Solvent Extraction of Polyphenolics from the Indigenous African Fruit Ximenia caffra and Characterization by LC-HRMS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
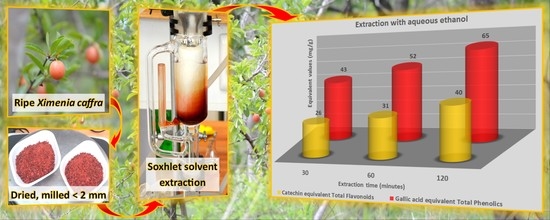
2.2. Raw Material Collection and Preparation
2.3. Experimental Design and Extraction Equipment
2.4. Extraction and Sampling Procedures
2.5. Analytical Methods
2.5.1. Total Phenolic Assay
2.5.2. Total Flavonoid Assay
2.5.3. DMPD Antioxidant Activity Assay
2.5.4. Identification of Phenolic Compounds
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
List of Compounds
| Catechin | PubChem CID: 9064 |
| Citric acid | PubChem CID: 311 |
| Gallic acid | PubChem CID: 370 |
| Hyperoside | PubChem CID: 5281643 |
| Isoquercitrin | PubChem CID: 5280804 |
| Procyanidin B1 | PubChem CID: 11250133 |
| Quercetin-3-O-glucoside | PubChem CID: 5748594 |
| Quercetin-3-O-robinobioside | PubChem CID: 10371536 |
| Rutin | PubChem CID: 5280805 |
References
- Ndhlala, A.R.; Muchuweti, M.; Mupure, C.; Chitindingu, K.; Murenje, T.; Kasiyamhuru, A.; Benhura, M.A. Phenolic content and profiles of selected wild fruits of zimbabwe: Ximenia caffra, Artobotrys brachypetalus and Syzygium cordatum. Int. J. Food Sci. Technol. 2008, 43, 1333–1337. [Google Scholar] [CrossRef]
- Haminiuk, C.W.I.; Maciel, G.M.; Plata-Oviedo, M.S.V.; Peralta, R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012, 47, 2023–2044. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.A.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed]
- Van Wyk, B.-E. The potential of South African plants in the development of new medicinal products. S. Afr. J. Bot. 2011, 77, 812–829. [Google Scholar] [CrossRef]
- Maroyi, A. Ximenia caffra sond. (ximeniaceae) in sub-saharan africa: A synthesis and review of its medicinal potential. J. Ethnopharmacol. 2016, 184, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Chivandi, E.; Davidson, B.C.; Erlwanger, K.H. A comparison of the lipid and fatty acid profiles from the kernels of the fruits (nuts) of Ximenia caffra and Ricinodendron rautenenii from Zimbabwe. Ind. Crops Prod. 2008, 27, 29–32. [Google Scholar] [CrossRef]
- Maroyi, A. Traditional use of medicinal plants in south-central Zimbabwe: Review and perspectives. J. Ethnobiol. Ethnomed. 2013, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.J.; Mulaudzi, R.B.; Chukwujekwu, J.C.; Van Heerden, F.R.; Van Staden, J. Antigonococcal activity of Ximenia caffra sond. (olacaceae) and identification of the active principle. S. Afr. J. Bot. 2013, 86, 111–115. [Google Scholar] [CrossRef]
- Maroyi, A. An ethnobotanical survey of medicinal plants used by the people in Nhema communal area, Zimbabwe. J. Ethnopharmacol. 2011, 136, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Rangasamy, J. Antimicrobial Activity of Zanthoxylum davyi and Ximenia caffra. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, October 2016. [Google Scholar]
- Motlhanka, D.M.T.; Motlhanka, P.; Selebatso, T. Edible indigenous wild fruit plants of eastern Botswana. Int. J. Poult. Sci. 2008, 7, 57–460. [Google Scholar] [CrossRef]
- Mabogo, D.E.N. The Ethnobotany of the Vhavenda. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, July 1990. [Google Scholar]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Liu, R.H. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003, 78, 517S–520S. [Google Scholar] [CrossRef] [PubMed]
