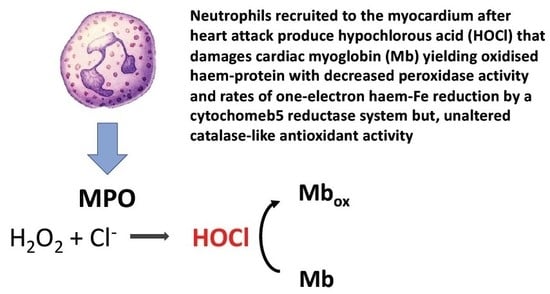
Catalase-Like Antioxidant Activity is Unaltered in Hypochlorous Acid Oxidized Horse Heart Myoglobin
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Experimental Section
2.2.1. Oxidation Reactions
2.2.2. Electrophoretic Mobility of Native and HOCl-Modified hhMb
2.2.3. UV-Vis Absorbance Spectroscopy
2.2.4. Assessment of 3-Chlorotyrosine (3-Cl-Tyr)
2.2.5. Liquid Chromatography and Electrospray Mass Spectrometry
- Proteins were separated at a flow rate of 0.4 mL/min on a C18 reverse-phase column (particle size 3 μm, 3 mm × 150 mm) using solvent A (0.1% v/v trifluoroacetic acid in water) and solvent B (0.1% v/v trifluoroacetic acid in CH3CN) and products were detected by absorbance at 210 nm to assess the impact of HOCl-oxidation on hhMb (as measured by changes in retention time for modified protein(s) eluting from the column).
- Where required, mass analyses were performed on desalted protein samples (Ziptips Cat# Z720070); Merck-Millipore in positive ion mode with a Finnigan LCQ Deca XP ion trap instrument (Thermo Fisher Scientific, San Jose, CA, USA) coupled to a Finnigan Surveyor HPLC system (Thermo Fisher Scientific, San Jose, CA, USA) as described in detail elsewhere [21]. Modified hhMb proteins were injected directly to the electrospray MS under the following parameters: Electrospray needle was held at 4500 V; sheath gas was nitrogen set at 80 units; collision gas was helium and the temperature of the heated capillary was 250 °C. This analytical approach consistently resulted in detection of the apo-protein without the haem moiety as the haem group is non-covalently bound to the protein.
2.2.6. Electron Paramagnetic Resonance and Spin Trapping Studies
2.2.7. Mb Catalase-Like Activity
2.2.8. Reduction of Myoglobin by Cytochrome b5 Reductase System
2.2.9. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bonaventura, A.; Montecucco, F.; Dallegri, F. Cellular recruitment in myocardial ischaemia/reperfusion injury. Eur. J. Clin. Investig. 2016, 46, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Dirksen, M.T.; Simoons, M.L.; Duncker, D.J.; Laarman, G.J. Reperfusion injury in humans: A review of clinical trials on reperfusion injury inhibitory strategies. Cardiovasc. Res. 2007, 74, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Winterbourn, C.C. Oxidation of neutrophil glutathione and protein thiols by myeloperoxidase-derived hypochlorous acid. Biochem. J. 1997, 327, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Pattison, D.; Davies, M. Reactions of Myeloperoxidase-Derived Oxidants with Biological Substrates: Gaining Chemical Insight into Human Inflammatory Diseases. Curr. Med. Chem. 2006, 13, 3271–3290. [Google Scholar] [CrossRef] [PubMed]
- Arnhold, J.; Hammerschmidt, S.; Wagner, M.; Mueller, S.; Arnold, K.; Grimm, E. On the action of hypochlorite on human serum albumin. Biomed. Biochim. Acta 1990, 49, 991–997. [Google Scholar]
- Wittenberg, B.A.; Wittenberg, J.B. Transport of oxygen in muscle. Annu. Rev. Physiol. 1989, 51, 857–878. [Google Scholar] [CrossRef]
