Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Study Design
2.3. Sarcopenia Diagnosis
2.4. Training Intervention
2.5. Body Composition
2.6. Functional Performance Evaluations
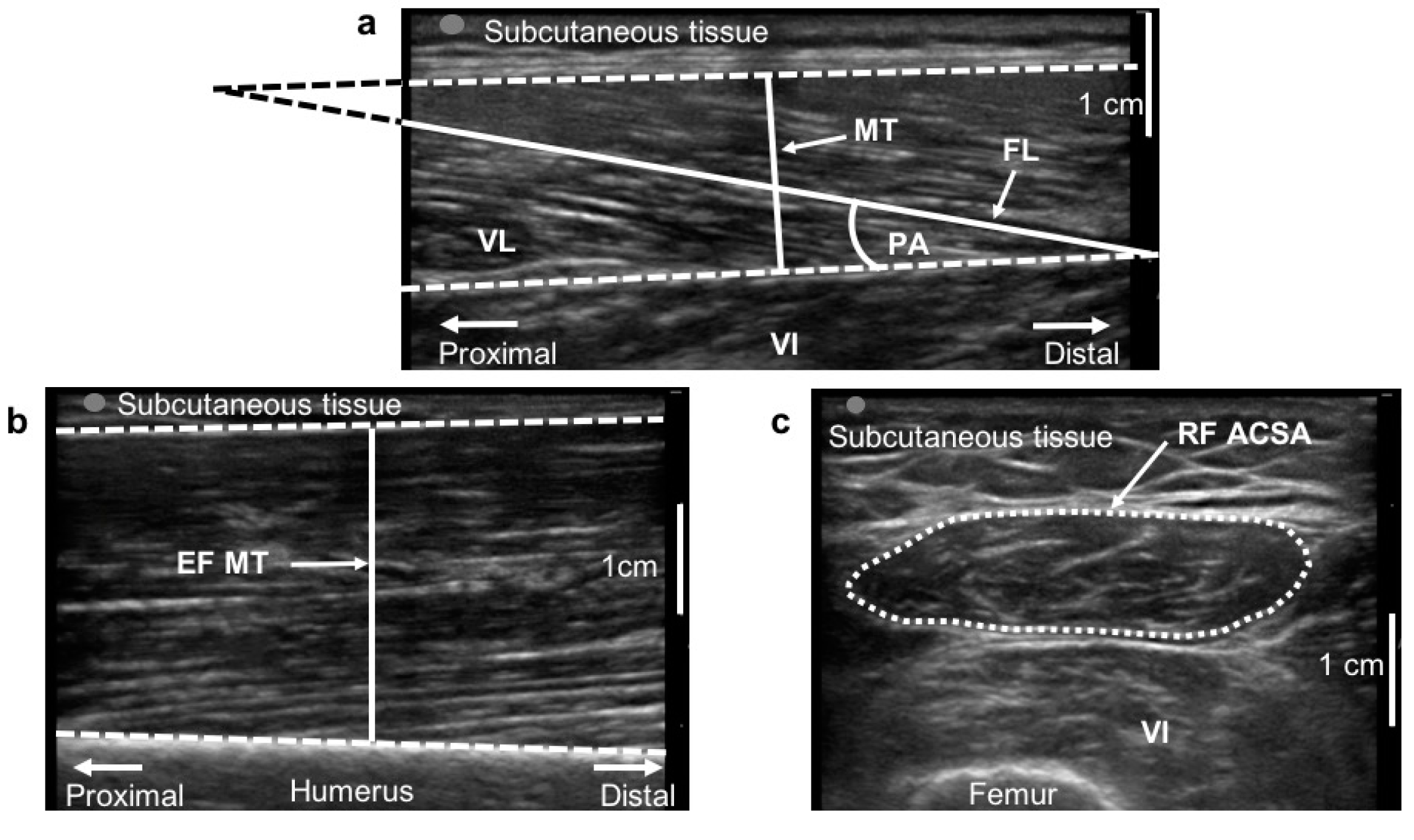
2.7. Muscle Morphology
2.8. Muscle Strength (1RM)
2.9. Biological Samples
2.10. Analytical Procedures
2.11. Statistical Analysis
3. Results
3.1. Sarcopenia Diagnosis
3.2. Body Composition and Functional Performance
3.3. Muscle Morphology
3.4. Muscle Strength
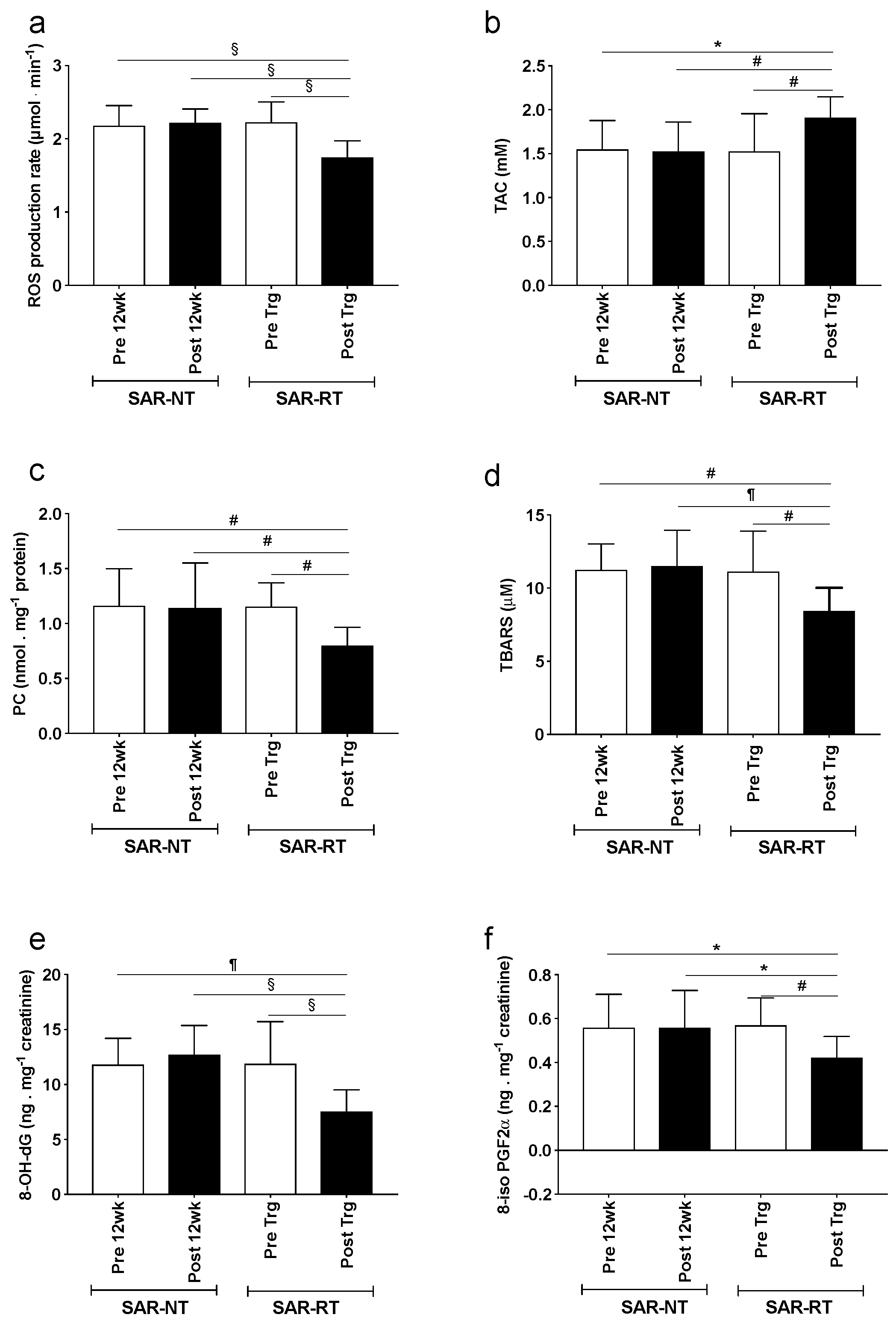
3.5. Biological Samples
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RT | resistive training |
| SAR | sarcopenic elders |
| SMI | skeletal muscle index |
| VL | vastus lateralis |
| EF | elbow flexors |
| MT | muscle thickness |
| PA | pennation angle |
| RF | rectus femoris |
| ACSA | anatomical cross-sectional area |
| EPR | electron paramagnetic resonance |
| ROS | reactive oxygen species |
| TAC | total Antioxidant Capacity |
| PC | protein carbonyls |
| TBARS | thiobarbituric acid-reactive substances |
| 8-iso-PGF2-α- | 8-isoprostane |
| 8-OH-dG | 8-OH-2-deoxyguanosine |
| OxS | markers of oxidative stress/damage |
References
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. JAGS 2002, 50, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Bajekal, M.; Wheeler, L.; Dix, D. Estimating residents and staff in communal establishments from the 2001census. Health Stat. Q. 2006, 31, 42–50. [Google Scholar]
- Wickham, C.; Cooper, C.; Margetts, B.M.; Barker, D.J. Muscle strength, activity, housing and the risk of falls in elderly people. Age Ageing 1989, 18, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.E.; Martin, H.J.; Dennison, E.M.; Anderson, F.H.; Cooper, C. Falls, sarcopenia, and growth in early life: Findings from the Hertfordshire cohort study. Am. J. Epidemiol. 2006, 164, 665–671. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevsky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Cederholm, T.; Morley, J.E. Sarcopenia: The new definitions. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Narici, M.V.; Maffulli, N. Sarcopenia: Characteristics, mechanisms and functional significance. Br. Med. Bull. 2010, 95, 139–159. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Rygiel, K.A.; Picard, M.; Turnbull, D.M. The ageing neuromuscular system and sarcopenia: A mitochondrial perspective. J. Physiol. 2016, 594, 4499–4512. [Google Scholar] [CrossRef]