- Ignat, I.; Volf, I.; Popa, V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011, 126, 1821–1835. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Berdahl, D.; Nahas, R.I.; Barren, J.P. Synthetic and Natural Additives in Food Stabilization: Current Applications and Future Research; Woodhead Publishing: Oxford, UK, 2010; pp. 272–320. [Google Scholar]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.-H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Mumber, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Lv, G.-P.; Chen, Y.W.; Li, S.P. Advanced development in analysis of phytochemicals from medicine and food dual purposes plants used in China. J. Chromatogr. 2011, 1218, 7453–7475. [Google Scholar] [CrossRef] [PubMed]
- Pompeu, D.R.; Silva, E.M.; Rogez, H. Optimisation of the solvent extraction of phenolic antioxidants from fruits of Euterpe oleracea using response surface methodology. Bioresour. Technol. 2009, 100, 6076–6082. [Google Scholar] [CrossRef] [PubMed]
- Carbonell-Capella, J.M.; Žlabur, J.Š.; Brnčić, S.R.; Francisco, J.B.; Grimi, N.; Koubaa, M.; Brnčić, M.; Vorobiev, E. Electrotechnologies, microwaves, and ultrasounds combined with binary mixtures of ethanol and water to extract steviol glycosides and antioxidant compounds from Stevia rebaudiana leaves. J. Food Process. Preserv. 2017, 41, e13179. [Google Scholar] [CrossRef]
- Pellegrini, N.; Colombi, B.; Salvatore, S.; Brenna, O.V.; Galaverna, G.; Del Rio, D.; Bianchi, M.; Bennett, R.N.; Brighenti, F. Evaluation of antioxidant capacity of some fruit and vegetable foods: Efficiency of extraction of a sequence of solvents. J. Sci. Food Agric. 2007, 87, 103–111. [Google Scholar] [CrossRef]
- Dhuique-Mayer, C.; Tbatou, M.; Carail, M.; Caris-Veyrat, C.; Dornier, M.; Amiot, M.J. Thermal degradation of antioxidant micronutrients in citrus juice: Kinetics and newly formed compounds. J. Agric. Food Chem. 2007, 55, 4209–4216. [Google Scholar] [CrossRef] [PubMed]
- Aspé, E.; Fernández, K. The effect of different extraction techniques on extraction yield, total phenolic, and anti-radical capacity of extracts from Pinus radiata bark. Ind. Crops Prod. 2011, 34, 838–844. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, C.Y. Extraction and isolation of polyphenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I.1.2.1–I.1.2.12. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Amado, I.R.; Franco, D.; Sanches, M.; Zapata, C.; Vazquez, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Stander, M.A.; Van Wyk, B.-E.; Taylor, M.J.C.; Long, H.S. Analysis of phenolic compounds in rooibos tea (Aspalathus linearis) with a comparison of flavonoid-based compounds in natural populations of plants from different regions. J. Agric. Food Chem. 2017, 65, 10270–10281. [Google Scholar] [CrossRef] [PubMed]
- Gironi, F.; Piemonte, V. Temperature and solvent effects on polyphenol extraction process from chestnut tree wood. Chem. Eng. Res. Des. 2011, 89, 857–862. [Google Scholar] [CrossRef]
- Trabelsi, N.; Megdiche, W.; Ksouri, R.; Falleh, H.; Oueslati, S.; Soumaya, B.; Hajlaoui, H.; Abdelly, C. Solvent effects on phenolic contents and biological activities of the halophyte Limoniastrum monopetalum leaves. LWT-Food Sci. Technol. 2010, 43, 632–639. [Google Scholar] [CrossRef]
- Hayouni, E.A.; Abedrabba, M.; Bouix, M.; Hamdi, M. The effects of solvents and extraction method on the phenolic contents and biological activities in vitro of tunisian Quercus coccifera L. and Juniperus phoenicea L. fruit extracts. Food Chem. 2007, 105, 1126–1134. [Google Scholar] [CrossRef]
