White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction of White Tea Polyphenols and Evaluation of the Antioxidant Activity in Vitro
2.2. Animal Experiment
2.3. Determination of Biochemical Indicators in Serum
2.4. Histological Observations
2.5. RT-qPCR Analysis
2.6. Western Blot
2.7. HPLC Analysis
2.8. Statistical Analysis
3. Results
3.1. Antioxidant Effects of WPPs in Vitro
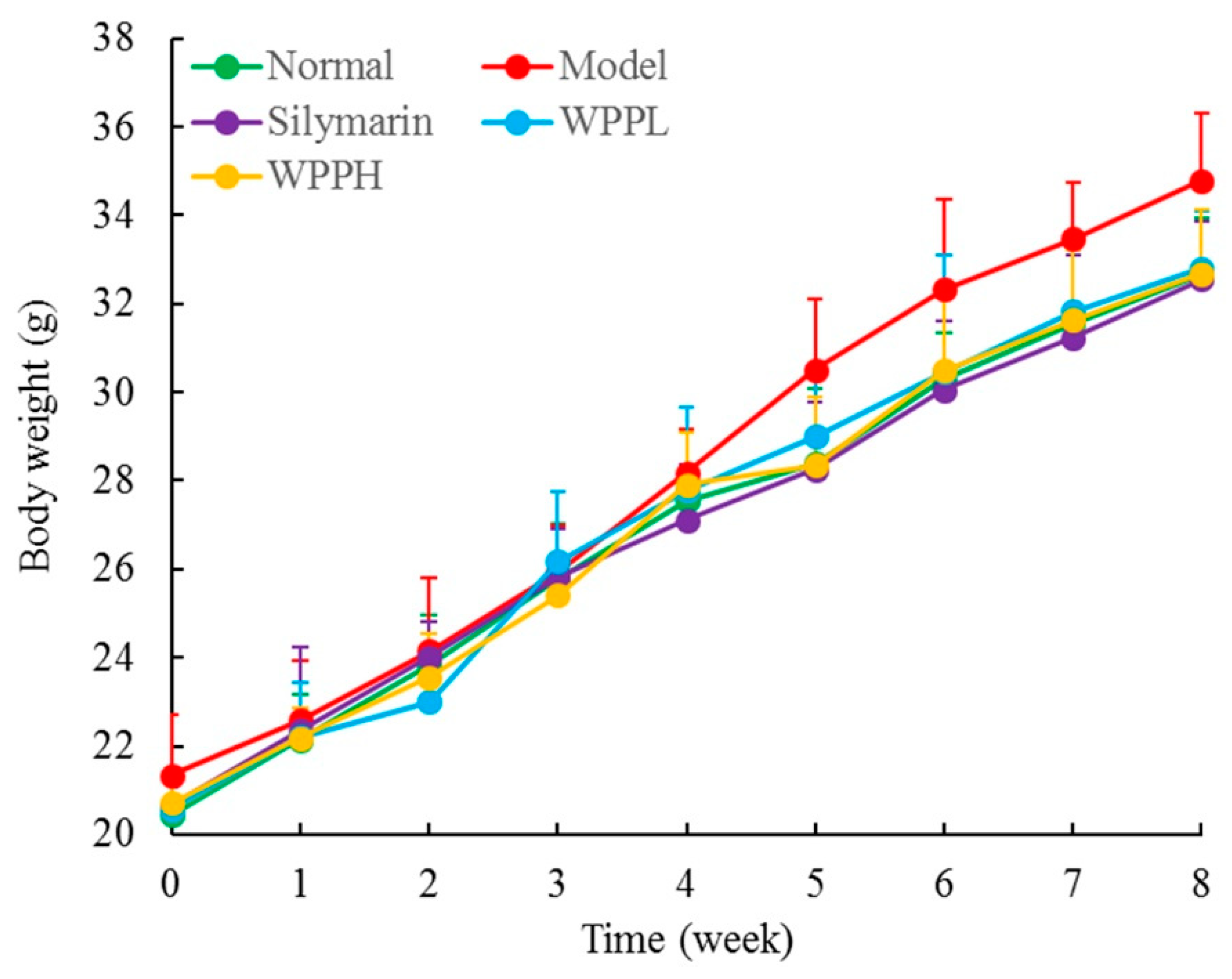
3.2. Liver Index
3.3. Liver Function-Related Serum Levels in Mice
3.4. Oxidation-Related Serum Levels in Mice
3.5. Cytokine Serum Levels in Mice
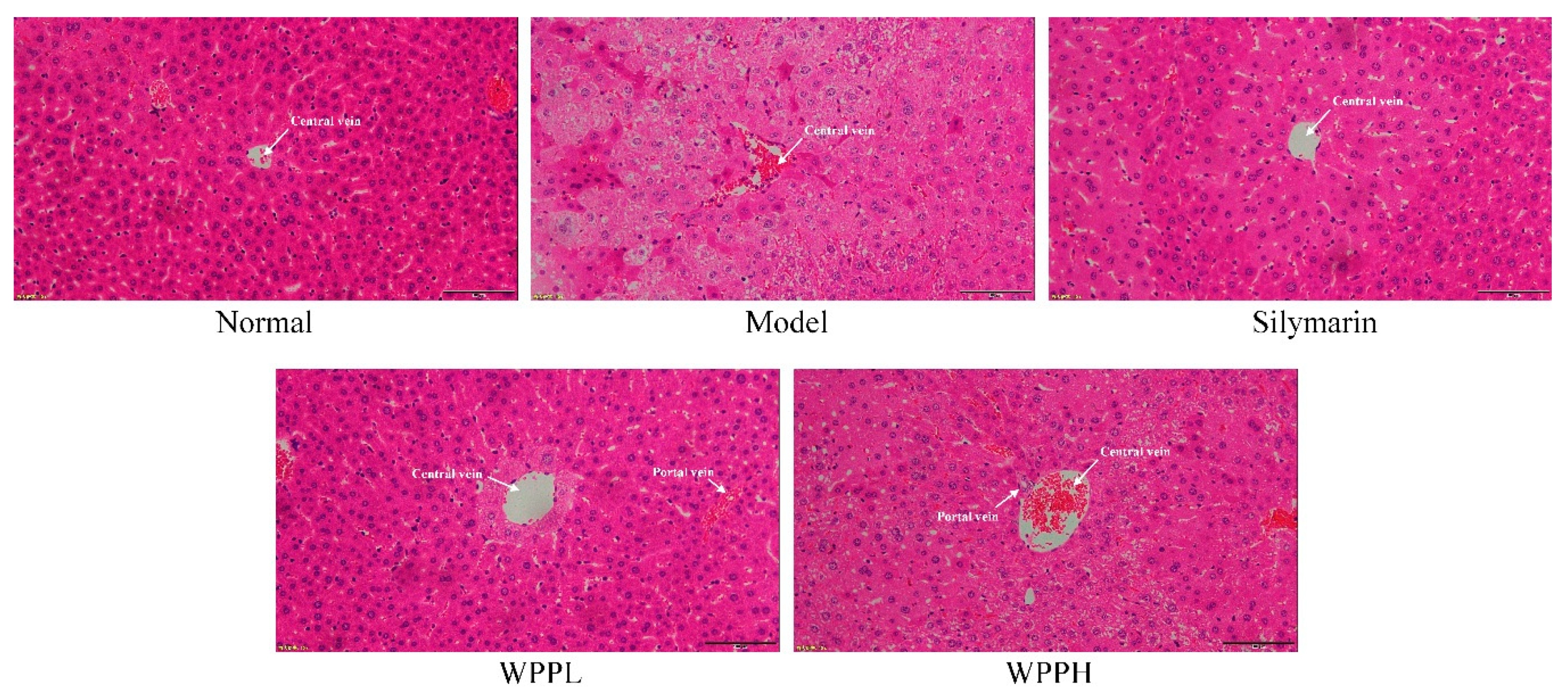
3.6. Histological Analyses
3.7. Expression of nNOS, eNOS, and iNOS in the Liver of Mice
3.8. Expression of Cu-Zn-SOD, Mn-SOD, and CAT in the Liver of Mice
3.9. Analysis of the Chemical Composition of WPPs
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Santana-Rios, G.; Orner, G.A.; Amantana, A.; Provost, C.; Wu, S.Y.; Dashwood, R.H. Potent antimutagenic activity of white tea in comparison with green tea in the Salmonella assay. Mutat. Res. 2001, 495, 61–74. [Google Scholar] [CrossRef]
- Yang, C.; Hu, Z.; Lu, M.; Li, P.; Tan, J.; Chen, M.; Lv, H.; Zhu, Y.; Zhang, Y.; Guo, L.; et al. Application of metabolomics profiling in the analysis of metabolites and taste quality in different subtypes of white tea. Food Res. Int. 2018, 106, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S. Effects of the aqueous extract of white tea (Camellia sinensis) in a streptozotocin-induced diabetes model of rats. Phytomedicine 2011, 19, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Almajano, M.P.; Vila, I.; Gines, S. Neuroprotective effects of white tea against oxidative stress-induced toxicity in striatal cells. Neurotox. Res. 2011, 20, 372–378. [Google Scholar] [CrossRef]
- Espinosa, C.; López-Jiménez, J.A.; Pérez-Llamas, F.; Guardiola, F.A.; Esteban, M.A.; Arnao, M.B.; Zamora, S. Long-term intake of white tea prevents oxidative damage caused by adriamycin in kidney of rats. J. Sci. Food Agric. 2016, 96, 3079–3087. [Google Scholar] [CrossRef]
- Yuan, D.S.; Li, L.M.; Yang, Z.J.; Yue, W.J.; Zheng, J.G. Effect of liver-protection with different drying temperatures for white tea processing. China Tea 2009, 31, 16–18. [Google Scholar]
