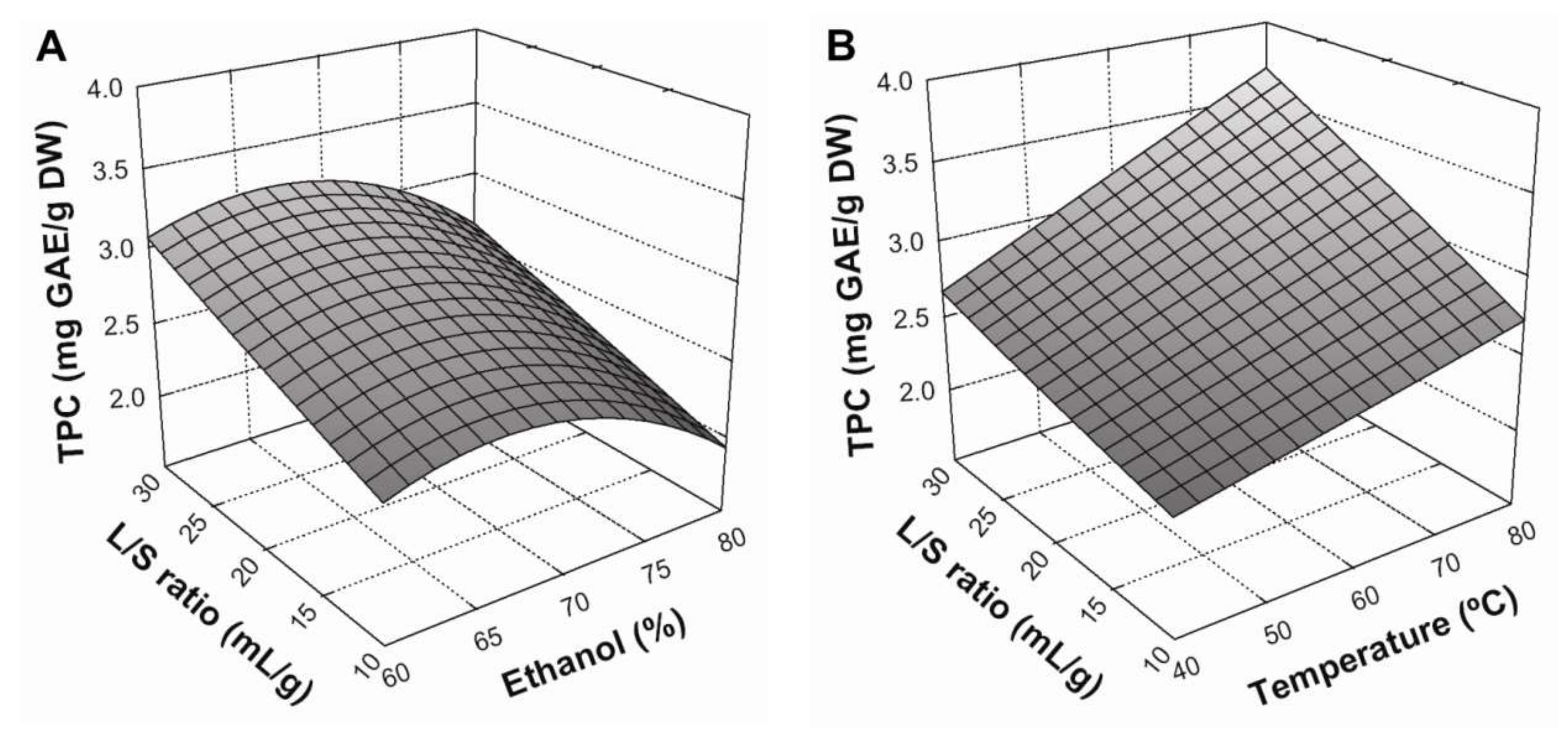
Figure 1 presents the response surface plots for the influence of extraction parameters on TPC yield. An increased extraction yield was observed when the liquid/solid (L/S) ratio increased from 10:1 to 30:1 mL/g (
Figure 1A). This is concordant with mass transfer principles, since a higher L/S ratio implies higher concentration gradient between the solid and the bulk of the liquid, resulting in a greater driving force for diffusion of compounds to the solvent. This effect was further improved by increasing extraction temperature from 40 to 80 °C (
Figure 1B), showing a considerable positive interaction between these variables. It is true that greater temperatures generally improve the solubility and diffusivity of compounds, thus increasing the mass transfer between the plant matrix and bulk solvent [