- Raschke, P.; Becker, B.F.; Leipert, B.; Schwartz, L.M.; Zahler, S.; Gerlach, E. Postischemic dysfunction of the heart induced by small numbers of neutrophils via formation of hypochlorous acid. Basic Res. Cardiol. 1993, 88, 321–339. [Google Scholar]
- Wittenberg, J.B. Myoglobin-facilitated oxygen diffusion: Role of myoglobin in oxygen entry into muscle. Physiol. Rev. 1970, 50, 559–636. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Wittenberg, B.A. Myoglobin function reassessed. J. Exp. Biol. 2003, 206, 2011–2020. [Google Scholar] [CrossRef] [Green Version]
- Brunori, M. Nitric oxide moves myoglobin centre stage. Trends Biochem. Sci. 2001, 26, 209–210. [Google Scholar] [CrossRef]
- Shiva, S.; Huang, Z.; Grubina, R.; Sun, J.; Ringwood, L.A.; MacArthur, P.H.; Xu, X.; Murphy, E.; Darley-Usmar, V.M.; Gladwin, M.T. Deoxymyoglobin Is a Nitrite Reductase That Generates Nitric Oxide and Regulates Mitochondrial Respiration. Circ. Res. 2007, 100, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Dungel, P.; Penzenstadler, C.; Ashmwe, M.; Dumitrescu, S.; Stoegerer, T.; Redl, H.; Bahrami, S.; Kozlov, A.V. Impact of mitochondrial nitrite reductase on hemodynamics and myocardial contractility. Sci. Rep. 2017, 7, 12092. [Google Scholar] [CrossRef] [PubMed]
- Vlasits, J.; Jakopitsch, C.; Bernroitner, M.; Zamocky, M.; Furtmüller, P.G.; Obinger, C. Mechanisms of catalase activity of heme peroxidases. Arch. Biochem. Biophys. 2010, 500, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Hagler, L.; Coppes, R.; Herman, R.H. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J. Biol. Chem. 1979, 254, 6505–6514. [Google Scholar]
- Witting, P.K.; Wu, B.J.; Raftery, M.; Southwell-Keely, P.; Stocker, R. Probucol protects against hypochlorite-induced endothelial dysfunction: Identification of a novel pathway of probucol oxidation to a biologically active intermediate. J. Biol. Chem. 2005, 280, 15612–15618. [Google Scholar] [CrossRef] [PubMed]
- Witting, P.K.; Willhite, C.; Davies, M.J.; Stocker, R. Lipid oxidation in human low-density lipoprotein induced by metmyoglobin/H2O2: Involvement of alpha-tocopheroxyl and phosphatidylcholine alkoxyl radicals. Chem. Res. Toxicol. 1999, 12, 1173–1181. [Google Scholar] [CrossRef]
- Sparks, D.L.; Phillips, M.C. Quantitative measurement of lipoprotein surface charge by agarose gel electrophoresis. J. Lipid Res. 1992, 33, 123–130. [Google Scholar]
- Hawkins, C.L.; Morgan, P.E.; Davies, M.J. Quantification of protein modification by oxidants. Free Radic. Biol. Med. 2009, 46, 965–988. [Google Scholar] [CrossRef]
- Shanu, A.; Groebler, L.; Kim, H.B.; Wood, S.; Weekley, C.M.; Aitken, J.B.; Harris, H.H.; Witting, P.K. Selenium Inhibits Renal Oxidation and Inflammation But Not Acute Kidney Injury in an Animal Model of Rhabdomyolysis. Antioxid. Redox Signal. 2013, 18, 756–769. [Google Scholar] [CrossRef] [Green Version]