- Teschke, R. Alcoholic liver disease: Current mechanistic aspects with focus on their clinical relevance. Biomedicines 2019, 7, 68. [Google Scholar] [CrossRef]
- Wu, D.; Cederbaum, A.I. Oxidative stress and alcoholic liver disease. Semin. Liver Dis. 2009, 29, 141–154. [Google Scholar] [CrossRef]
- Jeong, H.M.; Kim, D.J. Bone diseases in patients with chronic liver disease. Int. J. Mol. Sci. 2019, 20, 4270. [Google Scholar] [CrossRef]
- Gómez-Bañuelos, E.; Mukherjee, A.; Darrah, E.; Andrade, F. Rheumatoid arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J. Clin. Med. 2019, 8, 1309. [Google Scholar] [CrossRef]
- Drescher, H.K.; Weiskirchen, S.; Weiskirchen, R. Current status in testing for nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH). Cells 2019, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Lissi, E.A.; Modak, B.; Torres, R.; Escobar, J.; Urzua, A. Total antioxidant potential of resinous exudates from Heliotropium species, and a comparison of the ABTS and DPPH methods. Free Radic. Res. 1999, 30, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, J.; Yi, S.; Li, X.; Guo, Z.; Zhou, X.; Mu, J.; Yi, R. Lactobacillus plantarum CQPC02 prevents obesity in mice through the PPAR-α signaling pathway. Biomolecules 2019, 9, 407. [Google Scholar]
- Soares, D.G.; Andreazza, A.C.; Salvador, M. Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J. Agric. Food Chem. 2003, 51, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Martysiak-Żurowska, D.; Wenta, W. A comparison of ABTS and DPPH methods for assessing the total antioxidant capacity of human milk. Acta Sci. Pol. Technol. Aliment. 2012, 11, 83–89. [Google Scholar]
- Gao, B.; Bataller, R. Alcoholic liver disease: Pathogenesis and new therapeutic targets. Gastroenterology 2011, 141, 1572–1585. [Google Scholar] [CrossRef]
- Colica, C.; Boccuto, L.; Abenavoli, L. Silymarin: An option to treat non-alcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 8437–8438. [Google Scholar] [CrossRef]
- Wang, M.; Niu, J.; Ou, L.; Deng, B.; Wang, Y.; Li, S. Zerumbone protects against carbon tetrachloride (CCl4)-induced acute liver injury in mice via inhibiting oxidative stress and the inflammatory response: Involving the TLR4/NF-κB/COX-2 pathway. Molecules 2019, 24, 1964. [Google Scholar] [CrossRef]
- Iweala, E.E.J.; Evbakhavbokun, W.O.; Maduagwu, E.N. Antioxidant and hepatoprotective effect of Cajanus cajan in N-nitrosodiethylamine-induced liver damage. Sci. Pharm. 2019, 87, 24. [Google Scholar] [CrossRef]