5]. Regarding the effect of solvent composition, TPC yield slightly increased with an increase in ethanol concentration from 60% to about 68%, whilst ethanol concentrations greater than this led to a gradual decrease in TPC yields (
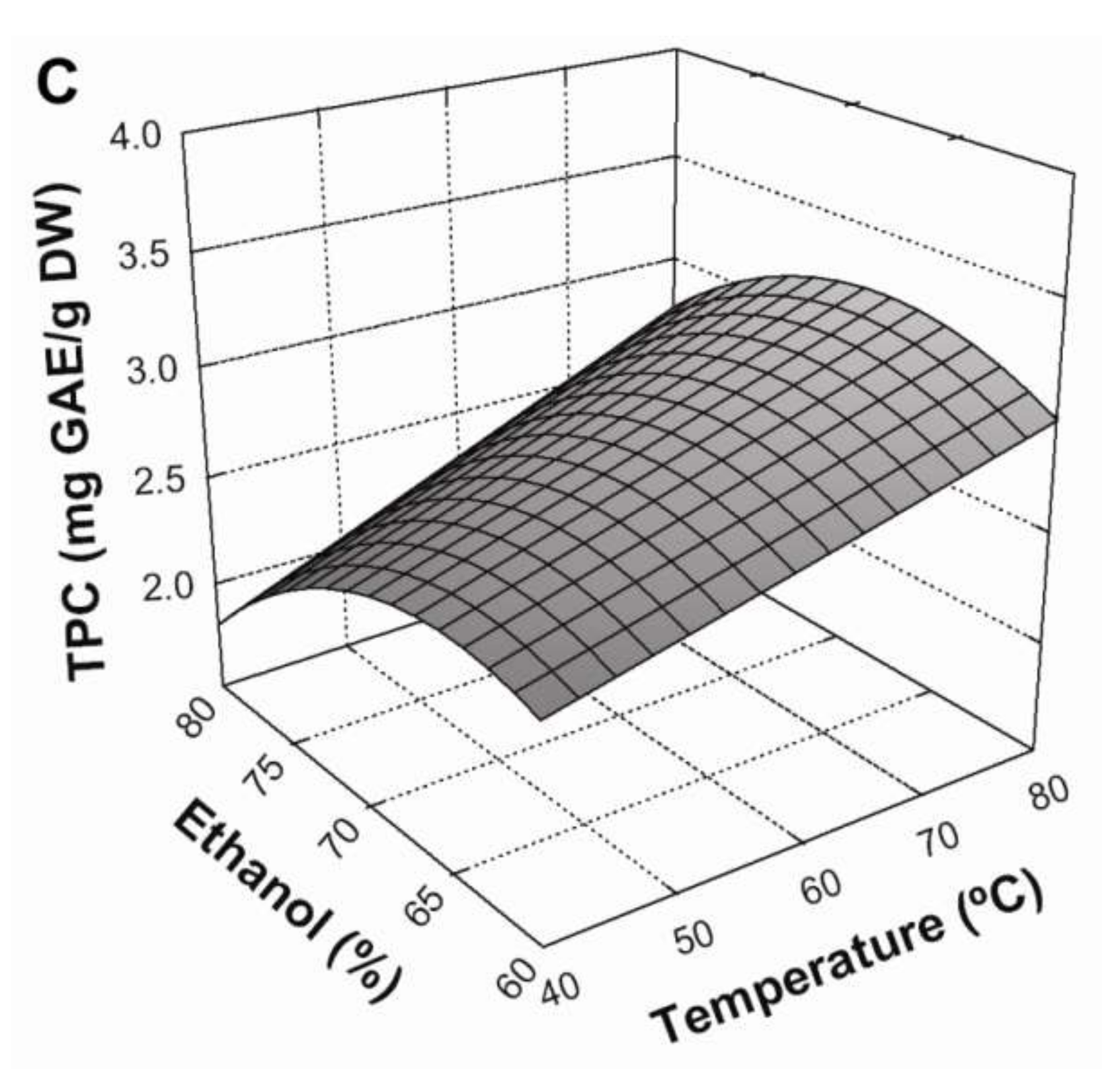
Figure 1C). This could be explained by different ethanol:water ratios modifying the polarity of the solvent system, thereby altering the solubility of different phenolic compounds and consequently determining which will be extracted [
12]. Thus, the conditions that maximized Equation (8) (
Table 5) in a BE process were: a 30:1 mL/g liquid/solid ratio, a temperature of 80 °C, and an ethanol concentration of 66.7% in the solvent system.