- Szuchman-Sapir, A.J.; Pattison, D.I.; Ellis, N.A.; Hawkins, C.L.; Davies, M.J.; Witting, P.K. Hypochlorous acid oxidizes methionine and tryptophan residues in myoglobin. Free Radic. Biol. Med. 2008, 45, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Witting, P.K.; Mauk, A.G.; Lay, P.A. Role of Tyrosine-103 in Myoglobin Peroxidase Activity: Kinetic and Steady-State Studies on the Reaction of Wild-Type and Variant Recombinant Human Myoglobins with H2O2. Biochemistry 2002, 41, 11495–11503. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Reinke, L.A.; Tanigawa, T.; Koshida, H. Determination of the rate of superoxide generation from biological systems by spin trapping: Use of rapid oxygen depletion to measure the decay rate of spin adducts. Free Radic. Biol. Med. 1994, 17, 215–223. [Google Scholar] [CrossRef]
- Cho, Y.S.; Kim, H.S.; Kim, C.H.; Cheon, H.G. Application of the ferrous oxidation–xylenol orange assay for the screening of 5-lipoxygenase inhibitors. Anal. Biochem. 2006, 351, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kuma, F. Properties of methemoglobin reductase and kinetic study of methemoglobin reduction. J. Biol. Chem. 1981, 256, 5518–5523. [Google Scholar] [PubMed]
- Livingston, D.J.; McLachlan, S.J.; La Mar, G.N.; Brown, W.D. Myoglobin: Cytochrome b5 interactions and the kinetic mechanism of metmyoglobin reductase. J. Biol. Chem. 1985, 260, 15699–15707. [Google Scholar] [PubMed]
- Charlton, T.S.; Domigan, N.M.; Duncan, M.W.; Winterbourn, C.C.; Kettle, A.J. Chlorination of Tyrosyl Residues in Peptides by Myeloperoxidase and Human Neutrophils. J. Biol. Chem. 1995, 270, 16542–16548. [Google Scholar] [Green Version]
- Zaia, J.; Annan, R.S.; Biemann, K. The correct molecular weight of myoglobin, a common calibrant for mass spectrometry. Rapid Commun. Mass Spectrom. 1992, 6, 32–36. [Google Scholar] [CrossRef]
- Manzanares, D.; Rodríguez-Capote, K.; Liu, S.; Haines, T.; Ramos, Y.; Zhao, L.; Doherty-Kirby, A.; Lajoie, G.; Possmayer, F. Modification of Tryptophan and Methionine Residues Is Implicated in the Oxidative Inactivation of Surfactant Protein B. Biochemistry 2007, 46, 5604–5615. [Google Scholar] [CrossRef]
- Wang, X.S.; Kim, H.B.; Szuchman-Sapir, A.; McMahon, A.; Dennis, J.M.; Witting, P.K. Neutrophils recruited to the myocardium after acute experimental myocardial infarct generate hypochlorous acid that oxidizes cardiac myoglobin. Arch. Biochem. Biophys. 2016, 612, 103–114. [Google Scholar] [CrossRef]
- Gunther, M.R.; Tschirret-Guth, R.; Witkowska, H.; Fann, Y.C.; Barr, D.P.; De Montellano, P.R.O.; Mason, R.P. Site-specific spin trapping of tyrosine radicals in the oxidation of metmyoglobin by hydrogen peroxide. Biochem. J. 1998, 330, 1293–1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Degray, J.; Gunther, M.R.; Tschirret-Guth, R.; De Montellano, P.R.O.; Mason, R.P. Peroxidation of a specific tryptophan of metmyoglobin by hydrogen peroxide. J. Biol. Chem. 1997, 272, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. Identification of a globin free radical in equine myoglobin treated with peroxides. Biochim. Biophys. Acta Protein Struct. Mol. Enzym. 1991, 1077, 86–90. [Google Scholar] [CrossRef]