- Jang, Y.C.; Lustgarten, M.S.; Liu, Y.; Muller, F.L.; Bhattacharya, A.; Liang, H.; Salmon, A.B.; Brooks, S.V.; Larkin, L.; Hayworth, C.R.; et al. Increased superoxide in vivo accelerates age-associated muscle atrophy through mitochondrial dysfunction and neuromuscular junction degeneration. FASEB J. 2010, 24, 1376–1390. [Google Scholar] [CrossRef]
- Jackson, M.J.; McArdle, A. Role of reactive oxygen species in age-related neuromuscular deficits. J. Physiol. 2016, 594, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, A.Z.; Caturegli, G.; Metter, E.J.; Makrogiannis, S.; Resnick, S.M.; Harris, T.B.; Ferrucci, L. Difference in muscle quality over the adult life span and biological correlates in the Baltimore Longitudinal Study of Aging. J. Am. Geriatr. Soc. 2014, 62, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Borde, R.; Hortobágyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Fiatarone, M.A.; Marks, E.C.; Ryan, N.D.; Meredith, C.N.; Lipsitz, L.A.; Evans, W.J. High-intensity strength training in nonagenarians. Effects on skeletal muscle. JAMA 1990, 263, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Men, Ageing and Health: Achieving Health across the Life Span; Ageing and Life Course; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 2008, 23, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.D.; Patrizi, R.M.; Cheek, D.J.; Wooten, J.S.; Barbee, J.J.; Mitchell, J.B. Resistance training reduces subclinical inflammation in obese, postmenopausal women. Med. Sci. Sports Exerc. 2012, 44, 2099–2110. [Google Scholar] [CrossRef] [PubMed]
- Vincent, H.K.; Bourguignon, C.; Vincent, K.R. Resistance training lowers exercise-induced oxidative stress and homocysteine levels in overweight and obese older adults. Obesity 2006, 14, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Exercise at old age: Does it increase or alleviate oxidative stress? Ann. Acad. Sci. 2001, 928, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Smythe, G.M.; Rando, T.A. Notch-mediated restoration of regenerative potential to aged muscle. Science 2003, 302, 1575–1577. [Google Scholar] [CrossRef] [PubMed]
- Conboy, I.M.; Conboy, M.J.; Wagers, A.J.; Girma, E.R.; Weissman, I.L.; Rando, T.A. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005, 433, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, I.M.; Conboy, M.J.; Shen, J.; Rando, T.A. A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell 2008, 2, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Blau, H.M.; Cosgrove, B.D.; Ho, A.T. The central role of muscle stem cells in regenerative failure with aging. Nat. Med. 2015, 21, 854–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouzid, M.A.; Filaire, E.; McCall, A.; Fabre, C. Radical Oxygen Species, Exercise and Aging: An Update. Sports Med. 2015, 45, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Taaffe, D.R.; Pruitt, L.; Pyka, G.; Guido, D.; Marcus, R. Comparative effects of high- and low-intensity resistance training on thigh muscle strength, fiber area, and tissue composition in elderly women. Clin. Physiol. 