3.3. Effect of Extraction Conditions on DPPH Radical Scavenging
According to Equation (9) (
Table 5), the liquid/solid ratio was the variable that had the greatest effect (
p < 0.05) on DPPH radical scavenging of extracts. This was followed by temperature, which had a lesser impact. The influence of extraction parameters on DPPH radical scavenging is shown in
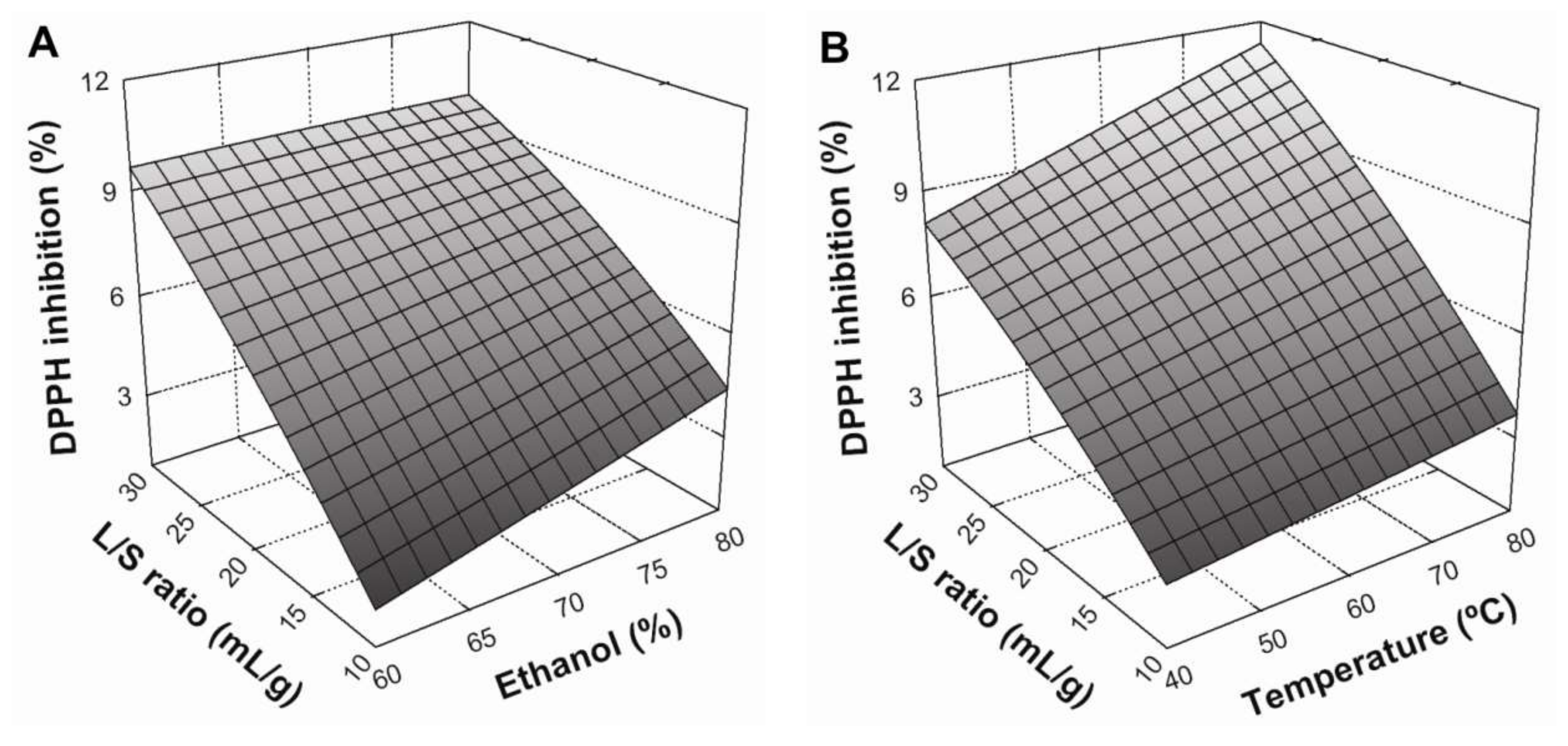
Figure 2. Similarly to TPC, DPPH inhibition increased when the L/S ratio increased from 10:1 to 30:1 mL/g and temperature increased from 40 to 80 °C (
Figure 2A,B, respectively). Furthermore, the interaction between these variables was significant (