- Albasher, G.; Almeer, R.; Al-Otibi, F.O.; Al-Kubaisi, N.; Mahmoud, A.M. Ameliorative effect of Beta vulgaris root extract on chlorpyrifos-induced oxidative stress, inflammation and liver injury in rats. Biomolecules 2019, 9, 261. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Z.; Zhang, J.; Mu, J.; Zhou, X.; Zhao, X. Liver injury induced by carbon tetrachloride in mice is prevented by the antioxidant capacity of Anji White tea polyphenols. Antioxidants 2019, 8, 64. [Google Scholar] [CrossRef]
- Tang, B.B.; Hu, D.H. Effect of early bedside hemofiltration on systemic inflammatory state as well as liver and kidney function in patients with severe acute pancreatitis. J. Hainan Med. Univ. 2016, 22, 124–127. [Google Scholar]
- Farbiszewski, R.; Radecka, A.; Chwiecko, M.; Holownia, A. The effect of heparegen on antioxidant enzyme activities in ethanol-induced liver injury in rats. Alcohol 1992, 9, 403–407. [Google Scholar] [CrossRef]
- Huang, Q.H.; Xu, L.Q.; Liu, Y.H.; Wu, J.Z.; Wu, X.; Lai, X.P.; Li, Y.C.; Su, Z.R.; Chen, J.N.; Xie, Y.L. Polydatin protects rat liver against ethanol-induced injury: Involvement of CYP2E1/ROS/Nrf2 and TLR4/NF-κB p65 pathway. Evid. Based Complement. Altern. Med. 2017, 2017, 7953850. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Ji, D.F.; Chen, S.; Hu, G.Y. Protective effects of sericin protein on alcohol-mediated liver damage in mice. Alcohol Alcohol. 2008, 43, 246–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Liu, M.; Zhang, C.; Li, S.; Yang, Q.; Zhang, J.; Gong, Z.; Han, J.; Jia, L. Antioxidant activity and protective effects of enzyme-extracted Oudemansiella radiata polysaccharides on alcohol-induced liver injury. Molecules 2018, 23, 481. [Google Scholar] [CrossRef]
- Sun, X.; Wang, P.; Yao, L.P.; Wang, W.; Gao, Y.M.; Zhang, J.; Fu, Y.J. Paeonol alleviated acute alcohol-induced liver injury via SIRT1/Nrf2/NF-κB signaling pathway. Environ. Toxicol. Pharmacol. 2018, 60, 110–117. [Google Scholar] [CrossRef]
- Clemens, M.G. Nitric oxide in liver injury. Hepatology 1999, 30, 1–5. [Google Scholar] [CrossRef]
- An, L.; Wang, X.; Cederbaum, A.I. Cytokines in alcoholic liver disease. Arch. Toxicol. 2012, 86, 1337–1338. [Google Scholar] [CrossRef]
