Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods
Abstract
:1. Introduction
2. Methods
2.1. Materials
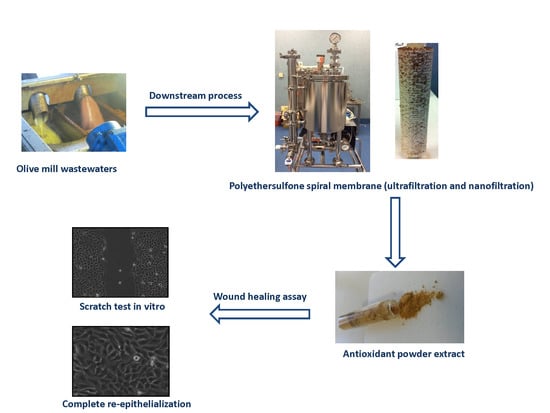
2.2. Downstream Process
2.2.1. Flocculation and Microfiltration
2.2.2. Ultrafiltration and Nano Filtration
2.3. Analytical Methods
2.3.1. Methods for the Quantification of the Phenolic Fraction Extracted from the Olive Mill Wastewaters
2.3.2. Determination of Chemical Oxygen Demand (COD) Residual
2.4. In Vitro Biological Assays
2.4.1. Cell Culture
2.4.2. Evaluation of the Cell Viability by MTT Assay after Stress Oxidative Induction
2.4.3. In Vitro Scratch Assay Using Time Lapse Video Microscopy (TLVM)
3. Results
3.1. Membrane Process Post Normal Filtration (First Strategy)
3.2. Membrane Process Post Centrifugation (Second Strategy)
3.3. Membrane Process Post Treatment with Adjuvant (Third Strategy)
3.4. HPLC Analysis
3.5. COD Analysis
3.6. MTT Test
3.7. Wound Healing Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| OMWs | olive mill wastewaters |
| TSS | total suspended solid concentration |
| COD | chemical oxygen demand |
| BOD | biological oxygen demand |
| MWCO | molecular weight cut-off |
| MF | microfiltration |
| UF | ultrafiltration |
| NF | nano filtration |
| RO | reverse osmosis |
| HPLC | high performance liquid chromatography |
| LMH | flux normalized on surface area |
| TMP | transmembrane pressure |
| Pin | inlet pressure |
| SE | standard error |
| HaCaT | human immortalized keratinocytes |
References
- Keys, A.; Menotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiol. 1986, 124, 903–915. [Google Scholar] [CrossRef] [PubMed]
- Visioli, F.; Bellomo, G.; Montedoro, G.; Galli, C. Low density lipoprotein oxidation is inhibited in vitro by olive oil constituents. Atherosclerosis 1995, 117, 25–32. [Google Scholar] [CrossRef]
- Graf, B.A.; Milbury, P.E.; Blumberg, J.B. Flavonols, flavonones, flavanones and human health: Epidemological evidence. J. Med. Food 2005, 8, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Hollman, P.C.H. Polyphenols and disease risk in epidemiological studies. Am. J. Clin. Nutr. 2005, 81, 317S–325S. [Google Scholar] [CrossRef] [PubMed]
- Damle, M.; Mallya, R. Development and evaluation of a novel delivery system containing phytophospholid complex for skin anging. AAPS PharmSciTech J. 2016, 17, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mackle, S.; Submann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.J.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, anti-oxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Mukherjeea, P.K.; Maitya, N.; Nemaa, N.K.; Sarkarb, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef] [PubMed]
- De Marco, E.; Bavarese, M.; Padano, A.; Sacchi, R. Characterization and fractionation of phenolic compounds entracte from olive oil mill wastewaters. Food Chem. 2007, 104, 858–867. [Google Scholar] [CrossRef]
- De La Puerta, R.; Gutierrez, V.R.; Hoult, J.R.S. Inhibition of leukocyte 5-lipoxygenase by phenolics from virgin olive oil. Biochem. Pharmacol. 1999, 57, 445–449. [Google Scholar] [CrossRef]
- Visioli, F.; Romani, A.; Mulinacci, N.; Zarini, S.; Conte, D.; Vincieri, F.F. Antioxidant and other biological activities of olive mill waste water. J. Agric. Food Chem. 1999, 47, 3397–3401. [Google Scholar] [CrossRef] [PubMed]
- Capasso, R.; Cristinzio, G.; Evidente, A.; Scognamiglio, F. Isolation spectoscopy and selective phytotoxic effects of polyphenols from vegetable wastewaters. Phytochemystry 1992, 31, 4125–4128. [Google Scholar] [CrossRef]
- Visioli, F.; Vinceri, F.; Galli, C. Wastewaters from olive production are rich in natural antioxidants. Experientia 1995, 51, 32–34. [Google Scholar] [PubMed]
- Visioli, F.; Poli, A.; Galli, C. Antioxidant and other biological activities of phenols from olives and olive oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, A. Acque di Vegetazione. Agricoltura e Innovazione. In Dossier: Acque di Vegetazione; ENEA: Roma, Italy, 1989. [Google Scholar]
- Di Lecce, G.; Cassano, A.; Bendini, A.; Conidi, C.; Giorno, L.; Toschi, T.G. Characterization of olive mill wastewater fractions treatment by integrated membrane process. J. Sci. Food Agric. 2014, 94, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Cassano, A.; Conidi, C.; Giorno, L.; Drioli, E. Fractionation of olive mill wastewaters by membrane separation techniques. J. Hazard. Mater. 2013, 248, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Paraskeva, C.A.; Papadakis, V.G.; Kanellopoulos, D.G.; Koutsoukos, P.G.; Angelopoulos, K.C. Membrane filtration of olive mill wastewater and exploitation of its fractions. Water Environ. Res. 2007, 79, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Marcellin, E.; Nielsen, L.K.; Abeydeera, P.; Krömer, J.O. Quantitative analysis of intracellular sugar phosphates and sugar nucleotides in encapsulated streptococci using HPAEC-PAD. Biotechnol. J. 2009, 4, 58–63. [Google Scholar] [CrossRef] [PubMed]
- ISO 6060:1989 Water Quality—Determination of the Chemical Oxygen Demand. Available online: https://www.iso.org/standard/12260.html (accessed on 5 April 2018).