p < 0.05), with positive effects on DPPH radical scavenging (
Figure 2B). Regarding the solvent effect,
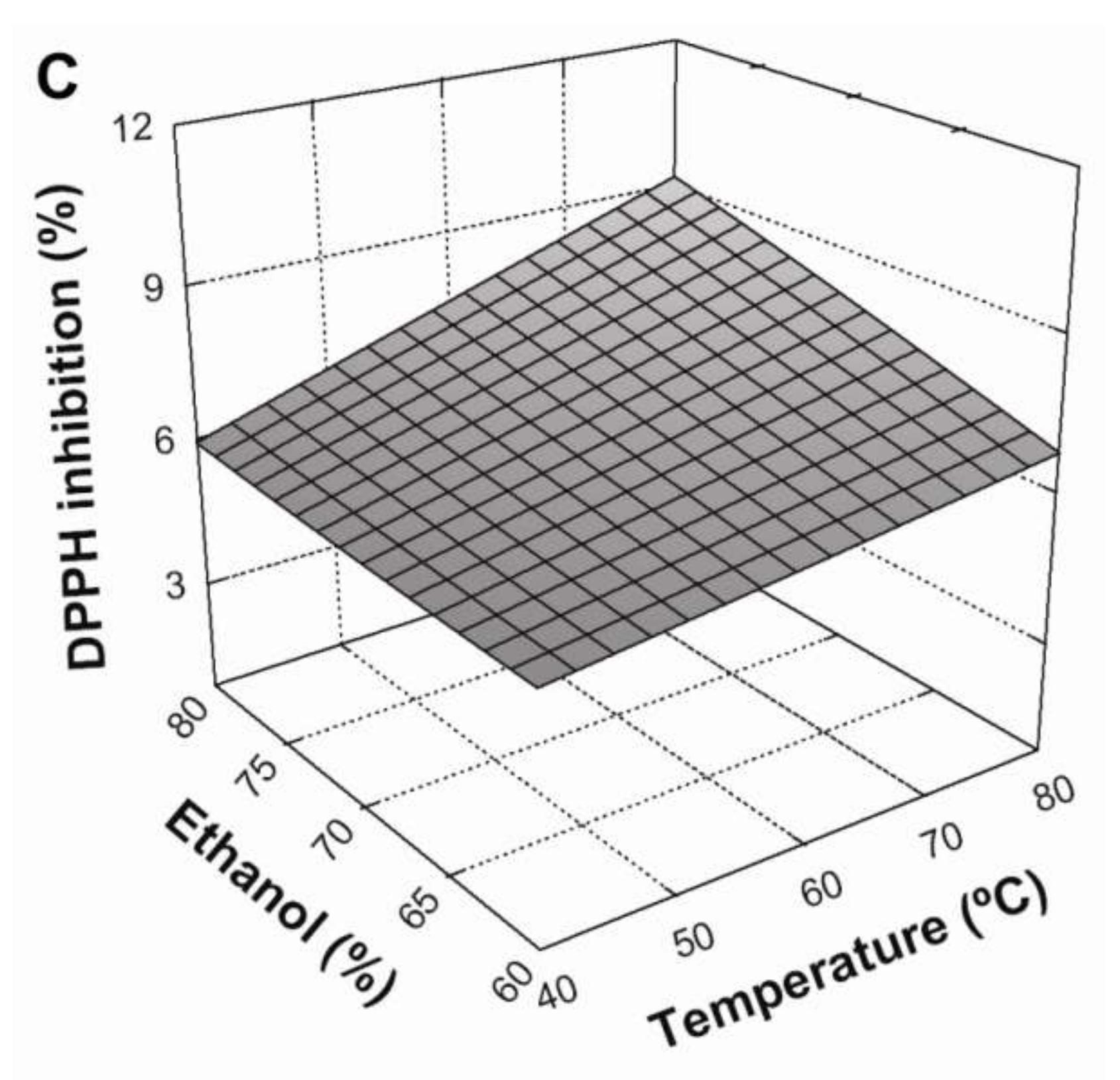
Figure 2C shows that an increase in ethanol concentration from 60 to 80% produced a positive, linear effect on antioxidant activity. Similarly, the interaction between the solvent system and temperature also produced a positive and statistically-significant (
p < 0.05) effect. According to these results, maximum DPPH radical scavenging in BE was be achieved under the following conditions: an L/S ratio of 30:1 mL/g at 80 °C, using 80% ethanol as a solvent system.