1996, 16, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Serra-Rexach, J.A.; Bustamante-Ara, N.; Hierro Villarán, M.; González Gil, P.; Sanz Ibáñez, M.J.; Blanco Sanz, N.; Ortega Santamaría, V.; Gutiérrez Sanz, N.; Marín Prada, A.B.; Gallardo, C.; et al. Short-term, light- to moderate-intensity exercise training improves leg muscle strength in the oldest old: A randomized controlled trial. J. Am. Geriatr. Soc. 2011, 59, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Burd, N.A.; Mitchell, C.J.; Churchward-Venne, T.A.; Phillips, S.M. Bigger weights may not beget bigger muscles: Evidence from acute muscle protein synthetic responses after resistance exercise. Appl. Physiol. Nutr. Metab. 2012, 37, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Breen, L.; Phillips, S.M. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr. Metab. 2011, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Selby, A.; Rankin, D.; Patel, R.; Atherton, P.; Hildebrandt, W.; Williams, J.; Smith, K.; Seynnes, O.; Hiscock, N.; et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J. Physiol. 2009, 587, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Greig, C.A.; Young, A.; Skelton, D.A.; Pippet, E.; Butler, F.M.; Mahmud, S.M. Exercise studies with elderly volunteers. Age Ageing 1994, 23, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Heymsfield, S.; Baumgartner, R.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Ferrucci, L.; Pieper, C.F.; Leveille, S.G.; Markides, K.S.; Ostir, G.V.; Studenski, S.; Berkman, L.F.; Wallace, R.B. Lower extremity function and subsequent disability: Consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M221–M231. [Google Scholar] [CrossRef] [PubMed]
- Franchi, M.V.; Atherton, P.J.; Reeves, N.D.; Flück, M.; Williams, J.; Mitchell, W.K.; Selby, A.; Beltran Valls, R.M.; Narici, M.V. Architectural, functional and molecular responses to concentric and eccentric loading in human skeletal muscle. Acta Physiol. 2014, 210, 642–654. [Google Scholar] [CrossRef] [PubMed]
