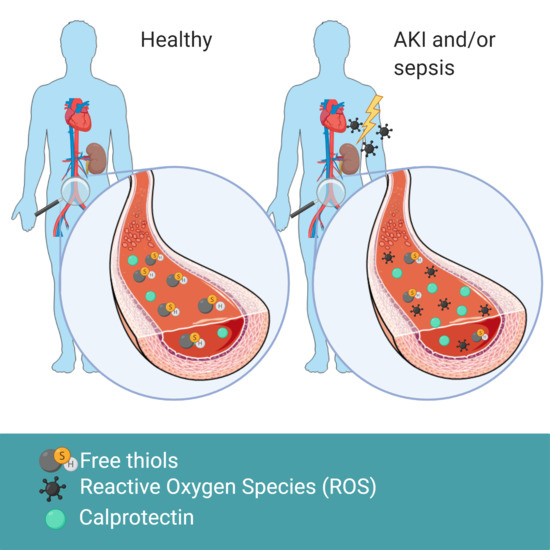
Acute Kidney Injury is Associated with Lowered Plasma-Free Thiol Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population, Data Collection, and Definitions
2.2. Measurement of Plasma-Free Thiol Levels
2.3. Measurement of Calprotectin
2.4. Measurement of Plasma NGAL
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Study Population
3.2. Reduced Plasma-Free Thiol Levels were Associated with AKI upon Admission
3.3. Association of Plasma-Free Thiol Levels with the Course of AKI during ICU Admission
3.4. Association of Plasma-Free Thiol Levels with Sepsis
3.5. Serum Calprotectin is Associated with AKI and Sepsis
3.6. Plasma-Free Thiol Levels were Associated with Age, Serum Albumin, Creatinine Levels, and Inflammatory Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AKI | Acute Kidney Injury |
| KDIGO | Kidney Disease: Improving Global Outcomes |
| ICU | Intensive Care Unit |
| ESRD | End-Stage Renal Disease |
| ROS | Reactive Oxygen Species |
| CRP | C-reactive protein |
| NGAL | calprotectin and neutrophil gelatinase-associated lipocalin |
Appendix A
| Stage | Serum Creatinine | Urine Output |
|---|---|---|
| 1 | 1.5–1.9-times baseline OR ≥ µmol/ increase | <0.5 ml/kg/h for 6–12 h |
| 2 | 2.0–2.9-times baseline | <0.5 ml/kg/h for ≥12 h |
| 3 | ≥3.0-times baseline OR increase in serum creatinine to ≥353.9 µmol/L OR renal replacement therapy | <0.3 ml/kg/h for ≥24 h of anuria for ≥12 h |
Appendix B
| Characteristics | No AKI Progression (n = 185 (78.7%)) | AKI Progression (n = 52 (21.3%)) | p-Value |
|---|---|---|---|
| Age (years) | 63 (55, 71) | 65 (50, 70) | 0.785 |
| Gender (% female) | 64 (34.6) | 25 (50.0) | 0.068 |
| BMI (kg/m2) baseline creatinine (µmol/L) | 22.4 (20.6, 24.9) 79 (69, 95) | 23.7 (20.7, 26.9) 92 (70, 101) | 0.086 0.113 |
| Comorbidities | |||
| CKD (%) | 10 (5.4) | 3 (6.0) | 1.000 |
| CVD (%) | 20 (10.9) | 9 (18.0) | 0.271 |
| DM (%) | 33 (17.8) | 11 (22.0) | 0.642 |
| Malignancy (%) | 16 (8.7) | 3 (6.0) | 0.736 |
| Operation (%) | 121 (65.4) | 33 (66.0) | 1.000 |
| Infection * (%) | 34 (18.4) | 13 (26.0) | 0.319 |
| Sepsis (%) | 28 (19.2) | 13 (26.0) | 0.384 |
| SIRS | 2 (1,3) | 3 (2, 3) | 0.565 |
| SOFA | 6 (2,7) | 7 (5, 9) | 0.001 |
| qSOFA | 0 (0,1) | 1 (0, 0) | 0.142 |
| Mechanical ventilation (%) | 127 (6.0) | 46 (92.0) | 0.002 |
| Vital parameters | |||
| Heart rate (bpm) | 80 (70,92) | 95 (80, 104) | 0.001 |
| MAP (mmHg) | 85.0 (73.3, 98.0) | 79.8 (69.1, 93.4) | 0.143 |
| Respiratory rate (resp/min) | 16.0 (14.0, 23.0) | 20.0 (16,0 25.0) | 0.114 |
| Body temperature (°C) | 36.1 (35.4, 37.2) | 37.1 (35.4, 37.4) | 0.630 |
| Laboratory values | |||
| CRP (mg/L) | 6.90 (2.05, 37.00) | 9.65 (6.45, 70.80) | 0.018 |
| Leucocytes(10^9/L) | 12.5 (9.3, 16.3) | 13.1 (10.8, 17.9) | 0.274 |
| Thrombocytes (10^9/L) | 188 (150, 242) | 185 (127, 253) | 0.801 |