3.4. Optimization of the Extraction Conditions
To optimize the process with two or more output responses, a multiresponse analysis was carried out using the desirability function in the chosen statistical software. The target was to obtain a BSG extract with a high content of phenolic compounds and antioxidant activity. Thus, through maximizing both responses, optimum extraction conditions were obtained: L/S ratio of 30:1 mL/g, 80 °C, and 72% ethanol concentration. Experimental verification of these conditions was performed in quintuplicate, obtaining values of 3.57 ± 0.08 mg GAE/g and 11.55 ± 0.08% DPPH inhibition, for TPC and DPPH assay, respectively. These values confirm the predicted values (3.59 mg GAE/g and 11.55% DPPH radical scavenging) within a 95% confidence level. Hence, under the aforementioned conditions, a BSG extract rich in antioxidant phenolic compounds was obtained.
3.5. Extraction Kinetics Study
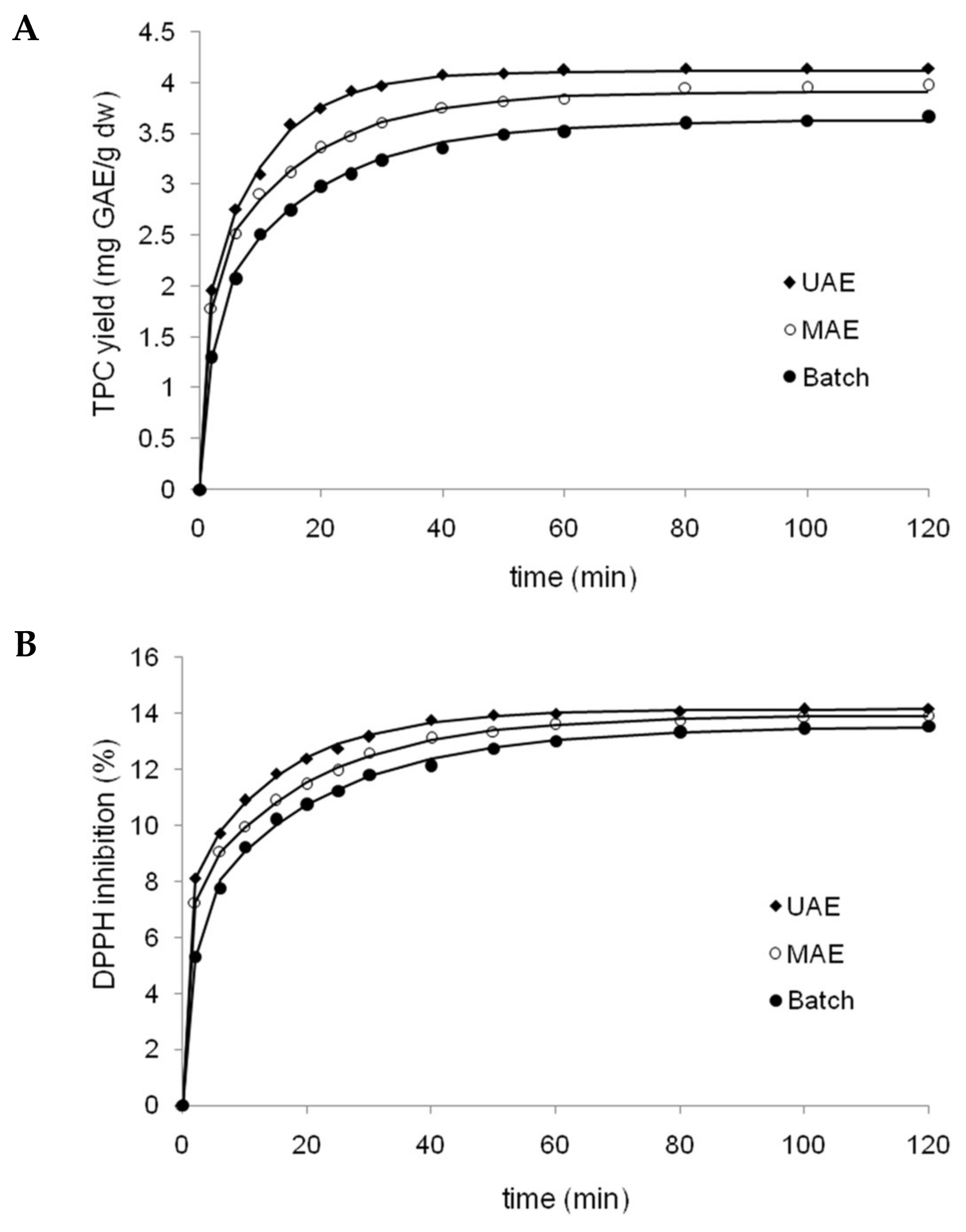
Extraction under optimized conditions was performed for a batch system (BE) as well as for assisted processes using ultrasound (UAE) and microwaves (MAE), in order to determine the influence of each extraction method on the yield of phenolic compounds (
Figure 3A), and the evolution of extract antioxidant capacity versus time (
Figure 3B). Both figures showed a clear, positive effect of assistance techniques, however this was more pronounced for ultrasound assistance than microwave assistance. The mean comparison by Tukey’s test showed significant differences (
p < 0.05) among the three extraction techniques from 10 to 50 min of extraction for TPC yields. In addition, the kinetics of each extraction method for both responses were fitted to the three equation models presented in
Table 2. The corresponding results of nonlinear regression and statistical parameters for BA, UAE and MAE fitted by all three models are shown in
Table 6. For both responses, Patricelli’s model was the most accurate fit, with the highest coefficient of determination and adjusted coefficient of determination, and the lowest root mean squared error (RMSE) and percentage average absolute relative deviation (%AARD), compared to Peleg and Page models.
The empirical model proposed by Patricelli [
13] involves two simultaneous processes with different kinetics coefficients: a washing stage and a diffusion stage (Equation (3),
