Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Processing
2.3. pH Measurement
2.4. Colorimetric Analysis
2.5. HS–GC/MS Analyses
2.6. Extraction of Polyphenols
2.7. HPLC-DAD Analysis
2.8. Total Phenolic Content (TPC), Total Flavonoid Content (TFC) and Antioxidant Assays
2.9. Statistical Analysis
3. Results and Discussion
3.1. Processing and Aim of the Work
3.2. pH Measurements
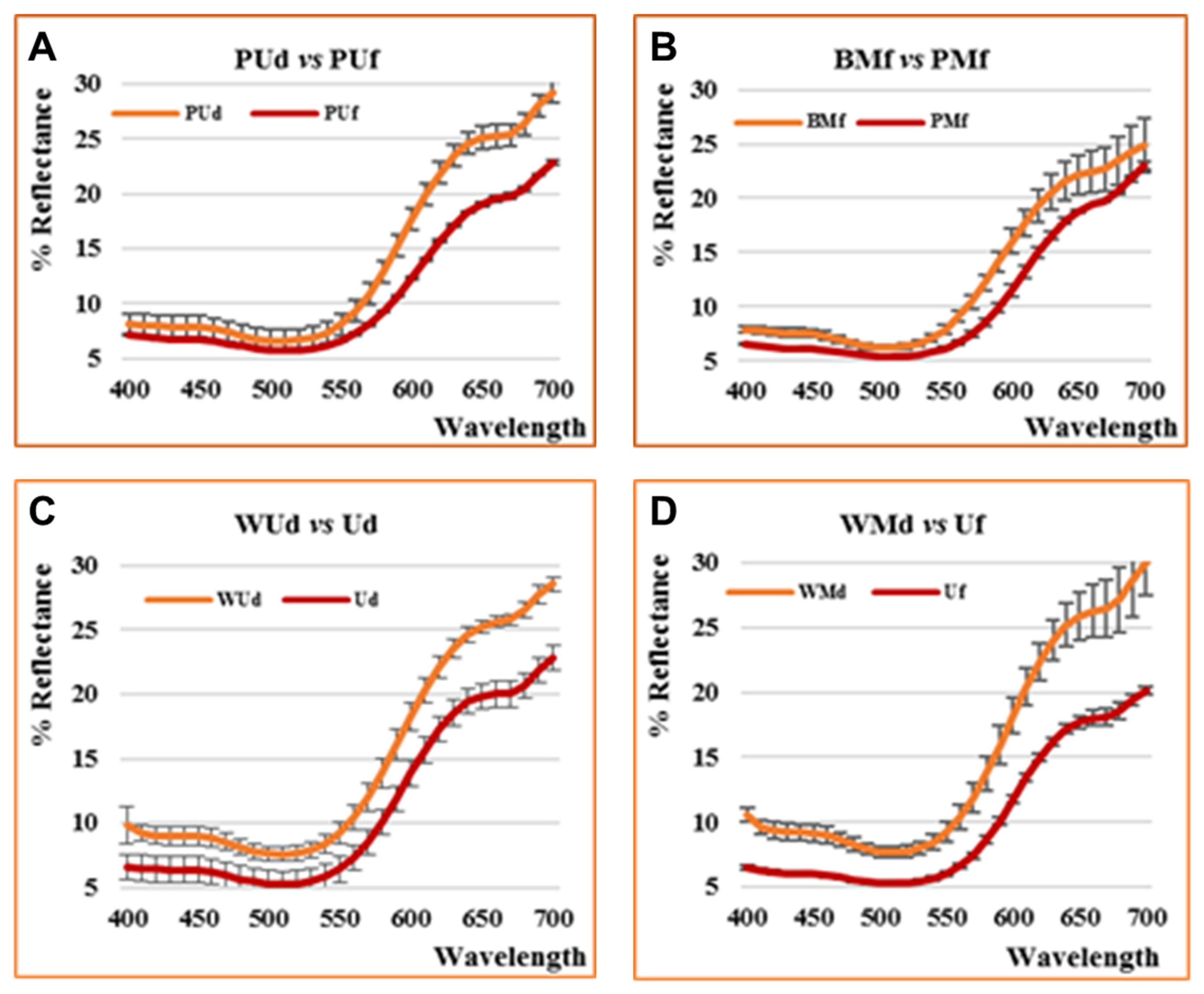
3.3. Color Analysis
3.4. HS-GC/MS Analysis
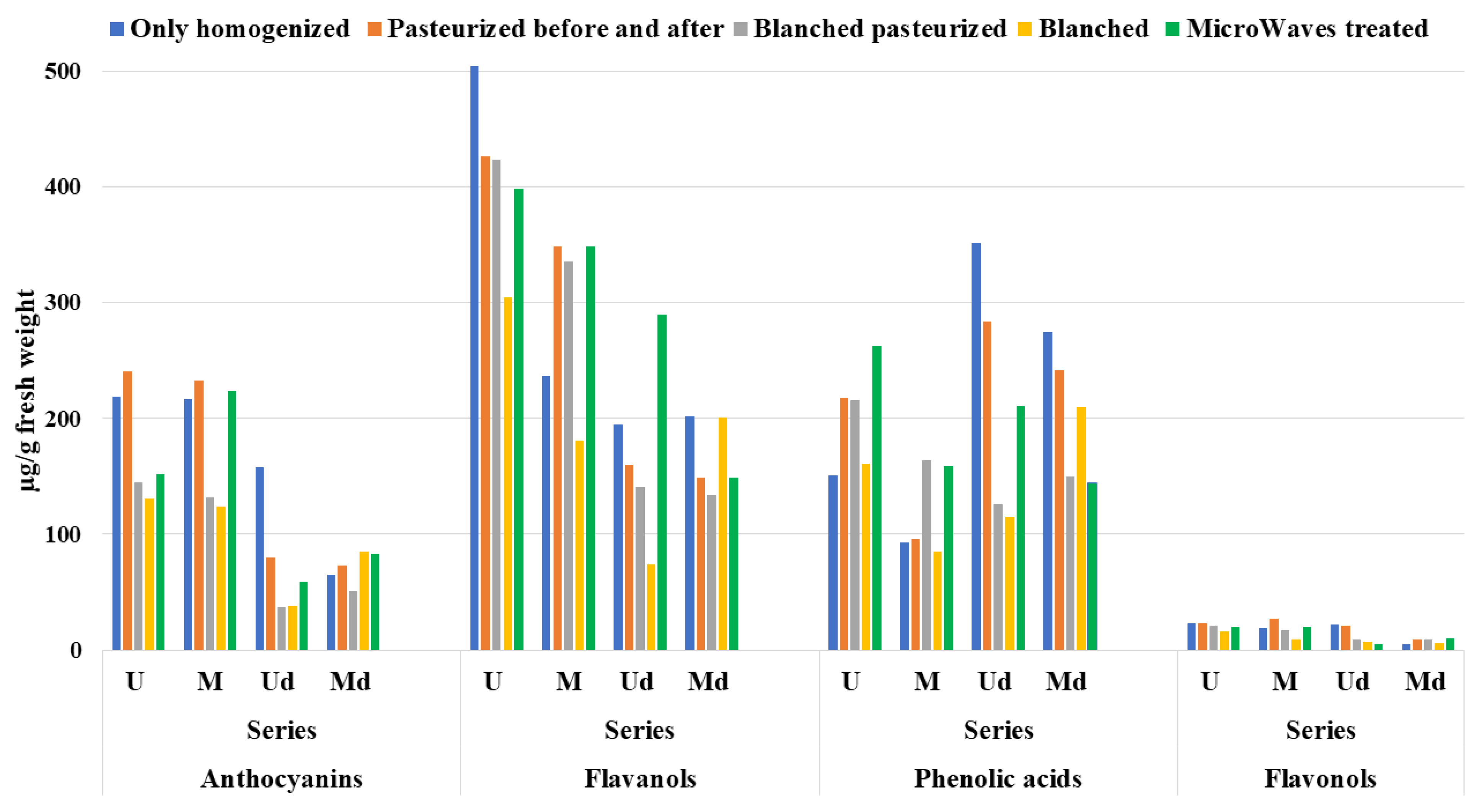
3.5. HPLC–Analysis
3.6. Total Phenolic Content and Total Flavonoid Content Evaluation
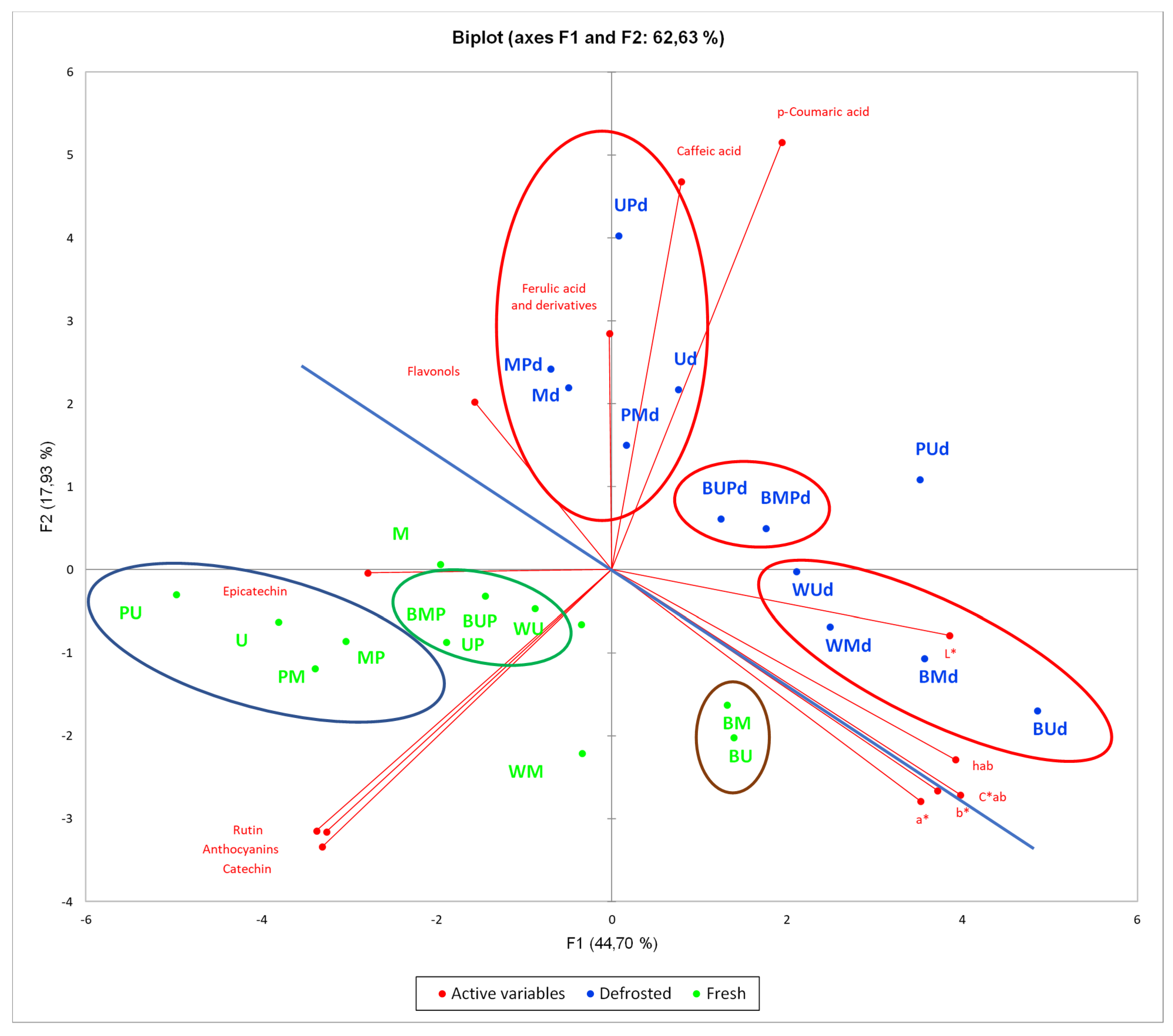
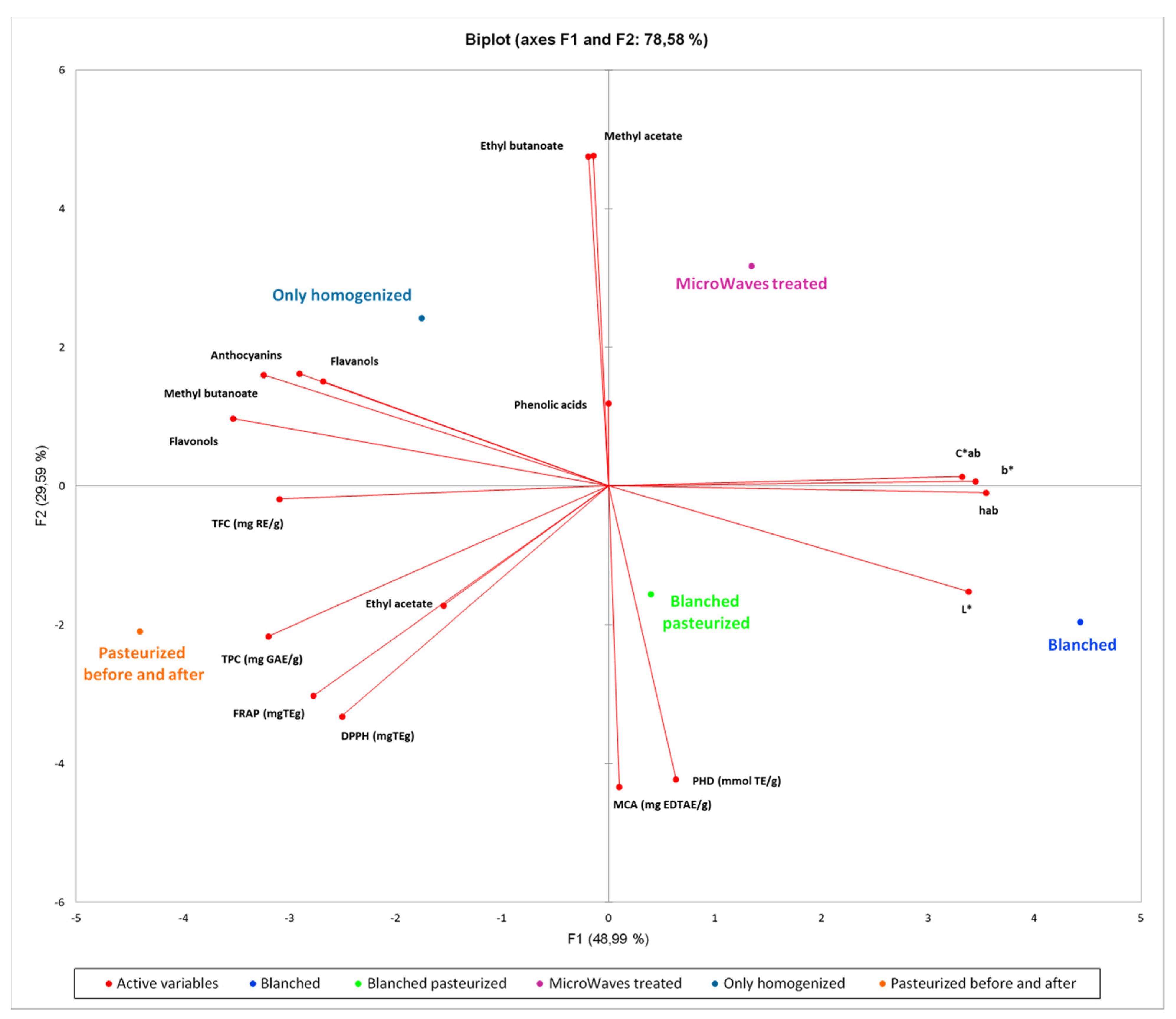
3.7. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703. [Google Scholar] [CrossRef]
- Pradas, I.; Medina, J.J.; Ortiz, V.; Moreno-Rojas, J.M. ‘Fuentepina’ and ‘Amiga’, two new strawberry cultivars: Evaluation of genotype, ripening and seasonal effects on quality characteristics and health-promoting compounds. J. Berry Res. 2015, 5, 157–171. [Google Scholar] [CrossRef] [Green Version]
- Kan, Y.A.W.; Tzvetkov, N.T.; Gokhan, Z.; Dongdong, W.; Suowen, X.; Goranka, M. The berries on the top. J. Berry Res. 2019, 9, 125–139. [Google Scholar]
- Afrin, S.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Reboredo-Rodriguez, P.; Mezzetti, B.; Varela-López, A.; Giampieri, F.; Battino, M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016, 64, 4435–4449. [Google Scholar] [CrossRef]