- Eipel, C.; Hardenberg, J.; Negendank, S.; Abshagen, K.; Vollmar, B. Thrombopoietin limits IL-6 release but fails to attenuate liver injury in two hepatic stress models. Eur. J. Gastroenterol. Hepatol. 2009, 21, 923–931. [Google Scholar] [CrossRef]
- Cheng, L.; Wang, J.; Li, X.; Xing, Q.; Du, P.; Su, L.; Wang, S. Interleukin-6 induces Gr-1+CD11b+ myeloid cells to suppress CD8+ T cell-mediated liver injury in mice. PLoS ONE 2011, 6, e17631. [Google Scholar] [CrossRef]
- Cheng, L.; Du, X.; Wang, Z.; Ju, J.; Jia, M.; Huang, Q.; Xing, Q.; Xu, M.; Tan, Y.; Liu, M.; et al. Hyper-IL-15 suppresses metastatic and autochthonous liver cancer by promoting tumour-specific CD8+ T cell responses. J. Hepatol. 2014, 61, 1297–1303. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.C.; Wang, H.; Shi, K.; Li, J.M.; Zong, Y.; Du, R. Hepatoprotective effect of baicalein against acetaminophen-induced acute liver injury in mice. Molecules 2019, 24, 131. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fang, Y.; Yi, R.; Zhao, X. Preventive effect of blueberry extract on liver injury induced by carbon tetrachloride in mice. Foods 2019, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.Y.; Li, H.W.; Liu, F.G.; Li, Y.C.; Tian, S.; Cao, L.H.; Hu, K.; Wu, X.X.; Miao, M.S. Effects of Portulaca Oleracea Extract on Acute Alcoholic Liver Injury of Rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef]
- Alexaki, V.I.; Charalampopoulos, I.; Kampa, M.; Vassalou, H.; Theodoropoulos, P.; Stathopoulos, E.N.; Hatzoglou, A.; Gravanis, A.; Castanas, E. Estrogen exerts neuroprotective effects via membrane estrogen receptors and rapid Akt/NOS activation. FASEB J. 2004, 18, 1594–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wickman, A.; Jonsdottir, I.H.; Bergstrom, G.; Hedin, L. GH and IGF-I regulate the expression of endothelial nitric oxide synthase (eNOS) in cardiovascular tissues of hypophysectomized female rats. Eur. J. Endocrinol. 2002, 147, 523–533. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y.; Abe, M.; Murai, A.; Shimizu, N.; Okamoto, I.; Katsuragi, T.; Tanaka, K. Comparison of effects of nitric oxide synthase (NOS) inhibitors on plasma nitrite/nitrate levels and tissue NOS activity in septic organs. Microbiol. Immunol. 2005, 49, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.I.; Wang, D.; Leu, F.J.; Chen, C.F.; Chen, H.I. Ischemia and reperfusion of liver induces eNOS and iNOS expression: Effects of a NO donor and NOS inhibitor. Chin. J. Physiol. 2004, 47, 121–127. [Google Scholar]