- Trezise, J.; Collier, N.; Blazevich, A.J. Anatomical and neuromuscular variables strongly predict maximum knee extension torque in healthy men. Eur. J. Appl. Physiol. 2016, 116, 1159–1177. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, Y.; Ikezoe, T.; Yamada, Y.; Tsukagoshi, R.; Nakamura, M.; Takagi, Y.; Kimura, M.; Ichihashi, N. Age-related ultrasound changes in muscle quantity and quality in women. Ultrasound Med. Biol. 2015, 41, 3013–3017. [Google Scholar] [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. Assessment of a standardized ROS production profile in humans by electron paramagnetic resonance. Oxid. Med. Cell. Longev. 2012. Available online: http://www.hindawi.com/journals/omcl/2012/973927 (accessed on 26 July 2012). [CrossRef] [PubMed]
- Mrakic-Sposta, S.; Gussoni, M.; Montorsi, M.; Porcelli, S.; Vezzoli, A. A quantitative method to monitor Reactive Oxygen Species production by Electron Paramagnetic Resonance in physiological and pathological conditions. Oxid. Med. Cell. Longev. 2014. Available online: http://www.hindawi.com/journals/omcl/2014/306179/abs (accessed on 12 October 2014). [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Mijnarends, D.M.; Meijers, J.M.; Halfens, R.J.; ter Borg, S.; Luiking, Y.C.; Verlaan, S.; Schoberer, D.; Jentoft, A.J.C.; van Loon, L.J.; Schols, J.M. Validity and reliability of tools to measure muscle mass, strength, and physical performance in community-dwelling older people: A systematic review. J. Am. Med. Dir. Assoc. 2013, 14, 170–178. [Google Scholar] [CrossRef]
- Ruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar]
- Franchi, M.V.; Longo, S.; Mallinson, J.; Quinlan, J.I.; Taylor, T.; Greenhaff, P.L.; Narici, M.V. Muscle thickness correlates to muscle cross-sectional area in the assessment of strength training-induced hypertrophy. Scand. J. Med. Sci. Sports 2018, 28, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Peterson, M.D.; Sen, A.; Gordon, P.M. Influence of resistance exercise on lean body mass in aging adults: A Meta-Analysis. Med. Sci. Sports Exerc. 2011, 43, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, A.; De Vito, G. Muscle strength, power and adaptations to resistance training in older people. Eur. J. Appl. Physiol. 2004, 91, 450–472. [Google Scholar] [CrossRef] [PubMed]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Bobeuf, F.; Labonte, M.; Dionne, I.J.; Khalil, A. Combined effect of antioxidant supplementation and resistance training on oxidative stress markers, muscle and body composition in an elderly population. J. Nutr. Health Aging 2011, 15, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Schilling, B.K.; Karlage, R.E.; Ledoux, M.S.; Pfeiffer, R.F.; Callegari, J. Effect of resistance training on blood oxidative stress in Parkinson disease. Med. Sci. Sports Exerc. 2008, 40, 1385–1389. [Google Scholar] [CrossRef]