- Alvarez-Suarez, J.M.; Giampieri, F.; Tulipani, S.; Casoli, T.; Di Stefano, G.; González-Paramás, A.M.; Santos-Buelga, C.; Busco, F.; Quiles, J.L.; Cordero, M.D.; et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014, 25, 289–294. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Bogunović, I.; Duralija, B.; Gadže, J.; Kisić, I. Biostimulant usage for preserving strawberries to climate damages. Hortic. Sci. 2015, 42, 132–140. [Google Scholar] [CrossRef] [Green Version]
- Gasperotti, M.; Masuero, D.; Mattivi, F.; Vrhovsek, U. Overall dietary polyphenol intake in a bowl of strawberries: The influence of Fragaria spp. in nutritional studies. J. Funct. Foods 2015, 18, 1057–1069. [Google Scholar] [CrossRef]
- Šaponjac, V.T.; Gironés-Vilaplana, A.; Djilas, S.; Mena, P.; Ćetković, G.; Moreno, D.A.; Čanadanović-Brunet, J.; Vulić, J.; Stajčić, S.; Vinčić, M. Chemical composition and potential bioactivity of strawberry pomace. RSC Adv. 2015, 5, 5397–5405. [Google Scholar] [CrossRef]
- Nowicka, A.; Kucharska, A.Z.; Sokół-Łętowska, A.; Fecka, I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019, 270, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Wein, S.; Behm, N.; Petersen, R.K.; Kristiansen, K.; Wolffram, S. Quercetin enhances adiponectin secretion by a PPAR-γ independent mechanism. Eur. J. Pharm. Sci. 2010, 41, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.; Oliveira, P.J.; Dias, A.; Malva, J.O. Quercetin, kaempferol and biapigenin from Hypericum perforatum are neuroprotective against excytotoxic insults. Neurotox. Res. 2008, 13, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.L.; Escribano-Bailón, M.T.; Alonso, J.J.P.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Anthocyanin pigments in strawberry. LWT Food Sci. Technol. 2007, 40, 374–382. [Google Scholar] [CrossRef]