- Liu, B.; Li, J.; Yi, R.; Mu, J.; Zhou, X.; Zhao, X. Preventive effect of alkaloids from Lotus plumule on acute liver injury in mice. Foods 2019, 8, 36. [Google Scholar] [CrossRef]
- Kanai, S.; Okano, H. Mechanism of the protective effects of sumac gall extract and gallic acid on the progression of CCl4-induced acute liver injury in rats. Am. J. Chin. Med. 1998, 26, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, M.D.; Nakagami, M.; Bradford, B.U.; Uesugi, T.; Mason, R.P.; Connor, H.D.; Dikalova, A.; Kadiiska, M.; Thurman, R.G. Overexpression of manganese superoxide dismutase prevents alcohol-induced liver injury in the rat. J. Biol. Chem. 2001, 276, 36664–36672. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Asokkumar, K.; Umamaheswari, M.; Sivashanmugam, A.T.; Subhadradevi, V. Antiulcerogenic effect of gallic acid in rats and its effect on oxidant and antioxidant parameters in stomach tissue. Indian J. Pharm. Sci. 2013, 75, 149–155. [Google Scholar] [PubMed]
- Rossetto, M.; Vanzani, P.; Mattivi, F.; Lunelli, M.; Scarpa, M.; Rigo, A. Synergistic antioxidant effect of catechin and malvidin 3-glucoside on free radical-initiated peroxidation of linoleic acid in micelles. Arch. Biochem. Biophys. 2002, 408, 239–245. [Google Scholar] [CrossRef]
- Kim, S.J.; Um, J.Y.; Lee, J.Y. Anti-inflammatory activity of hyperoside through the suppression of nuclear factor-κB activation in mouse peritoneal macrophages. Am. J. Chin. Med. 2011, 39, 171–181. [Google Scholar] [CrossRef] [PubMed]




| Gene Name | Sequence |
|---|---|
| nNOS | Forward: 5’-GAATACCAGCCTGATCCATGGAA-3’ |
| Reverse: 5’-TCCTCCAGGAGGGTGTCCACCGCATG-3’ | |
| eNOS | Forward: 5’-TCAGCCATCACAGTGTTCCC-3’ |
| Reverse: 5’-ATAGCCCGCATAGCGTATCAG-3’ | |
| iNOS | Forward: 5’-GTTCTCAGCCCAACAATACAAGA-3’ |
| Reverse: 5’-GTGGACGGGTCGATGTCAC-3’ | |
| Cu–Zn-SOD | Forward: 5’–AACCAGTTGTGTTGTCAGGAC–3’ |
| Reverse: 5′–CCACCATGTTTCTTAGAGTGAGG–3’ | |
| Mn-SOD | Forward: 5’-CAGACCTGCCTTACGACTATGG-3’ |
| Reverse: 5’-CTCGGTGGCGTTGAGATTGTT-3’ | |
| CAT | Forward: 5’-GGAGGCGGGAACCCAATAG-3’ |
| Reverse: 5’-GTGTGCCATCTCGTCAGTGAA-3’ | |
| GAPDH | Forward: 5’-AGGTCGGTGTGAACGGATTTG-3’ |
