Selenium-Rich Ricegrass Juice Improves Antioxidant Properties and Nitric Oxide Inhibition in Macrophage Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Plantation and Plant Extract Preparation
2.3. Selenium Content Determination
2.4. Total Polyphenol Content
2.5. Phenolic Profiles Identification Using UHPLC-DAD-ESI-MS
2.6. Cell Culture Model
2.7. Cell Viability Assay
2.8. Preparation of Endogenous Cellular Extracts
2.9. Determination of Endogenous Antioxidant Enzyme Activity
2.10. Determination of Lipid Peroxidation
2.11. Nitric Oxide Synthesis Inhibition Determination
2.12. Statistical Analysis
3. Results and Discussion
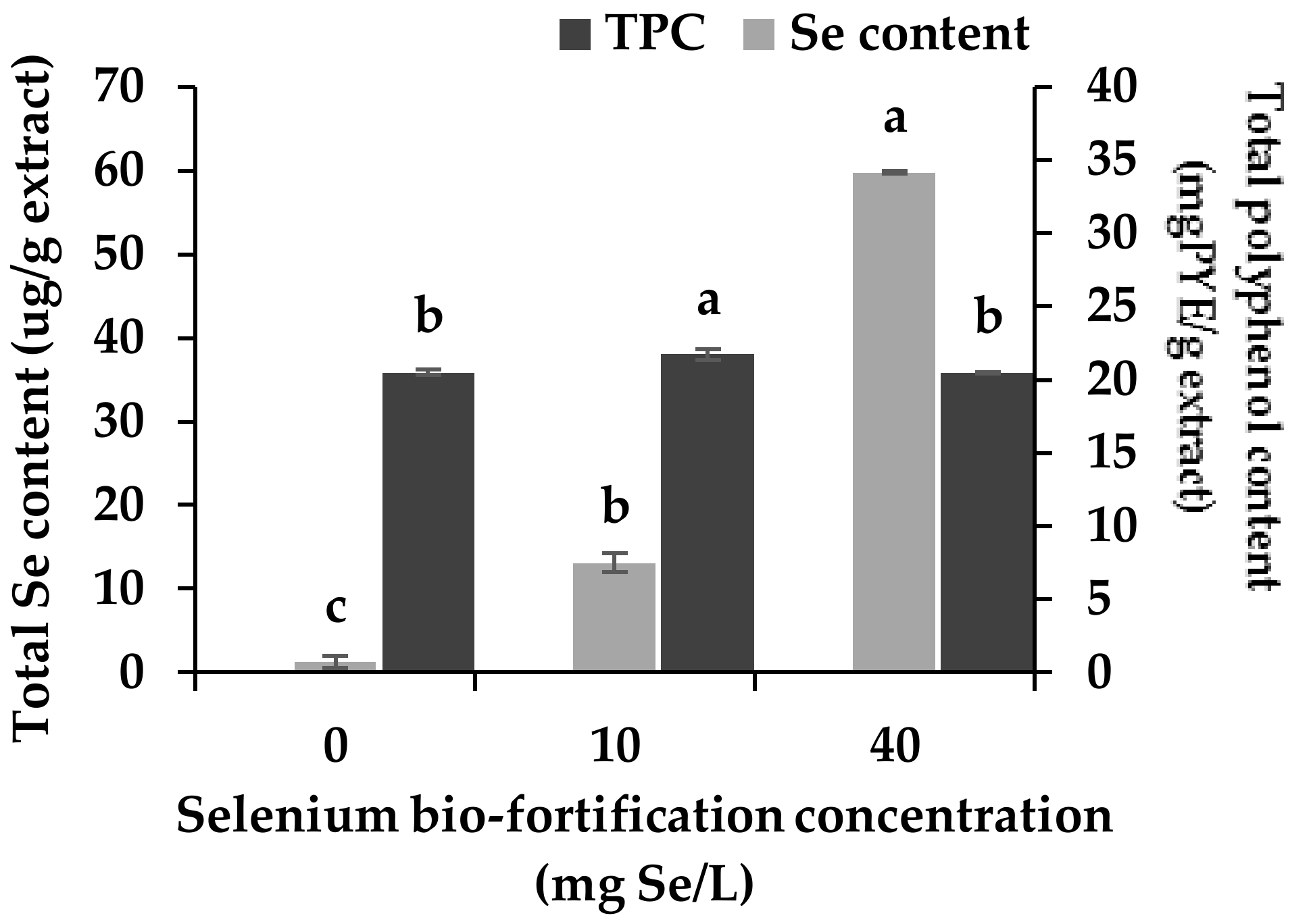
3.1. Changes in Se Content and Total Polyphenol Content
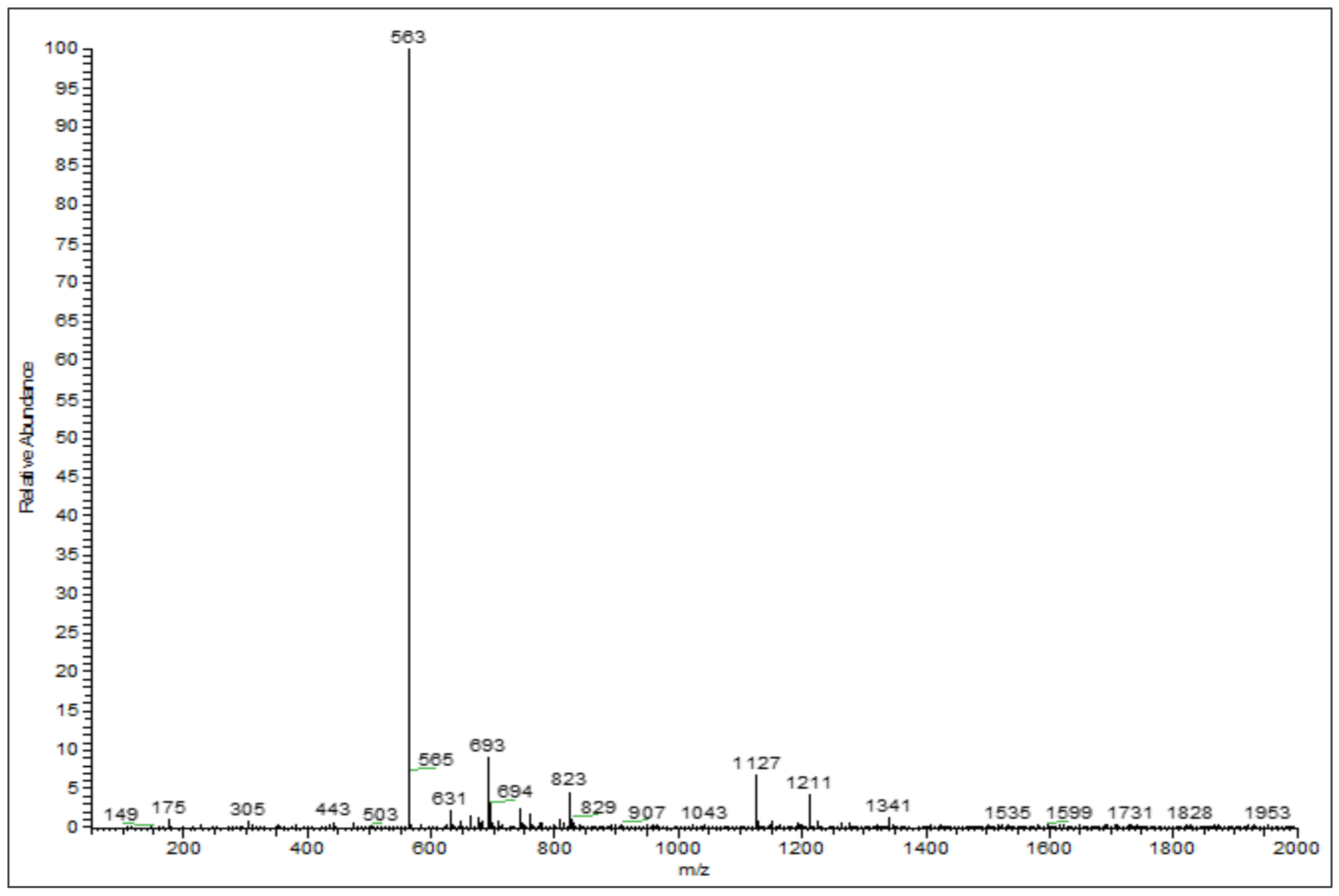
3.2. Phenolic Profiles Identification Using UHPLC-DAD-ESI-MS
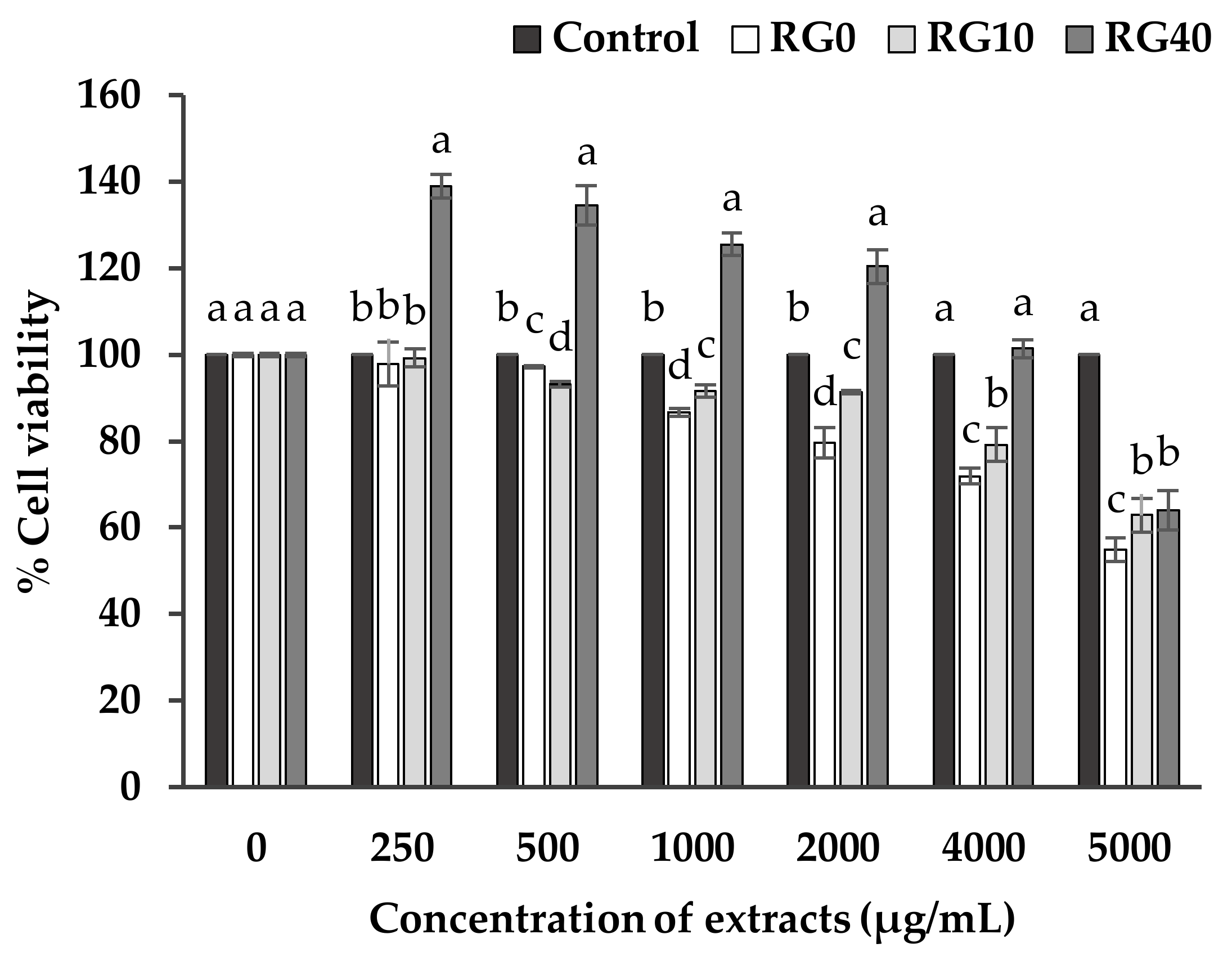
3.3. Cell Viability and Cell Proliferation
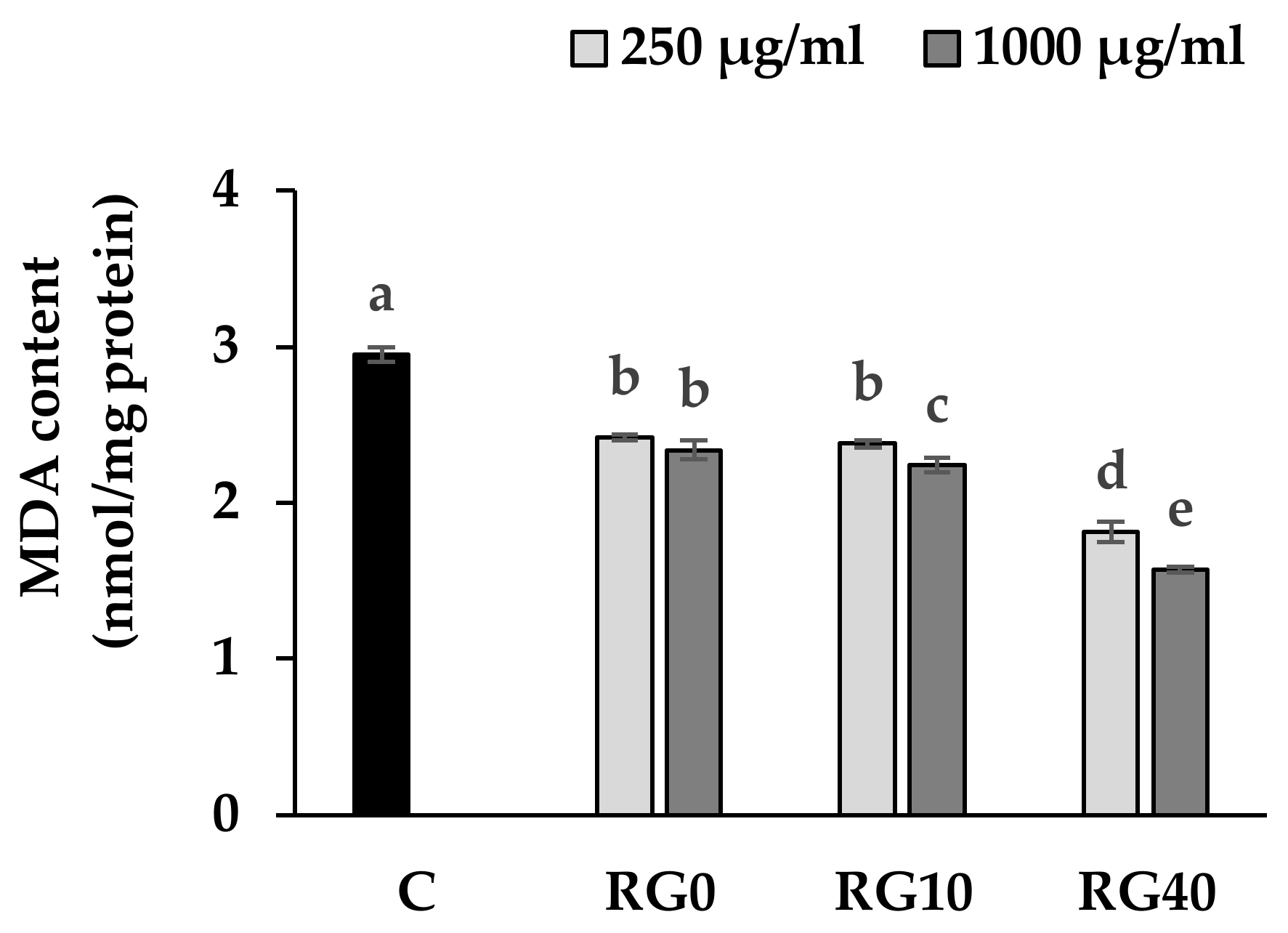
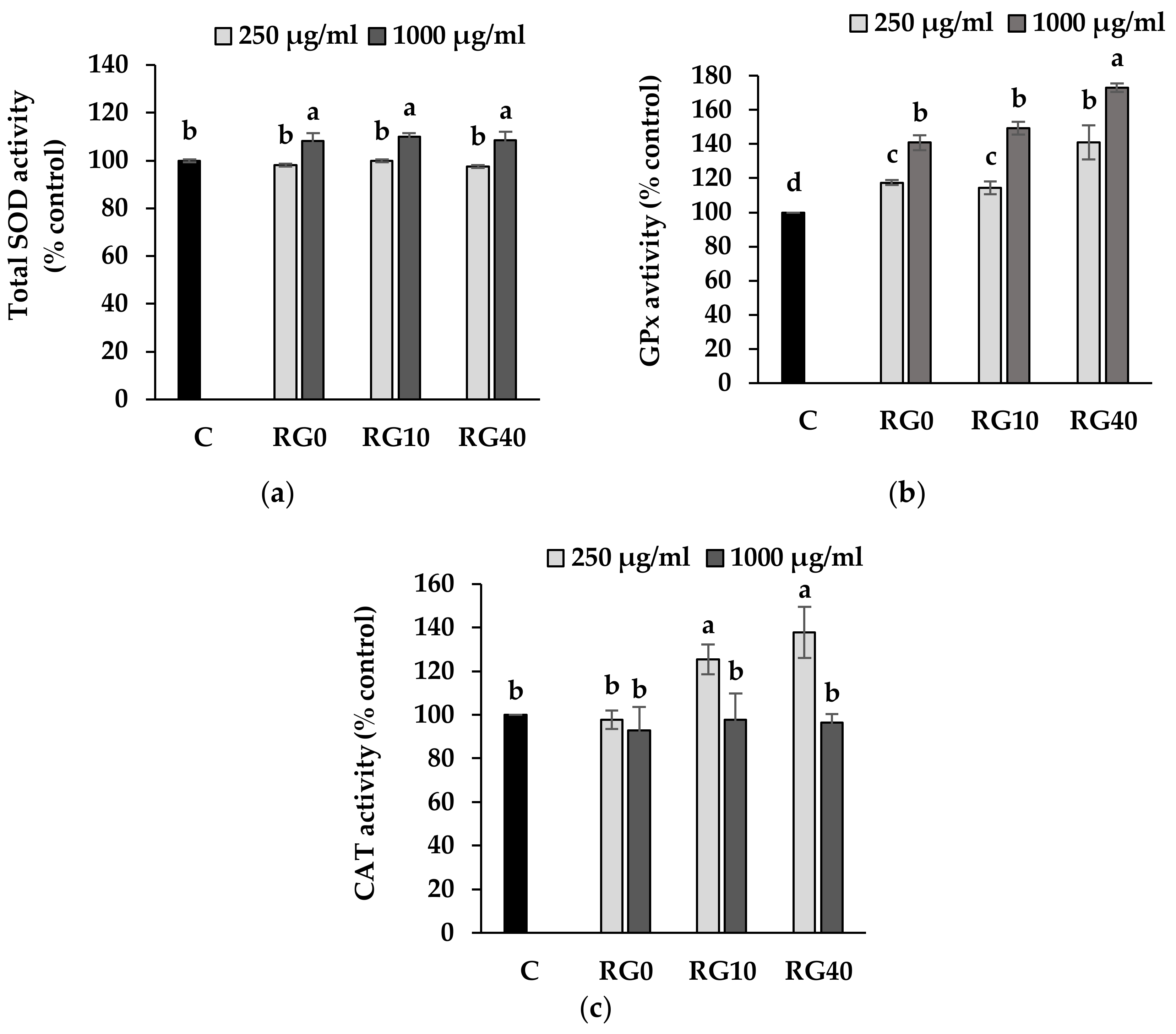
3.4. Lipid Peroxidation and Antioxidant Enzymes Activity
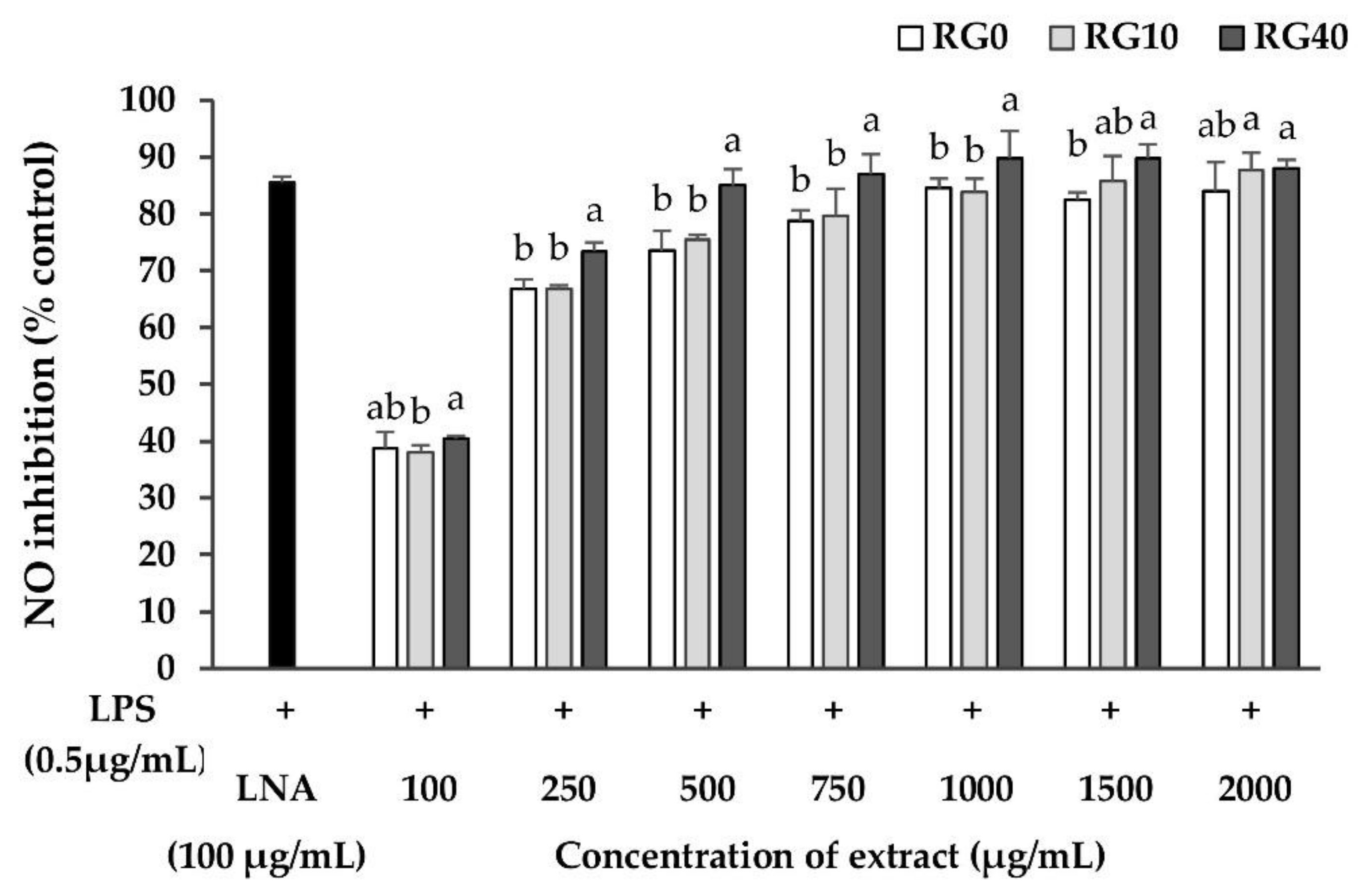
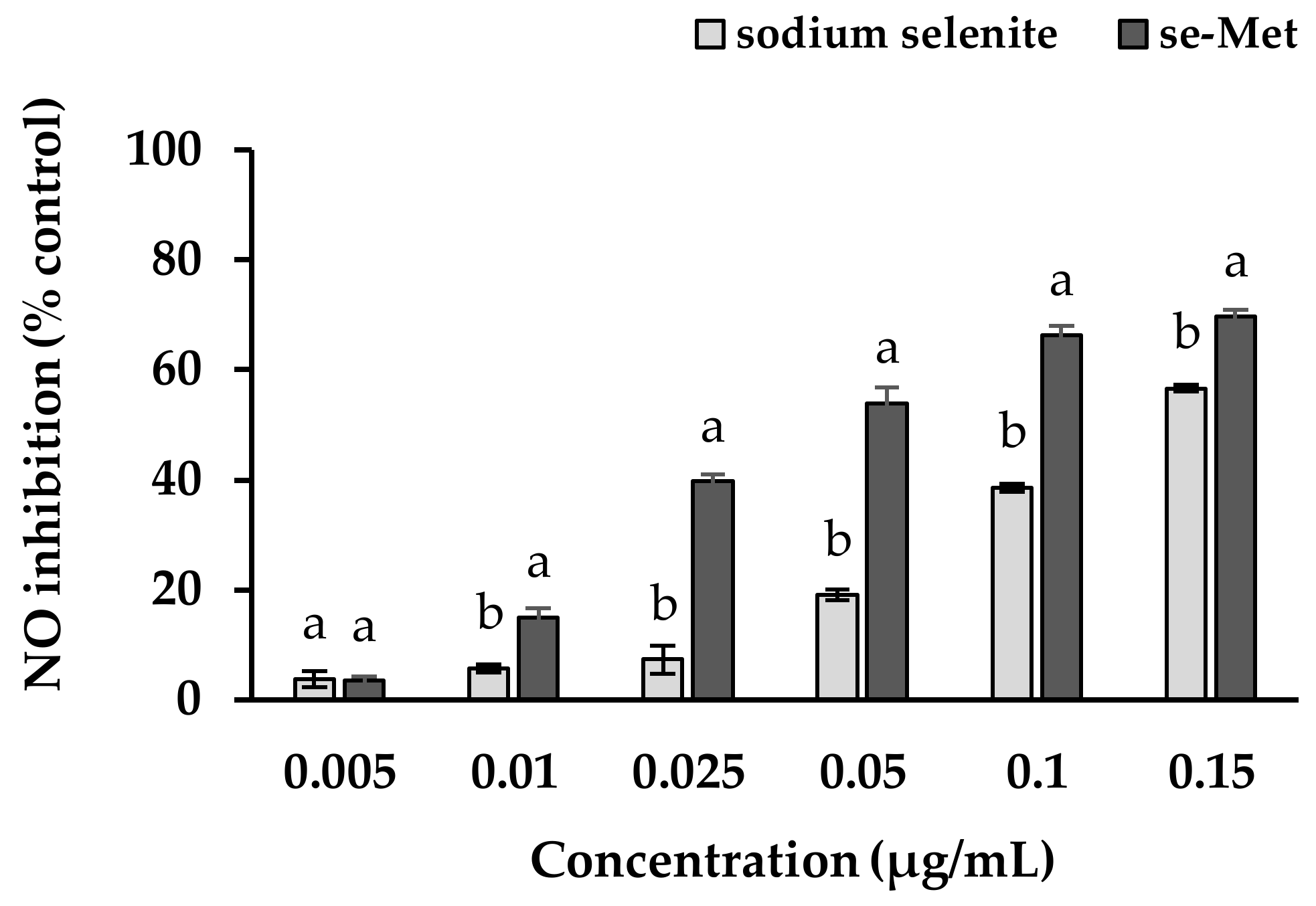
3.5. Nitric Oxide Inhibition Properties
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fardet, A.; Rock, E. Toward a new philosophy of preventive nutrition: From a reductionist to a holistic paradigm to improve nutritional recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef] [PubMed]