- Tomić, J.; Pesakovic, M.; Milivojevic, J.; Miletic, R.; Karaklajic-Stajic, Z.; Paunovic, S.M.; Milinkovic, M. Changes in anthocyanins and total phenols in fruit of three strawberry cultivars during five harvests. Acta Hortic. 2016, 1139, 633–638. [Google Scholar] [CrossRef]
- Weber, N.; Schmitzer, V.; Jakopic, J.; Stampar, F. First fruit in season: Seaweed extract and silicon advance organic strawberry (Fragaria × ananassa Duch.) fruit formation and yield. Sci. Hortic. 2018, 242, 103–109. [Google Scholar] [CrossRef]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Patsilinakos, A.; Ragno, R.; Carradori, S.; Petralito, S.; Cesa, S. Carotenoid content of Goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018, 268, 49–56. [Google Scholar] [CrossRef]
- Oliva, A.; Costantini, S.; De Angelis, M.; Garzoli, S.; Bozovic, M.; Mascellino, M.T.; Vullo, V.; Ragno, R. High potency of Maleleuca alternifolia essential oil against multi-drug resistent Gram-negative bacteria and methicillin–resistant Staphyloccocus aureus. Molecules 2018, 23, 2584. [Google Scholar] [CrossRef] [Green Version]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Andreotti, E.; Tadic, V.; Costantini, S.; Ragno, R.; Bozovic, M. Chemical composition and antimicrobial activity of essential oil of Helichrysum italicum (Roth) G. Don fil. (Asteraceae). Nat. Prod. Res. 2020, 34, 445. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Laghezza Masci, V.; Ovidi, E. Liquid and Vapour Phase of Lavandin (Lavandula × intermedia) Essential Oil: Chemical Composition and Antimicrobial Activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carradori, S.; Cairone, F.; Garzoli, S.; Fabrizi, G.; Iazzetti, A.; Giusti, A.M.; Menghini, L.; Uysal, S.; Ak, G.; Zengin, G.; et al. Phytocomplex Characterization and Biological Evaluation of Powdered Fruits and Leaves from Elaeagnus angustifolia. Molecules 2020, 25, 2021. [Google Scholar] [CrossRef] [PubMed]
- Balli, D.; Cecchi, L.; Khatib, M.; Bellumori, M.; Cairone, F.; Carradori, S.; Zengin, G.; Cesa, S.; Innocenti, M.; Mulinacci, N. Characterization of arils juice and peel decoction of fifteen varieties of Punica granatum L.: A focus on anthocyanins, ellagitannins and polysaccharides. Antioxidants 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra-and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.C.N.; Brecht, J.K.; Morais, A.M.; Sargent, S.A. Possible influences of water loss and polyphenol oxidase activity on anthocyanin content and discoloration in fresh ripe strawberry (cv. Oso Grande) during storage at 1 C. J. Food Sci. 2005, 70, S79–S84. [Google Scholar] [CrossRef]
- Cesa, S.; Casadei, M.A.; Cerreto, F.; Paolicelli, P. Infant milk formulas: Effect of storage conditions on the stability of powdered products towards autoxidation. Foods 2015, 4, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Cutzu, R.; Bardi, L. Production of bioethanol from agricultural wastes using residual thermal energy of a cogeneration plant in the distillation phase. Fermentation 2017, 3, 24. [Google Scholar] [CrossRef] [Green Version]