Table 2). The total amount of extracted solute (equilibrium yield) is equal to the sum of the amounts extracted during both stages. An estimate of the initial extraction rate is given by the first derivative of the equation when
t = 0 [
14]. Thus, according to Patricelli’s model, the equilibrium yields of TPC are 4.11, 3.91, and 3.62 mg GAE/g for UAE, MAE and BE, respectively. These results showed that UAE and MAE significantly increased polyphenol extraction relative to BE, with increases of 13% and 8% for equilibrium conditions, respectively. Additionally, polyphenol extraction was faster using UAE and MAE, according to higher extraction rates (2.42 and 1.66 mg/g/min, respectively) in comparison to BE (0.98 mg/g/min). The observed increase in polyphenol extraction could be due to mechanical effects induced on BSG cell walls, produced during the collapse of the cavitation bubbles (shockwave-induced damage and microjet impact on the surface of the solid material), in relation to MAE where the extraction principle is based on the synergistic combination of heat and mass transfers working from the inside to the outside of the solid sample (in contrast to conventional extraction, in which both transport phenomena occur in different directions) [
15,
16].
According to transfer coefficients calculated for Patricelli’s model (
Table 6), the coefficients of the washing stage (k1) for both responses (TPC and DPPH radical scavenging) were much greater than those of the diffusion stage (k2) for all extraction methods tested. As can be seen in
Figure 3A,B, all extraction methods showed a quick extraction rate at the outset which was subsequently reduced. This is explained well by Patricelli’s model, since the washing stage allows for quick dissolution of the target components located both at the surface and within broken matrix cells. On the other hand, the diffusion stage is slower due to mass transfer limitations, where the remaining active compounds diffuse from the interior of intact cells into the solvent [
5,
14]. In addition, a comparison of k values among three methods (
Table 6) evidenced greater coefficients for assisted methods relative to BE, mainly in relation to the washing stage. Thus, the assistance of microwaves and especially ultrasound greatly accelerated the washing phase of extraction.