- Wigmore, A. The Sprouting Book; Penguin Putnam Inc.: East Rutherford, NJ, USA, 1986. [Google Scholar]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Investigation of phytochemical constituents, phenolic profiles and antioxidant activities of ricegrass juice compared to wheatgrass juice. Funct. Food. Health Dis. 2016, 6, 822–835. [Google Scholar]
- Rattanapon, R.; Siripongvutikorn, S.; Usawakesmanee, W.; Thongraung, C. Improvement nutritional value and bioactivity of ricegrass as affected of priming induced by fish protein hydrolysate. Funct. Foods Health Dis. 2016, 6, 219–233. [Google Scholar]
- Khanthapoka, P.; Muangpromb, A.; Sukronga, S. Antioxidant activity and DNA protective properties of rice grass juices. Sci. Asia 2015, 41, 119–129. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortifying crops with essential mineral elements. Trends Plant Sci. 2005, 10, 586–593. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R.; Wilkinson, N.; Madison, R.; Felix, T. Vitamins and Minerals Functioning as Antioxidants with Supplementation Considerations; Florida Ruminant Nutrition Symposium, Best Western Gateway Grand: Gainesville, FL, USA, 2007; pp. 30–31. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P. Selenium bio-fortification: An alternative to improve phytochemicals and bioactivities of plant foods. Funct. Foods Health Dis. 2017, 7, 263–279. [Google Scholar]
- Devasagayam, T.P.A.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals, and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India 2004, 52, 794–804. [Google Scholar] [PubMed]