- Irwin, J.A.; Østdal, H.; Davies, M.J. Myoglobin-Induced Oxidative Damage: Evidence for Radical Transfer from Oxidized Myoglobin to Other Proteins and Antioxidants. Arch. Biochem. Biophys. 1999, 362, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Szuchman-Sapir, A.J.; Pattison, D.I.; Davies, M.J.; Witting, P.K. Site-specific hypochlorous acid-induced oxidation of recombinant human myoglobin affects specific amino acid residues and the rate of cytochrome b5-mediated heme reduction. Free Radic. Biol. Med. 2010, 48, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Lardinois, O.M.; De Montellano, P.R.O. Autoreduction of Ferryl Myoglobin: Discrimination among the Three Tyrosine and Two Tryptophan Residues as Electron Donors. Biochemistry 2004, 43, 4601–4610. [Google Scholar] [CrossRef] [PubMed]
- Gunther, M.R.; Sturgeon, B.E.; Mason, R.P. A long-lived tyrosyl radical from the reaction between horse metmyoglobin and hydrogen peroxide. Free Radic. Biol. Med. 2000, 28, 709–719. [Google Scholar] [CrossRef]
- Catalano, C.; Choe, Y.S.; De Montellano, P.R.O. Reactions of the protein radical in peroxide-treated myoglobin. Formation of a heme-protein cross-link. J. Biol. Chem. 1989, 264, 10534–10541. [Google Scholar]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Dunford, H.B. Horseradish peroxidase: Structure and kinetic properties. In Peroxidases in Chemistry and Biology; Everse, K.E., Grisham, M.B., Eds.; CRC Press: Boca Raton, FL, USA, 1991; Volume 2. [Google Scholar]
- Watanabe, M.; Kanai, Y.; Nakamura, S.; Nishimura, R.; Shibata, T.; Momotake, A.; Yanagisawa, S.; Ogura, T.; Matsuo, T.; Hirota, S.; et al. Synergistic Effect of Distal Polar Interactions in Myoglobin and Their Structural Consequences. Inorg. Chem. 2018, 57, 14269–14279. [Google Scholar] [CrossRef]
- Ozaki, S.-I.; Roach, M.P.; Matsui, T.; Watanabe, Y. Investigations of the Roles of the Distal Heme Environment and the Proximal Heme Iron Ligand in Peroxide Activation by Heme Enzymes via Molecular Engineering of Myoglobin. Acc. Chem. Res. 2001, 34, 818–825. [Google Scholar] [CrossRef]
- Kanai, Y.; Nishimura, R.; Nishiyama, K.; Shibata, T.; Yanagisawa, S.; Ogura, T.; Matsuo, T.; Hirota, S.; Neya, S.; Suzuki, A.; et al. Effects of Heme Electronic Structure and Distal Polar Interaction on Functional and Vibrational Properties of Myoglobin. Inorg. Chem. 2016, 55, 1613–1622. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.C.; Samuni, A.; Taira, J.; Goldstein, S.; Mitchell, J.B.; Russo, A. Stimulation by nitroxides of catalase-like activity of hemeproteins. Kinetics and mechanism. J. Biol. Chem. 1996, 271, 26018–26025. [Google Scholar] [CrossRef] [PubMed]
- Adachi, S.; Nagano, S.; Ishimori, K.; Watanabe, Y.; Morishima, I.; Egawa, T.; Kitagawa, T.; Makino, R. Roles of proximal ligand in heme proteins: Replacement of proximal histidine of human myoglobin with cysteine and tyrosine by site-directed mutagenesis as models for P-450, chloroperoxidase, and catalase. Biochemistry 1993, 32, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Ueno, T.; Fukuzumi, S.; Watanabe, Y. Catalase reaction by myoglobin mutants and native catalase: Mechanistic investigation by kinetic isotope effect. J. Biol. Chem. 2004, 279, 52376–52381. [Google Scholar] [CrossRef] [PubMed]