- Ndhlala, A.R.; Mupure, C.H.; Benhura, M.A.N.; Muchuweti, M. Antioxidant potentials and degrees of polymerization of six wild fruits. Sci. Res. Essays 2006, 1, 87–92. [Google Scholar]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- De Castro, M.D.L.; García-Ayuso, L.E. Soxhlet extraction of solid materials: An outdated technique with a promising innovative future. Anal. Chim. Acta 1998, 369, 1–10. [Google Scholar] [CrossRef]
- Spigno, G.; Tramelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Zhang, H.-C.; Liu, W.-X.; Li, C.-H. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B 2012, 13, 94–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, E.; Poerner, N.; Rockenbach, I.I.; Gonzaga, L.V.; Mendes, C.R.; Fett, R. Phenolic compounds and antioxidant activity of blueberry cultivars grown in Brazil. Food Sci. Technol. 2011, 34, 911–917. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.S.; Moyá, M.L.; Troncoso, A.M.; García-Parrilla, M.C. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Han, X.; Piao, M.J.; Oh, M.C.; Fernando, P.M.D.J.; Kang, K.A.; Ryu, Y.S.; Jung, U.; Kim, I.G.; Hyun, J.W. Hyperoside induces endogenous antioxidant system to alleviate oxidative stress. J. Cancer Prev. 2016, 21, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentová, K.; Vrba, J.; Bancîřová, M.; Ulrichová, J.; Křen, K. Isoquercitrin: Pharmacology, toxicology, and metabolism. Food Chem. Toxicol. 2014, 68, 267–282. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomás-Barberán, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Rostamzad, H.; Shabanpour, B.; Kashaninejad, M.; ShabaniI, A. Antioxidative activity of citric and ascorbic acids and their preventive effect on lipid oxidation in frozen Persian sturgeon fillets. Lat. Am. Appl. Res. 2011, 41, 135–140. [Google Scholar]
- Lampila, P.; Van Lieshout, M.; Gremmen, B.; Lähteenmäki, L. Consumer attitudes towards enhanced flavonoid content in fruit. Food Res. Int. 2009, 42, 122–129. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.-O. Consideration on equivalent chemicals in total phenolic assay of chlorogenic acid-rich plums. Food Res. Int. 2004, 37, 337–342. [Google Scholar] [CrossRef]
- Galato, D.; Ckless, K.; Susin, M.F.; Giacomelli, C.; Ribeiro-do-Valle, R.M.; Spinelli, A. Antioxidant capacity of phenolic and related compounds: Correlation among electrochemical, visible spectroscopy methods and structure-antioxidant activity. Redox Rep. 2001, 6, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Jeong, S.W.; Lee, C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003, 81, 321–326. [Google Scholar] [CrossRef]
- Tawaha, K.; Alali, F.Q.; Gharaibeh, M.; Mohammad, M.; El-Elimat, T. Antioxidant activity and total phenolic content of selected Jordanian plant species. Food Chem. 2007, 104, 1372–1378. [Google Scholar] [CrossRef]



| Compound Number | Compound Name | m/z1 | Retention Time (min) | [M − H]− | MSE Fragments 2 | Individual Compound Concentrations (μg mL−1) | |||
|---|---|---|---|---|---|---|---|---|---|
| Ethanol: 30 min | Ethanol: 60 min | Ethanol: 120 min | Methanol Extraction | ||||||
| Positively identified compounds | |||||||||
| 1 | Catechin | 289.0713 | 11.48 | C15H13O6 | 289,125,203,245,151 | 36.8 | 51.9 | 54.9 | 68.0 |
| 2 | Citric acid | 191.0187 | 3.12 | C6H7O7 | 191,111,87,173 | 1042 | 1313 | 1288 | 2133 |
| 3 | Epicatechin 3 | 289.0698 | 13.57 | C15H13O6 | weak | - | - | - | - |