| Bilirubin (umol/L) | 9 (6, 14) | 12 (8, 19) | 0.038 |
| Albumin (g/L) | 30 (25, 34) | 28 (24, 33) | 0.084 |
| Creatinine (µmol/L) | 72 (59, 88) | 91 (72, 121) | 0.001 |
| Calprotectin (μg/mL) | 4.33 (2.45, 6.62) | 4.60 (2.64, 8.08) | 0.300 |
| Thiols (µmol/L) | 256.24 (162.72, 359.70) | 230.49 (116.14, 326.12) | 0.073 |
| APACHE II | 15 (11, 20) | 17 (13, 21) | 0.058 |
| APACHE IV | 46 (34, 66) | 60 (46, 91) | <0.001 |
| <7-day Mortality (%) | 8 (4.3) | 4 (8.0) | 0.493 |
| <28-day Mortality (%) | 18 (9.7) | 7 (14.0) | 0.542 |
References
- Ronco, C.; Bellomo, R.; Kellum, A. Acute kidney injury. Lancet 2019, 394, 1949–1964. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Pavlakou, P.; Liakopoulos, V.; Eleftheriadis, T.; Mitsis, M.; Dounousi, E. Oxidative Stress and Acute Kidney Injury in Critical Illness: Pathophysiologic Mechanisms—Biomarkers—Interventions, and Future Perspectives. Oxidative Med. Cell. Longev. 2017, 2017, 6193694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Case, J.; Khan, S.; Khalid, R.; Khan, A. Epidemiology of acute kidney injury in the intensive care unit. Crit. Care Res. Pract. 2013, 2013, 479730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Singer, M.; Deutschman, C.S.; Seymour, C.C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.J.; Coopersmith, C.C.; et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, A.; Duranteau, J. Pathophysiology of Sepsis-Induced Acute Kidney Injury: The Role of Global Renal Blood Flow and Renal Vascular Resistance. In Contributions to Nephrology; Karger: Basel, Switzerland, 2011; Volume 174, pp. 89–97. [Google Scholar]
- Wan, L.; Bagshaw, S.M.; Langenberg, C.; Saotome, T.; May, C.; Bellomo, R. Pathophysiology of septic acute kidney injury: What do we really know? Crit. Care Med. 2008, 36, S198–S203. [Google Scholar] [CrossRef]
- Gomez, H.; Ince, C.; De Backer, D.; Pickkers, P.; Payen, D.; Hotchkiss, J.; Kellum, J.A. A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 2014, 41, 3–11. [Google Scholar] [CrossRef] [Green Version]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Kerkhoff, C.; Nacken, W.; Benedyk, M.; Dagher, M.C.; Sopalla, C.; Doussiere, J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005, 19, 1–28. [Google Scholar] [CrossRef]
- Exline, M.C.; Crouser, E.D. Mitochondrial mechanisms of sepsis-induced organ failure. Front. Biosci. 2008, 13, 5030–5041. [Google Scholar] [PubMed]
- Abilés, J.; de La Cruz, A.P.; Castaño, J.; Rodríguez-Elvira, M.; Aguayo, E.; Moreno-Torres, R.; Llopis, J.E.; Aranda, P.; Argüelles, S.; Ayala, A.; et al. Oxidative stress is increased in critically ill patients according to antioxidant vitamins intake, independent of severity: A cohort study. Crit. Care 2006, 10, R146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Litwak, J.J.; Cho, N.; Nguyen, H.B.; Moussavi, K.; Bushell, T.R. Vitamin C, Hydrocortisone, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Analysis of Real-World Application. J. Clin. Med. 2019, 8, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiruppathi, C.; Finnegan, A.; Malik, A.B. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Balcerczyk, A.; Grzelak, A.; Janaszewska, A.; Jakubowski, W.; Koziol, S.; Marszalek, M.; Rychlik, B.; Soszyński, M.; Bilinski, T.; Bartosz, G. Thiols as major determinants of the total antioxidant capacity. BioFactors 2003, 17, 75–82. [Google Scholar] [CrossRef]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced Level of Serum Thiols in Patients with a Diagnosis of Active Disease. J. Anti Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef]
- Turell, L.; Radi, R.; Alvarez, B. The thiol pool in human plasma: The central contribution of albumin to redox processes. Free Radic. Biol. Med. 2013, 65, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Závada, J.; Hoste, E.; Cartin-Ceba, R.; Calzavacca, P.; Gajic, O.; Clermont, G.; Bellomo, R.; A Kellum, J. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol. Dial. Transpl. 2010, 25, 3911–3918. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonça, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar] [PubMed]
- Plotnikov, E.Y.; Kazachenko, A.V.; Vyssokikh, M.Y.; Vasileva, A.K.; Tcvirkun, D.V.; Isaev, N.K.; Kirpatovsky, V.; Zorov, D.B. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007, 72, 1493–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrezenmeier, E.V.; Barasch, J.; Budde, K.; Westhoff, T.; Schmidt-Ott, K.M. Biomarkers in acute kidney injury—pathophysiological basis and clinical performance. Acta Physiol. 2017, 219, 556–574. [Google Scholar] [CrossRef]
- Kashani, K.; Cheungpasitporn, W.; Ronco, C. Biomarkers of acute kidney injury: The pathway from discovery to clinical adoption. Clin. Chem. Lab. Med. 2017, 55, 1074–1089. [Google Scholar] [CrossRef]
- Uchino, S. Creatinine. Curr. Opin. Crit. Care 2010, 16, 562–567. [Google Scholar] [CrossRef]
- Mårtensson, J.; Bellomo, R.R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef]
- Koeze, J.; Van Der Horst, I.C.C.; Keus, F.; Wiersema, R.; Dieperink, W.; Kootstra-Ros, J.E.; Zijlstra, J.G.; Van Meurs, M. Plasma neutrophil gelatinase-associated lipocalin at intensive care unit admission as a predictor of acute kidney injury progression. Clin. Kidney J. 2020, 1–9. [Google Scholar] [CrossRef]
- Qian, J.; Fang, J.; Zhu, Q.; Ma, S.; Wang, W.; Zheng, Y.; Hao, G.; Deng, B.; Zhao, X.; Ding, F. Serum Protein Thiol Levels in Patients with Hospital-Acquired Acute Kidney Injury. Kidney Blood Press. Res. 2015, 40, 623–629. [Google Scholar] [CrossRef]
- Ayar, G.; Sahin, S.; Yazici, M.U.; Neselioglu, S.; Erel, O.; Bayrakci, U.S. Effects of Hemodialysis on Thiol-Disulphide Homeostasis in Critically Ill Pediatric Patients with Acute Kidney Injury. BioMed Res. Int. 2018, 2018, 1898671. [Google Scholar] [CrossRef] [Green Version]