- D’Agostino, A.; Stellavato, A.; Busico, T.; Papa, A.; Tirino, V.; Papaccio, G.; La Gatta, A.; De Rosa, M.; Schiraldi, C. In vitro analysis of the effects on wound healing of high and low molecular weight chains of hyaluronan and their hybrid H-HA/L-HA complexes. BMC Cell Biol. 2015, 16, 19. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival application to proliferation and citotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- ISO 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. Available online: https://www.iso.org/standard/36406.html (accessed on 5 April 2018).
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ginos, A.; Manios, T.; Mantzavinos, D. Treatment of olive mill by coagulation-flocculation-hydrogen peroxide oxidation and effect phytotoxicity. J. Hazard. Mater. 2005, 133, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Amaral-Silva, N.; Martins, R.C.; Castro-Silva, S.; Quinta-Ferreira, R.M. Integration of traditional systems and advanced oxidation process technologies for the industrial treatment of olive mill wastewaters. Environ. Technol. 2016, 37, 2524–2535. [Google Scholar] [CrossRef] [PubMed]
- Coskun, T.; Debik, E.; Demir, N.M. Treatment of olive mill wastewaters by nanofiltration and reverse osmosis membranes. Desalination 2010, 259, 65–70. [Google Scholar] [CrossRef]












| Centrifugation | Pellet Weight (g/L) | % Weight Removed |
|---|---|---|
| I | 0.471 | 71 |
| II | 0.096 | 14.5 |
| III | 0.073 | 11 |
| IV | 0.019 | 2.8 |
| Treatment | To (mg) | UFret (mg) | UFperm (mg) | Polyphenols Recovery UF Step (%) | NFret (mg) | NFperm (mg) | Final Recovery (%) |
|---|---|---|---|---|---|---|---|
| 1° | 784 ± 15 | 125 ± 15 | 658 ± 15 | 84 | 250 ± 10 | 400 ± 12 | 38 |
| 2° | 768 ± 15 | 153 ± 15 | 614 ± 10 | 80 | 250 ± 10 | 368 ± 15 | 40 |
| 3° | 770 ± 15 | 115 ± 10 | 664 ± 15 | 85 | 430 ± 10 | 235 ± 10 | 64.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfano, A.; Corsuto, L.; Finamore, R.; Savarese, M.; Ferrara, F.; Falco, S.; Santabarbara, G.; De Rosa, M.; Schiraldi, C. Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants 2018, 7, 72. https://doi.org/10.3390/antiox7060072
Alfano A, Corsuto L, Finamore R, Savarese M, Ferrara F, Falco S, Santabarbara G, De Rosa M, Schiraldi C. Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants. 2018; 7(6):72. https://doi.org/10.3390/antiox7060072
Chicago/Turabian StyleAlfano, Alberto, Luisana Corsuto, Rosario Finamore, Maria Savarese, Filomena Ferrara, Salvatore Falco, Giuseppe Santabarbara, Mario De Rosa, and Chiara Schiraldi. 2018. "Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods" Antioxidants 7, no. 6: 72. https://doi.org/10.3390/antiox7060072
APA StyleAlfano, A., Corsuto, L., Finamore, R., Savarese, M., Ferrara, F., Falco, S., Santabarbara, G., De Rosa, M., & Schiraldi, C. (2018). Valorization of Olive Mill Wastewater by Membrane Processes to Recover Natural Antioxidant Compounds for Cosmeceutical and Nutraceutical Applications or Functional Foods. Antioxidants, 7(6), 72. https://doi.org/10.3390/antiox7060072