- McGeer, P.L.; McGeer, E.G. Inflammation and the degenerative diseases of aging. Ann. N. Y. Acad. Sci. 2004, 1035, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Wang, X.; Wong, Y.S. Generation of selenium-enriched rice with enhanced grain yield, selenium content, and bioavailability through fertilisation with selenite. Food Chem. 2013, 141, 2385–2393. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Louis, K.S.; Siegel, A.C. Cell viability analysis using trypan blue: Manual and automated methods. Mamm. Cell Viability Methods Protoc. 2011, 740, 7–12. [Google Scholar]
- Du, Y.; Esfandi, R.; Willmore, W.G.; Tsopmo, A. Antioxidant activity of oat proteins derived peptides in stressed hepatic HepG2 cells. Antioxid 2016, 5, 39. [Google Scholar] [CrossRef] [PubMed]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Günzler, W.A. Assays of glutathione peroxidase. Methods Enzymol. 1984, 105, 114–120. [Google Scholar] [PubMed]
- Brennan, T.; Frenkel, C. Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol. 1977, 59, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation and membrane fluidity in HepG2 cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Chomchan, R.; Siripongvutikorn, S.; Puttarak, P.; Rattanapon, R. Influence of selenium bio-fortification on nutritional compositions, bioactive compounds content and anti-oxidative properties of young ricegrass (Oryza sativa L.). Funct. Foods Health Dis. 2017, 7, 195–209. [Google Scholar]