- Williams, A.; Ryan, D.; Guasca, A.O.; Marriott, P.; Pang, E. Analysis of strawberry volatiles using comprehensive two-dimensional gas chromatography with headspace solid-phase microextraction. J. Chrom. B 2005, 817, 97–107. [Google Scholar] [CrossRef]
- Oz, A.T.; Baktemur, G.; Kargi, S.P.; Kafkas, E. Volatile compounds of strawberry varieties. Chem. Nat. Compd. 2016, 52, 507–509. [Google Scholar] [CrossRef]
- Kafkas, E.; Cabaroglu, T.; Selli, S.; Bozdoğan, A.; Kürkçüoğlu, M.; Paydaş, S.; Başer, K.H.C. Identification of volatile aroma compounds of strawberry wine using solid-phase microextraction techniques coupled with gas chromatography–mass spectrometry. Flavour Frag. J. 2006, 21, 68–71. [Google Scholar] [CrossRef]
- Kelebek, H.; Selli, S. Characterization of phenolic compounds in strawberry fruits by RP-HPLC-DAD and investigation of their antioxidant capacity. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 2495–2504. [Google Scholar] [CrossRef]
- Andrianjaka-Camps, Z.N.; Heritier, J.; Ancay, A.; Andlauer, W.; Carlen, C. Evolution of the taste-related and bioactive compound profiles of the external and internal tissues of strawberry fruits (Fragaria × ananassa) cv. ‘Clery’ during ripening. J. Berry Res. 2017, 7, 11–22. [Google Scholar] [CrossRef]