- Marzouki, L.; Jarry, G.; Janati-Idrissi, R.; Amri, M. The Role of Myoglobin in Retarding Oxygen Depletion in Anoxic Heart. Arch. Physiol. Biochem. 2002, 110, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Gödecke, A.; Flögel, U.; Zanger, K.; Ding, Z.; Hirchenhain, J.; Decking, U.K.M.; Schrader, J. Disruption of myoglobin in mice induces multiple compensatory mechanisms. Proc. Natl. Acad. Sci. USA 1999, 96, 10495–10500. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.A. Functional activity of Na+, K(+)-pump in normal and pathological tissues. Mol. Chem. Neuropathol. 1993, 19, 83–93. [Google Scholar] [CrossRef]
- Reeder, B.J.; Svistunenko, D.A.; Cooper, C.E.; Wilson, M.T. The Radical and Redox Chemistry of Myoglobin and Hemoglobin: From In Vitro Studies to Human Pathology. Antioxid. Redox Signal. 2004, 6, 954–966. [Google Scholar]
- Hendgen-Cotta, U.B.; Merx, M.W.; Shiva, S.; Schmitz, J.; Becher, S.; Klare, J.P.; Steinhoff, H.-J.; Goedecke, A.; Schrader, J.; Gladwin, M.T.; et al. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc. Natl. Acad. Sci. USA 2008, 105, 10256–10261. [Google Scholar] [CrossRef] [Green Version]
- Hendgen-Cotta, U.B.; Esfeld, S.; Coman, C.; Ahrends, R.; Klein-Hitpass, L.; Flögel, U.; Rassaf, T.; Totzeck, M. A novel physiological role for cardiac myoglobin in lipid metabolism. Sci. Rep. 2017, 7, 43219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Totzeck, M.; Hendgen-Cotta, U.B.; Luedike, P.; Berenbrink, M.; Klare, J.P.; Steinhoff, H.-J.; Semmler, D.; Shiva, S.; Williams, D.; Kipar, A.; et al. Nitrite Regulates Hypoxic Vasodilation via Myoglobin–Dependent Nitric Oxide Generation. Circulation 2012, 126, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S.; Salinas, E.P.; Darley-Usmar, K.; Eiserich, J.P.; Freeman, B.A.; Darley-Usmar, V.M.; Anderson, P.G. Concentration-dependent Effects of Nitric Oxide on Mitochondrial Permeability Transition and Cytochrome cRelease. J. Biol. Chem. 2000, 275, 20474–20479. [Google Scholar] [CrossRef] [PubMed] [Green Version]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, G.; Chami, B.; El Kazzi, M.; Wang, X.; Moreira, M.T.S.; Hamilton, N.; Maw, A.M.; Hambly, T.W.; Witting, P.K. Catalase-Like Antioxidant Activity is Unaltered in Hypochlorous Acid Oxidized Horse Heart Myoglobin. Antioxidants 2019, 8, 414. https://doi.org/10.3390/antiox8090414
Ahmad G, Chami B, El Kazzi M, Wang X, Moreira MTS, Hamilton N, Maw AM, Hambly TW, Witting PK. Catalase-Like Antioxidant Activity is Unaltered in Hypochlorous Acid Oxidized Horse Heart Myoglobin. Antioxidants. 2019; 8(9):414. https://doi.org/10.3390/antiox8090414
Chicago/Turabian StyleAhmad, Gulfam, Belal Chami, Mary El Kazzi, Xiaosuo Wang, Maria Tereza S. Moreira, Natasha Hamilton, Aung Min Maw, Thomas W. Hambly, and Paul K. Witting. 2019. "Catalase-Like Antioxidant Activity is Unaltered in Hypochlorous Acid Oxidized Horse Heart Myoglobin" Antioxidants 8, no. 9: 414. https://doi.org/10.3390/antiox8090414
APA StyleAhmad, G., Chami, B., El Kazzi, M., Wang, X., Moreira, M. T. S., Hamilton, N., Maw, A. M., Hambly, T. W., & Witting, P. K. (2019). Catalase-Like Antioxidant Activity is Unaltered in Hypochlorous Acid Oxidized Horse Heart Myoglobin. Antioxidants, 8(9), 414. https://doi.org/10.3390/antiox8090414