- Schiavon, M.; dall’Acqua, S.; Mietto, A.; Pilon-Smits, E.A.; Sambo, P.; Masi, A.; Malagoli, M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.). J. Agric. Food Chem. 2013, 61, 10542–10554. [Google Scholar] [CrossRef] [PubMed]
- Sae-Lee, N.; Kerdchoechuen, O.; Laohakunjit, N. Chemical qualities and phenolic compounds of Assam tea after soil drench application of selenium and aluminium. Plant Soil 2012, 356, 381–393. [Google Scholar] [CrossRef]
- Hahlbrock, K.; Scheel, D. Physiology and molecular biology of phenylpropanoid metabolism. Annu. Rev. Plant Biol. 1989, 40, 347–369. [Google Scholar] [CrossRef]
- Besson, E.; Dellamonica, G.; Chopin, J.; Markham, K.R.; Kim, M.; Koh, H.-S.; Fukami, H. C-glycosylflavones from Oryza sativa. Phytochemistry 1985, 24, 1061–1064. [Google Scholar] [CrossRef]
- Yang, Z.; Nakabayashi, R.; Okazaki, Y.; Mori, T.; Takamatsu, S.; Kitanaka, S.; Kikuchi, J.; Saito, K. Toward better annotation in plant metabolomics: Isolation and structure elucidation of 36 specialized metabolites from Oryza sativa (rice) by using MS/MS and NMR analyses. Metabolomics 2014, 10, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Cheon, Y.M.; Kim, B.-G.; Ahn, J.-H. Analysis of flavonoids and characterization of the OsFNS gene involved in flavone biosynthesis in rice. J. Plant Biol. 2008, 51, 97. [Google Scholar] [CrossRef]
- Moheb, A.; Agharbaoui, Z.; Kanapathy, F.; Ibrahim, R.K.; Roy, R.; Sarhan, F. Tricin biosynthesis during growth of wheat under different abiotic stresses. Plant Sci. 2013, 201, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q.; Wang, W.