- Vincent, K.R.; Vincent, H.K.; Braith, R.W.; Lennon, S.L.; Lowenthal, D.T. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur. J. Appl. Physiol. 2002, 87, 416–423. [Google Scholar] [CrossRef]
- Parise, G.; Phillips, S.M.; Kaczor, J.J.; Tarnopolsky, M.A. Antioxidant enzyme activity is up-regulated after unilateral resistance exercise training in older adults. Free Radic. Biol. Med. 2005, 39, 289–295. [Google Scholar] [CrossRef]
- Parise, G.; Brose, A.N.; Tarnopolsky, M.A. Resistance exercise training decreases oxidative damage to DNA and increases cytochrome oxidase activity in older adults. Exp. Gerontol. 2005, 40, 173–180. [Google Scholar] [CrossRef]
- Rall, L.C.; Roubenoff, R.; Meydani, S.N.; Han, S.N.; Meydani, M. Urinary 8-hydroxy20-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: Effect of progressive resistance training. J. Nutr. Biochem. 2000, 11, 581–584. [Google Scholar] [CrossRef]
- Powers, S.K.; Nelson, W.B.; Hudson, M.B. Exercise-induced oxidative stress in humans: Cause and consequences. Free Radic. Biol. Med. 2011, 51, 942–950. [Google Scholar] [CrossRef] [PubMed]
- Prochniewicz, E.; Thomas, D.D.; Thompson, L.V. Age-related decline in actomyosin function. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Response of the ubiquitin-proteasome pathway to changes in muscle activity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1423–R1431. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative protein damage is associated with poor grip strength among older women living in the community. J. Appl. Physiol. 2007, 103, 17–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltran Valls, M.R.; Wilkinson, D.J.; Narici, M.V.; Smith, K.; Phillips, B.E.; Caporossi, D.; Atherton, P.J. Protein carbonylation and heat shock proteins in human skeletal muscle: Relationships to age and sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 174–181. [Google Scholar] [CrossRef]


| SAR-NT | SAR-RT | |||
|---|---|---|---|---|
| n = 15 9 F/6 M | n = 20 10 F/10 M | |||
| Pre 12 Weeks | Post 12 Weeks | Pre Training | Post Training | |
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| Age (years) | 71.7 ± 3.4 | 71.7 ± 3.4 | 73.0 ± 5.5 | 73.0 ± 5.5 |
| Body Mass (kg) | 69.8 ± 15.0 | 70.2 ± 15.4 | 76.3 ± 16 | 75.7 ± 16.8 |
| Stature (m) | 1.62 ± 0.1 | 1.62 ± 0.1 | 1.65 ± 0.1 | 1.65 ± 0.1 |
| BMI (kg m−2) | 26.6 ± 3.5 | 26.8 ± 3.6 | 27.7 ± 4.4 | 27.5 ± 4.7 |
| Skeletal Muscle Mass (kg) | 20.8 ± 6.3 | 21.2 ± 5.9 | 22.5 ± 6.3 | 22.9 ± 6.4 |
| SMI% | 29.6 ± 3.8 | 29.8 ± 3.6 | 29.4 ± 4.7 | 30.2 ± 4.2 # |