| Reverse: 5’-GGGGTCGTTGATGGCAACA-3’ |
| Group | Body Weight | Live Weight | Liver Index |
|---|---|---|---|
| Normal | 32.67 ± 1.25 a | 1.28 ± 0.11 d | 3.92 ± 0.18 d |
| Model | 34.79 ± 1.51 a | 2.93 ± 0.48 a | 8.42 ± 0.33 a |
| Silymarin | 32.55 ± 1.33 a | 1.75 ± 0.27 c | 5.38 ± 0.24 c |
| WPPL | 32.81 ± 1.29 a | 2.32 ± 0.34 b | 7.07 ± 0.29 b |
| WPPH | 32.69 ± 1.44 a | 1.88 ± 0.22 c | 5.75 ± 0.23 c |
| Group | ALT (U/L) | AST (U/L) | ALP (K-A) | TG (mmol/L) | TC (mmol/L) | BUN (mg/dL) | ALB (g/dL) |
|---|---|---|---|---|---|---|---|
| Normal | 16.33 ± 1.45 d | 10.89 ± 0.38 d | 5.80 ± 0.49 d | 0.36 ± 0.03 e | 1.38 ± 0.15 e | 20.18 ± 2.03 d | 4.12 ± 0.14 a |
| Model | 74.97 ± 3.62 a | 61.28 ± 3.06 a | 19.32 ± 1.96 a | 2.24 ± 0.26 a | 6.37 ± 0.42 a | 53.69 ± 3.44 a | 1.78 ± 0.10 d |
| Silymarin | 31.25 ± 2.87 c | 28.39 ± 2.62 c | 10.66 ± 1.21 c | 0.92 ± 0.06 d | 2.89 ± 0.25 d | 32.69 ± 2.71 c | 2.86 ± 0.09 b |
| WPPL | 55.69 ± 3.20 b | 46.05 ± 2.89 b | 15.12 ± 1.02 b | 1.79 ± 0.18 b | 4.83 ± 0.36 b | 41.09 ± 2.42 b | 2.39 ± 0.08 c |
| WPPH | 33.17 ± 3.08 c | 30.27 ± 2.70 c | 11.34 ± 1.19 c | 1.20 ± 0.08 c | 3.31 ± 0.22 c | 33.25 ± 2.16 c | 2.77 ± 0.10 b |
| Group | SOD (U/mL) | NO (µmol/L) | CAT (U/mL) | MDA (µmol/L) | GSH-Px (U/mL) |
|---|---|---|---|---|---|
| Normal | 148.39 ± 11.35 a | 50.33 ± 2.98 e | 42.05 ± 2.77 a | 5.77 ± 0.52 d | 279.52 ± 22.09 a |
| Model | 48.39 ± 4.02 d | 169.80 ± 9.23 a | 9.83 ± 0.76 d | 21.19 ± 1.12 a | 93.52 ± 13.71 d |
| Silymarin | 97.25 ± 4.68 b | 83.26 ± 4.57 d | 28.93 ± 2.10 b | 9.89 ± 0.60 c | 187.96 ± 18.35 b |
| WPPL | 65.09 ± 4.39 c | 133.05 ± 5.09 b | 15.32 ± 1.76 c | 15.27 ± 0.71 b | 127.58 ± 15.50 c |
| WPPH | 95.07 ± 4.26 b | 97.37 ± 3.92 c | 27.47 ± 1.92 b | 10.36 ± 0.48 c | 178.07 ± 17.46 b |
| Group | IL-6 (pg/mL) | IL-12 (pg/mL) | TNF-α (pg/mL) | IFN-γ (pg/mL) |
|---|---|---|---|---|
| Normal | 26.05 ± 2.15 e | 188.08 ± 12.59 e | 20.85 ± 1.97 e | 16.22 ± 1.81 e |
| Model | 238.93 ± 17.73 a | 855.67 ± 25.78 a | 118.57 ± 7.23 a | 89.30 ± 3.92 a |
| Silymarin | 87.36 ± 8.36 d | 369.07 ± 18.33 d | 51.22 ± 4.36 d | 38.77 ± 2.63 d |
| WPPL | 179.20 ± 10.55 b | 628.48 ± 20.36 b | 88.05 ± 4.93 b | 57.08 ± 2.50 b |
| WPPH | 110.25 ± 7.69 c | 400.36 ± 19.65 c | 67.82 ± 3.06 c | 43.17 ± 2.01 c |
| Group | GAPDH | nNOS | eNOS | iNOS | |||
|---|---|---|---|---|---|---|---|