-S.; Zhang, F.; Zhang, T.; Zhao, W.; Fu, B.-Y.; Li, Z.-K. Temporal profiling of primary metabolites under chilling stress and its association with seedling chilling tolerance of rice (Oryza sativa L.). Rice 2013, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Yu, S.; Prior, R.L. LC/MS/MS characterization of phenolic constituents in dried plums. J. Agric. Food Chem. 2002, 50, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, C.; Gopalakrishnan, V.; Balachandran, I. Reverse phase liquid chromatography coupled with quadra pole-time of flight mass spectrometry for the characterization of phenolics from Acacia catechu (L.f.) willd. Int. J. Phytomed. 2012, 4, 403–408. [Google Scholar]
- Markham, K.R.; Tanner, G.J.; Caasi-Lit, M.; Whitecross, M.I.; Nayudu, M.; Mitchell, K.A. Possible protective role for 3′,4′-dihydroxyflavones induced by enhanced UV-B in a UV-tolerant rice cultivar. Phytochemistry 1998, 49, 1913–1919. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontology 2000 2014, 64, 57–80. [Google Scholar] [CrossRef] [PubMed]
- Ioset, J.-R.; Brun, R.; Wenzler, T.; Kaiser, M.; Yardley, V. Drug Screening for Kinetoplastids Diseases—A Training Manual for Screening in Neglected Diseases. Available online: https://www.dndi.org/wp-content/uploads/2009/04/kinetoplastid_drug_screening_manual_final.pdf (accessed on 20 February 2018).
- Genuardi, J.; Eskew, M.; Zeligs, B.; Bellanti, J. The effects of vitamin E and selenium on the nitric oxide production of macrophages. Pediatr. Res. 1999, 45, 771. [Google Scholar] [CrossRef][Green Version]