- Tan, X.; Zheng, X.; Huang, Z.; Lin, J.; Xie, C.; Lin, Y. Involvement of S100A8/A9-TLR4-NLRP3 Inflammasome Pathway in Contrast-Induced Acute Kidney Injury. Cell Physiol. Biochem. 2017, 43, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Nikolakopoulou, Z.; Hector, L.R.; Creagh-Brown, B.C.; Evans, T.W.; Quinlan, G.J.; Burke-Gaffney, A. Plasma S100A8/A9 heterodimer is an early prognostic marker of acute kidney injury associated with cardiac surgery. Biomark. Med. 2019, 13, 205–218. [Google Scholar] [CrossRef] [PubMed]




| Characteristics | Total (n = 286) | No AKI at Admission (n = 253 (88.4%)) | AKI at Admission (n = 33 (11.6%)) | p-Value |
|---|---|---|---|---|
| Age (years) | 62 (53, 71) | 62 (53,70) | 66 (55, 74) | 0.096 |
| Gender (% female) | 109 (38.1) | 96 (37.9) | 13 (39.4) | 1.000 |
| BMI (kg/m2) | 22.8 (20.7, 25,3) | 22.7 (20.8, 25.0) | 23.2 (20.1, 25.8) | 0.664 |
| Baseline creatinine (µmol/L) | 78 (68, 95) | 78 (68, 94) | 83 (71, 104) | 0.072 |
| Comorbidities | ||||
| COPD (%) | 34 (12.0) | 28 (11.2) | 9 (21.4) | 0.339 |
| CVD (%) | 31 (12.0) | 28 (11.2) | 6 (18.8) | 0.231 |
| DM (%) | 46 (7.8) | 34 (13.4) | 16 (37.2) | 0.002 |
| Malignancy (%) | 22 (7.8) | 21 (8.4) | 1 (3.1) | 0.255 |
| Operation (%) | 192 (67.1) | 183 (72.3) | 9 (27.3) | <0.001 |
| Infection (%) | 49 (17.1) | 34 (13.4) | 15 (45.5) | <0.001 |
| Sepsis (%) | 40 (18.3) | 27 (14.2) | 13 (44.8) | <0.001 |
| SIRS score | 3 (1, 3) | 2 (1, 3) | 3 (3, 3) | 0.619 |
| SOFA score | 6 (3, 7) | 5 (3, 7) | 8 (6, 10) | <0.001 |
| Mechanical ventilation (%) | 203 (71.7) | 174 (69.3) | 29 (90.6) | 0.021 |
| RRT (within 24 h) | 6 (2.1) | 2 (0.8) | 4 (12.1) | <0.001 |
| Vital parameters | ||||
| Heart rate (bpm) | 80 (70, 100) | 80 (70, 100) | 90 (76, 102) | 0.078 |
| MAP (mmHg) | 84.0 (72.3, 98.3) | 84.5 (73.3, 98.7) | 78.3 (68.2, 93.8) | 0.416 |
| Respiratory rate (resp/min) | 18 (15, 24) | 16 (14, 20) | 24 (18, 33) | 0.006 |
| Body temperature (°C) | 36.2 (35.4, 37.3) | 36.4 (35.5, 37.3) | 35.4 (3501, 36.5) | 0.285 |
| Glasgow coma scale | 0.013 | |||
| 15 | 229 (80.9) | 209 (83.3) | 20 (62.5) | |
| 13–14 | 14 (4.9) | 10 (4.0) | 4 (12.5) | |
| ≤12 | 40 (14.1) | 32 (12.7) | 8 (25.0) | |
| Laboratory values | ||||
| CRP (mg/L) | 10.20 (2.70, 61.47) | 9.00 (2.40, 50.65) | 89.60 (43.10, 130.20) | <0.001 |
| Leucocytes (10^9/L) | 12.7 (9.5, 16.5) | 12.5 (9.3, 16.1) | 13.9 (11.1, 19.2) | 0.064 |
| Thrombocytes (10^9/L) | 189 (145, 244) | 187 (144, 241) | 207 (155, 304) | 0.268 |
| Bilirubin (µmol/L) | 9 (6, 15) | 9 (6, 15) | 12 (7, 17) | 0.233 |
| Albumin (g/L) | 30 (25, 34) | 30 (26, 34) | 28 (22, 33) | 0.084 |
| Creatinine (µmol/L) | 73 (58, 90) | 69 (58, 82) | 162 (136, 208) | <0.001 |
| PO2//FiO2 | 32.7 (23.7, 43.9) | 33.1 (25.2, 44.6) | 26.1 (18.5, 36.3) | 0.005 |