| Handgrip (kg) | 27.8 ± 9.4 | 29.4 ± 9.6 | 32.4 ± 10.7 | 30.9 ± 10.6 |
| SPPB Score | 11.7 ± 0.4 | 11.6 ± 0.4 | 11.0 ± 1.8 | 11.2 ± 1.2 |
| Get-Up and go Score | n = 15 freely mobile | n = 15 freely mobile | n = 19 freely mobile n = 1 mostly independent | n = 20 freely mobile |
| Stair Climbing (W) | 256.1 ± 87.6 | 262.4 ± 84.5 | 299.8 ± 114.0 | 322.8 ± 148.2 ## |
| Classification | ||||
| Non sarcopenic | n = 0 | n = 0 | n = 0 | n = 3 |
| Class I sarcopenia | n = 14 | n = 14 | n = 14 | n = 14 |
| Class II sarcopenia | n = 1 | n = 1 | n = 6 | n = 3 |
| SAR-NT | SAR-RT | |||||||
|---|---|---|---|---|---|---|---|---|
| Muscle Morphology | Pre 12 Weeks | Post 12 Weeks | P | Pre Training | Post Training | Difference % | P | MDC95% |
| VL—PA (°) | 15.7 ± 2.5 | 15.6 ± 2.3 | 0.092 | 13.5 ± 3.1 | 15.3 ± 2.8 | +13.4% | <0.001 | 9.6% |
| VL—MT (cm) | 1.6 ± 0.2 | 1.7 ± 0.3 | 0.202 | 1.7 ± 0.3 | 1.8 ± 0.4 | +5.5% | 0.002 | 4.9% |
| RF—ACSA (cm2) | 3.7 ± 1.1 | 3.7 ± 1.1 | 0.226 | 4.0 ± 1.3 | 4.5 ± 1.4 | +14.5% | <0.001 | 10.0% |
| EF—MT (cm) | 2.4 ± 0.5 | 2.4 ± 0.4 | 0.123 | 2.6 ± 0.6 | 2.8 ± 0.9 | +10.4% | <0.001 | 8.3% |
| 1 RM (kg) | Pre | Post | Difference | P | MDC95% |
|---|---|---|---|---|---|
| Chest press | 22.2 ± 12.3 | 44.6 ± 15.4 | +101.0% | <0.001 | 15.0% |
| Leg press | 90.0 ± 32.2 | 167.2 ± 87.3 | +85.8% | <0.001 | 9.4% |
| Vertical row | 26.5 ± 15.5 | 50.2 ± 17.4 | +88.7% | <0.001 | 15.6% |
| Lateral rise | 2.6 ± 1.5 | 4.3 ± 1.8 | +66.6% | <0.001 | 39.3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vezzoli, A.; Mrakic-Sposta, S.; Montorsi, M.; Porcelli, S.; Vago, P.; Cereda, F.; Longo, S.; Maggio, M.; Narici, M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants 2019, 8, 431. https://doi.org/10.3390/antiox8100431
Vezzoli A, Mrakic-Sposta S, Montorsi M, Porcelli S, Vago P, Cereda F, Longo S, Maggio M, Narici M. Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants. 2019; 8(10):431. https://doi.org/10.3390/antiox8100431
Chicago/Turabian StyleVezzoli, Alessandra, Simona Mrakic-Sposta, Michela Montorsi, Simone Porcelli, Paola Vago, Ferdinando Cereda, Stefano Longo, Marcello Maggio, and Marco Narici. 2019. "Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals" Antioxidants 8, no. 10: 431. https://doi.org/10.3390/antiox8100431
APA StyleVezzoli, A., Mrakic-Sposta, S., Montorsi, M., Porcelli, S., Vago, P., Cereda, F., Longo, S., Maggio, M., & Narici, M. (2019). Moderate Intensity Resistive Training Reduces Oxidative Stress and Improves Muscle Mass and Function in Older Individuals. Antioxidants, 8(10), 431. https://doi.org/10.3390/antiox8100431