- Halliwell, B. Reactive oxygen species in living systems: Source, biochemistry, and role in human disease. Am. J. Med. 1991, 91, S14–S22. [Google Scholar] [CrossRef]
- Ray Halder, S.; Bhattacharyya, M. Oxidative stress: Lipid peroxidation products as predictors in disease progression. J. Exp. Integr. Med. 2014, 4, 151–164. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Mates, J. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Yeh, C.T.; Yen, G.C. Induction of hepatic antioxidant enzymes by phenolic acids in rats is accompanied by increased levels of multidrug resistance-associated protein 3 mRNA expression. J. Nutr. 2006, 136, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Park, W. Anti-inflammatory effect of quercetin on raw 264.7 mouse macrophages induced with polyinosinic-polycytidylic acid. Molecules 2016, 21, 450. [Google Scholar] [CrossRef] [PubMed]
- Panico, A.; Cardile, V.; Avondo, S.; Garufi, F.; Gentile, B.; Puglia, C.; Bonina, F.; Santagati, N.; Ronsisvalle, G. The in vitro effect of a lyophilized extract of wine obtained from Jacquez grapes on human chondrocytes. Phytomed 2006, 13, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Terra, X.; Montagut, G.; Bustos, M.; Llopiz, N.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L. Grape-seed procyanidins prevent low-grade inflammation by modulating cytokine expression in rats fed a high-fat diet. J. Nutr. Biochem. 