| Calprotectin (µg/mL) | 4.17 ( 2.46, 7.00) | 3.91 (2.36, 6.62) | 5.06 (3.94, 9.92) | 0.011 |
| Thiols (µmol/L) | 255.58 (161.63, 374.88) | 275.57 (166.96, 395.21) | 182.75 (75.78, 260.17) | <0.001 |
| APACHE II | 15 (11, 19) | 14 (11, 18) | 22 (18, 28.5) | <0.001 |
| APACHE IV | 48 (34, 65) | 44 (33, 58) | 89 (66, 104) | <0.001 |
| <7-day Mortality (%) | 17 (5.9) | 11 (4.3) | 6 (18.2) | 0.006 |
| <28-day Mortality (%) | 30 (10.5) | 20 (7.9) | 10 (30.3) | <0.001 |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | p-Value | Odds Ratio (95% CI) | p-Value | |
| Constant | −3.848 (−5.283 to –2.577) | <0.001 | ||
| Plasma thiols (µmol/g) | −0.119 (−0.208 to –0.035) | 0.007 | −0.106 (−0.246 to –0.005) | 0.047 |
| APACHE IV | 0.043 (0.030 to 0.056) | <0.001 | 0.039 (0.026 to 0.054) | <0.001 |
| Diabetes mellitus | 1.056 (0.352 to 1.735) | 0.003 | 1.032 (0.122 to 1.924) | 0.024 |
| Admission via OR | −1.649 (−2.327 to –1.006) | <0.001 | ||
| CRP (mg/L) | 0.006 (0.003 to 0.010) | <0.001 | ||
| Sepsis | 1.510 (0.779 to2.239) | <0.001 | ||
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| B (95% CI) | p-Value | B (95% CI) | p-Value | |
| Constant | 6.158 (2.953 to 9.364) | <0.001 | ||
| Age (in 10 years) | −1.065 (−1.401 to −0.728) | <0.001 | −0.719 (−1.019 to −0.421) | <0.001 |
| APACHE IV | −0.037 (−0.057 to −0.018) | <0.001 | ||
| CRP (mg/L) | −0.018 (−0.025 to −0.010) | <0.001 | −0.008 (−0.015 to −0.001) | 0.023 |
| Serum albumin (g/L) | 0.498 (0.426 to 0.567) | <0.001 | 0.470 (0.396 to0.544) | <0.001 |
| Serum creatinine (µmol/L) | −0.007 (−0.010 to −0.001) | 0.050 | −0.009 (−0.015 to −0.003) | 0.004 |
| Serum NGAL (mg/mL) | −0.001 (−0.002 to −0.001) | 0.215 | −0.002 (<−0.001 to −0.003) | 0.007 |
| Infection | −2.364 (−3.790 to −0.937) | 0.001 | ||
| Sepsis | −4.295 (−2.635 to −0.985) | 0.002 | ||
| Diabetes Mellitus | −1.832 (−3.268 to −0.395) | 0.013 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boekhoud, L.; Koeze, J.; van der Slikke, E.C.; Bourgonje, A.R.; Moser, J.; Zijlstra, J.G.; Muller Kobold, A.C.; Bulthuis, M.L.C.; van Meurs, M.; van Goor, H.; et al. Acute Kidney Injury is Associated with Lowered Plasma-Free Thiol Levels. Antioxidants 2020, 9, 1135. https://doi.org/10.3390/antiox9111135
Boekhoud L, Koeze J, van der Slikke EC, Bourgonje AR, Moser J, Zijlstra JG, Muller Kobold AC, Bulthuis MLC, van Meurs M, van Goor H, et al. Acute Kidney Injury is Associated with Lowered Plasma-Free Thiol Levels. Antioxidants. 2020; 9(11):1135. https://doi.org/10.3390/antiox9111135
Chicago/Turabian StyleBoekhoud, Lisanne, Jacqueline Koeze, Elisabeth C. van der Slikke, Arno R. Bourgonje, Jill Moser, Jan G. Zijlstra, Anneke C. Muller Kobold, Marian L. C. Bulthuis, Matijs van Meurs, Harry van Goor, and et al. 2020. "Acute Kidney Injury is Associated with Lowered Plasma-Free Thiol Levels" Antioxidants 9, no. 11: 1135. https://doi.org/10.3390/antiox9111135