| 4 | Gallic acid | 169.0129 | 5.8 | C7H5O5 | 125,169,111 | 4.8 | 5.2 | 5.8 | 8.5 |
| 5 | Hesperetin | 301.1643 | 24.49 | C15H25O6 | weak | 0.1 | 0.1 | 0.1 | 0.1 |
| 6 | Hyperoside | 463.0878 | 17.51 | C21H19O12 | 300,463,271,255 | 34.6 | 42.9 | 40.8 | 102.8 |
| 7 | Isoquercitrin | 463.0876 | 17.51 | C21H19O12 | 300,463,271,301,255, | 43.2 | 53.5 | 50.8 | 128.2 |
| 8 | Kaempferol glucoside | 447.0938 | 18.06 | C21H19O11 | 285,169,447 | 12.1 | 12.3 | 16.7 | 33.1 |
| 9 | Luteolin-7-O-glucoside | 447.0935 | 18.06 | C21H19O11 | 285,284,169,125,447 | 0.3 | 0.3 | 0.3 | 1.1 |
| 10 | Procyanidin B1 | 577.1317 | 10.68 | C30H25O12 | 289,407,425,577 | 36.8 | 52.5 | 60.0 | 203.8 |
| 11 | Procyanidin B2 3 | 577.1345 | 12.72 | C30H25O12 | 289,407,425,577 | - | - | - | - |
| 12 | Quercetin-3-O-glucoside | 463.0886 | 17.81 | C21H19O12 | 300,271,463,255,125 | 2.7 | 3.4 | 3.1 | 9.0 |
| 13 | Quercetin-3-O-robinobioside | 609.1432 | 17.06 | C27H29O16 | 300,609,271,125 | 3.9 | 3.7 | 5.8 | 15.4 |
| 14 | Quercetin | 301.0353 | 23.99 | C15H9O7 | 125,169 | 2.0 | 2.2 | 1.8 | 1.9 |
| 15 | Rutin | 609.1458 | 17.27 | C27H29O16 | 300,609,271,255 | 11.5 | 13.8 | 13.1 | 38.3 |
| 16 | Trilobatin | 435.1284 | 18.75 | C21H23O10 | 315,345 | 0.5 | 0.7 | 0.8 | 2.1 |
| Tentatively identified and unknown compounds 3 | |||||||||
| Aconitic acid | 173.0089 | 9.14 | C6H5O6 | 111 | |||||
| Dihydroxy hexadecanoic acid | 287.2236 | 24.5 | C16H31O4 | 287 | |||||
| p-Coumaroylquinic acid | 337.0916 | 11.18 | C16H17O8 | 163,119,191,337 | |||||
| Procyanidin | 577.1344 | 10.68 | C30H25O12 | 289,407,425,577 | |||||
| Quercetin galloyl glucoside | 615.0979 | 16.63 | C28H23O16 | 300,615,463,255,169 | |||||
| Quercetin galloyl glucoside | 615.0977 | 16.94 | C28H23O16 | 300,463,615 | |||||
| Quercetin rhamnoside | 447.0927 | 19.63 | C21H19O11 | 300,271,255,447,243 | |||||
| Quercetin rhamnoside | 447.0927 | 18.89 | C21H19O11 | 300,271,255,447 | |||||
| Quercetin-3-O-pentoside | 433.0764 | 18.83 | C20H17O11 | 300,271,255,433,315 | |||||
| unknown | 340.1035 | 14.37 | C15H18NO8 | 161,101,85 | |||||
| unknown | 443.1913 | 11.37 | C21H31O10 | 443,289,303 | |||||
| unknown | 515.1246 | 5.49 | C18H27O17 | 515,111,173 | |||||
| unknown | 515.1245 | 5.68 | C18H27O17 | 515,111,173 | |||||
| unknown | 515.1255 | 5.83 | C18H27O17 | 515,111,173 | |||||
| unknown | 515.1253 | 5.93 | C18H27O17 | 515,111,173 | |||||
| unknown | 435.107 | 21.403 | C24H19O8 | 341,189,125,435 | |||||
| unknown | 219.0506 | 9.37 | C8H11O7 | 111,219,87 | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosthuizen, D.; Goosen, N.J.; Stander, M.A.; Ibrahim, A.D.; Pedavoah, M.-M.; Usman, G.O.; Aderinola, T. Solvent Extraction of Polyphenolics from the Indigenous African Fruit Ximenia caffra and Characterization by LC-HRMS. Antioxidants 2018, 7, 103. https://doi.org/10.3390/antiox7080103
Oosthuizen D, Goosen NJ, Stander MA, Ibrahim AD, Pedavoah M-M, Usman GO, Aderinola T. Solvent Extraction of Polyphenolics from the Indigenous African Fruit Ximenia caffra and Characterization by LC-HRMS. Antioxidants. 2018; 7(8):103. https://doi.org/10.3390/antiox7080103
Chicago/Turabian StyleOosthuizen, Dewald, Neill J. Goosen, Maria A. Stander, Aliyu D. Ibrahim, Mary-Magdalene Pedavoah, Grace O. Usman, and Taiwo Aderinola. 2018. "Solvent Extraction of Polyphenolics from the Indigenous African Fruit Ximenia caffra and Characterization by LC-HRMS" Antioxidants 7, no. 8: 103. https://doi.org/10.3390/antiox7080103