2009, 20, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.C.; McIntosh, M.K. Potential mechanisms by which polyphenol-rich grapes prevent obesity-mediated inflammation and metabolic diseases. Annu. Rev. Nutr. 2011, 31, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Shen, S.C.; Lee, W.R.; Hou, W.C.; Yang, L.L.; Lee, T.J. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide-induced inducible nos and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW264.7 macrophages. J. Cell. Biochem. 2001, 82, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Zamamiri-Davis, F.; Stewart, J.B.; Thompson, J.T.; Sordillo, L.M.; Reddy, C.C. Selenium deficiency increases the expression of inducible nitric oxide synthase in RAW264.7 macrophages: Role of nuclear factor-κb in up-regulation. Biochem. J. 2002, 366, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Kumar, A.; Rahal, A.; Kumar, V.; Roy, D. Inorganic versus organic selenium supplementation: A review. Pak. J. Biol. Sci. 2012, 15, 418–425. [Google Scholar]
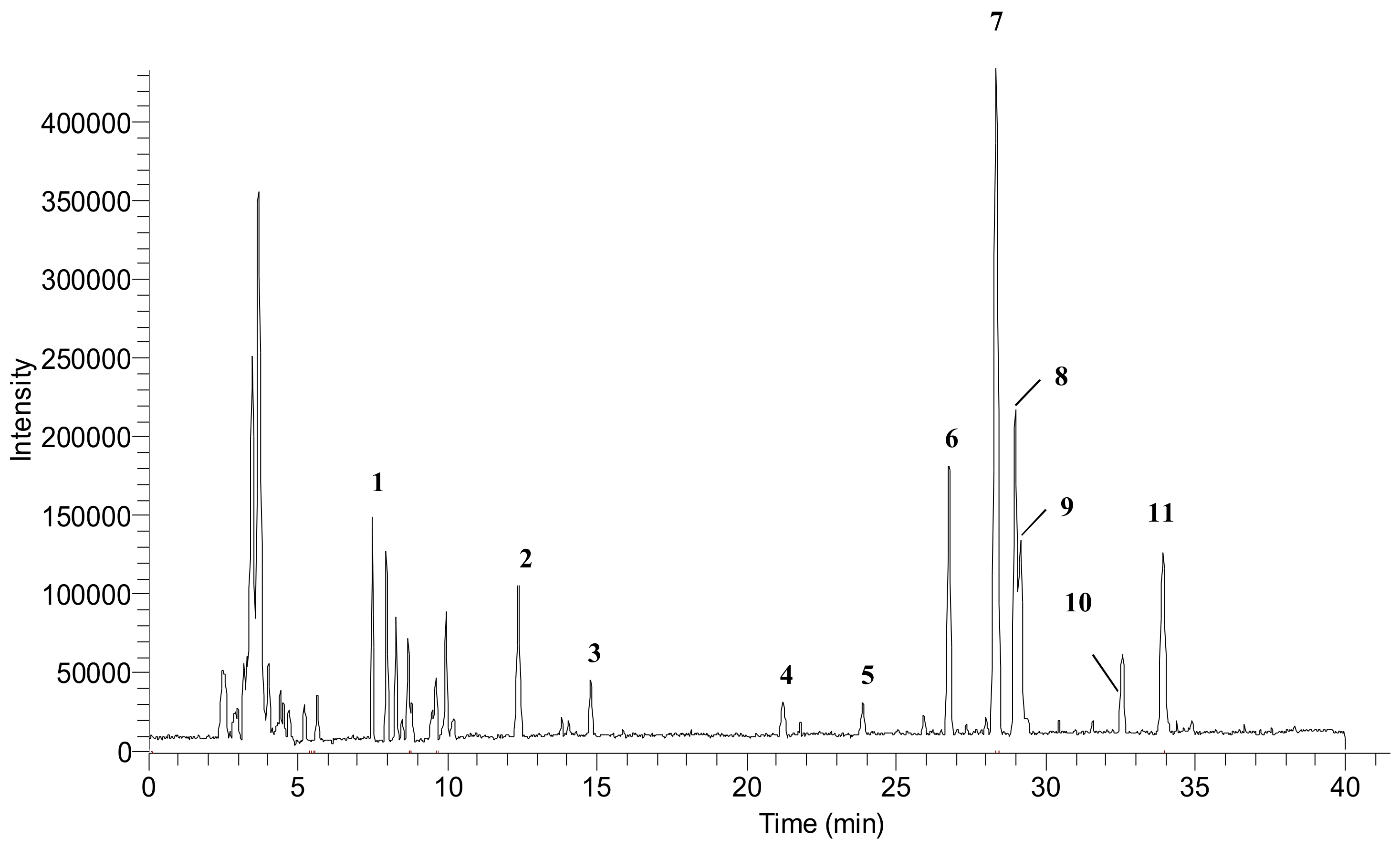
| Peak No. a | RT (min) | Tentative Compounds | MW | [M − H] + (m/z) | Fragment Ion | Ref. |
|---|---|---|---|---|---|---|
| 1 | 7.95 | Quinic acid | 192 | 191 | 111, 192, 613 | [30] |
| 2 | 12.38 | Tricin | 330 | 329 | 175, 461 | [27,28] |
| 3 | 14.79 | Protocatechuic glucoside | 316 | 315 | 175, 445, 575 | [31,32] |
| 4 | 21.20 | 1-o-Sinapoyl-β-d-glucose | 386 | 385 | 175, 469, 599 | [27] |
| 5 | 23.87 | 3-o-Feruloylquinic acid | 368 | 367 | 193, 435, 497 | [27,33] |
| 6 | 26.75 | Chrysoeriol arabinosyl arabinoside derivatives | 564 | 563 | 175, 305, 693 | [26,27] |