| Ct value | Ct value | Relative Expression | Ct Value | Relative Expression | Ct Value | Relative Expression | |
| Normal | 24.06 ± 0.03 a | 24.83 ± 0.08 e | 4.20 ± 0.19 a | 24.94 ± 0.01 d | 3.64 ± 0.06 a | 28.86 ± 0.06 a | 0.23 ± 0.01 e |
| Model | 24.07 ± 0.01 a | 26.90 ± 0.08 a | 1.00 ± 0.01 d | 26.81 ± 0.09 a | 1.00 ± 0.01 d | 26.73 ± 0.08 e | 1.00 ± 0.01 a |
| Silymarin | 24.03 ± 0.03 a | 25.23 ± 0.03 d | 3.09 ± 0.14 b | 25.47 ± 0.03 c | 2.46 ± 0.11 b | 27.86 ± 0.04 b | 0.45 ± 0.02 d |
| WPPL | 24.02 ± 0.01 a | 25.86 ± 0.06 b | 1.99 ± 0.10 c | 25.94 ± 0.05 b | 1.78 ± 0.07 c | 27.06 ± 0.05 d | 0.78 ± 0.03 b |
| WPPH | 24.04 ± 0.01 a | 25.30 ± 0.02 c | 2.98 ± 0.09 b | 25.51 ± 0.02 c | 2.42 ± 0.07 b | 27.56 ± 0.05 c | 0.55 ± 0.01 c |
| Group | GAPDH | Cu/Zn-SOD | Mn-SOD | CAT | |||
|---|---|---|---|---|---|---|---|
| Ct Value | Ct Value | Relative Expression | Ct Value | Relative Expression | Ct Value | Relative Expression | |
| Normal | 24.06 ± 0.03 a | 23.54 ± 0.02 e | 6.82 ± 0.22 a | 23.91 ± 0.02 d | 5.66 ± 0.19 a | 24.19 ± 0.05 e | 4.66 ± 0.12 a |
| Model | 24.07 ± 0.01 a | 26.32 ± 0.07 a | 1.00 ± 0.01 d | 26.42 ± 0.01 a | 1.00 ± 0.01 d | 26.42 ± 0.18 a | 1.00 ± 0.01 d |
| Silymarin | 24.03 ± 0.03 a | 23.78 ± 0.09 d | 5.65 ± 0.47 b | 24.22 ± 0.03 c | 4.45 ± 0.21 b | 24.57 ± 0.01 d | 3.51 ± 0.07 b |
| WPPL | 24.02 ± 0.01 a | 24.02 ± 0.01 b | 3.14 ± 0.09 c | 24.91 ± 0.04 b | 2.77 ± 0.12 c | 24.91 ± 0.04 b | 2.54 ± 0.15 c |
| WPPH | 24.04 ± 0.01 a | 23.95 ± 0.01 c | 5.05 ± 0.11 b | 24.21 ± 0.01 c | 4.52 ± 0.06 b | 24.65 ± 0.04 c | 3.34 ± 0.13 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Tan, F.; Li, C.; Li, W.; Liao, W.; Li, Q.; Qin, G.; Liu, W.; Zhao, X. White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation. Antioxidants 2019, 8, 524. https://doi.org/10.3390/antiox8110524
Zhou Y, Tan F, Li C, Li W, Liao W, Li Q, Qin G, Liu W, Zhao X. White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation. Antioxidants. 2019; 8(11):524. https://doi.org/10.3390/antiox8110524
Chicago/Turabian StyleZhou, Yalin, Fang Tan, Chong Li, Wenfeng Li, Wei Liao, Qin Li, Guohui Qin, Weiwei Liu, and Xin Zhao. 2019. "White Peony (Fermented Camellia sinensis) Polyphenols Help Prevent Alcoholic Liver Injury via Antioxidation" Antioxidants 8, no. 11: 524. https://doi.org/10.3390/antiox8110524