| 7 | 28.31 | Chrysoeriol arabinosyl arabinoside derivatives | 564 | 563 | 565, 693 | [26,27] |
| 8 | 29.00 | Chrysoeriol arabinosyl arabinoside derivatives | 564 | 563 | 693 | [26,27] |
| 9 | 29.14 | Chrysoeriol arabinosyl arabinoside derivatives | 564 | 563 | 693 | [26,27] |
| 10 | 32.56 | Swertisin | 446 | 445 | 175, 305 | [27] |
| 11 | 33.90 | Tricin-7-o-β-d-glucopyranoside | 492 | 491 | 769, 983 | [27] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chomchan, R.; Puttarak, P.; Brantner, A.; Siripongvutikorn, S. Selenium-Rich Ricegrass Juice Improves Antioxidant Properties and Nitric Oxide Inhibition in Macrophage Cells. Antioxidants 2018, 7, 57. https://doi.org/10.3390/antiox7040057
Chomchan R, Puttarak P, Brantner A, Siripongvutikorn S. Selenium-Rich Ricegrass Juice Improves Antioxidant Properties and Nitric Oxide Inhibition in Macrophage Cells. Antioxidants. 2018; 7(4):57. https://doi.org/10.3390/antiox7040057
Chicago/Turabian StyleChomchan, Rattanamanee, Panupong Puttarak, Adelheid Brantner, and Sunisa Siripongvutikorn. 2018. "Selenium-Rich Ricegrass Juice Improves Antioxidant Properties and Nitric Oxide Inhibition in Macrophage Cells" Antioxidants 7, no. 4: 57. https://doi.org/10.3390/antiox7040057
APA StyleChomchan, R., Puttarak, P., Brantner, A., & Siripongvutikorn, S. (2018). Selenium-Rich Ricegrass Juice Improves Antioxidant Properties and Nitric Oxide Inhibition in Macrophage Cells. Antioxidants, 7(4), 57. https://doi.org/10.3390/antiox7040057