- Buendia, B.; Gil, M.I.; Tudela, J.A.; Gady, A.L.; Medina, J.J.; Soria, C.; López, J.M.; Tomás-Barberán, F. HPLC-MS analysis of proanthocyanidin oligomers and other phenolics in 15 strawberry cultivars. J. Agric. Food Chem. 2010, 58, 3916–3926. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.L.; Feneuil, A.; Acosta, O.; Pérez, A.M.; Vaillant, F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. 2011, 44, 2243. [Google Scholar] [CrossRef]
- Chamorro, M.F.; Reiner, G.; Theoduloz, C.; Ladio, A.; Schmeda-Hirschmann, G.; Gómez-Alonso, S.; Jiménez-Aspee, F. Polyphenol Composition and (Bio) Activity of Berberis Species and Wild Strawberry from the Argentinean Patagonia. Molecules 2019, 24, 3331. [Google Scholar] [CrossRef] [Green Version]
- Oszmiański, J.; Wojdyło, A.; Kolniak, J. Effect of L-ascorbic acid, sugar, pectin and freeze–thaw treatment on polyphenol content of frozen strawberries. LWT-Food Sci. Technol. 2009, 42, 581. [Google Scholar] [CrossRef]
- Holzwarth, M.; Korhummel, S.; Carle, R.; Kammerer, D.R. Evaluation of the effects of different freezing and thawing methods on color, polyphenol and ascorbic acid retention in strawberries (Fragaria × ananassa Duch.). Food Res. Int. 2012, 48, 241. [Google Scholar] [CrossRef]

| Components 1 | LRI 2 | LRI 3 | Sliced | U | M | WU | WM | BM | BU |
|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 650 | 655 | 3.8 | 12.9 | 4.4 | 5.7 | 8.5 | 2.8 | - |
| Methyl acetate | 826 | 828 | 40.0 | 34.6 | 36.7 | 38.7 | 38.8 | - | - |
| Acetone | 838 | 842 | 1.9 | 9.9 | 4.4 | 6.9 | 4.9 | - | 11.5 |
| Ethyl acetate | 880 | 885 | 19.8 | 5.8 | 30.4 | 27.1 | 25.4 | 23.9 | 20.4 |
| Methyl butanoate | 986 | 985 | 17.7 | 22.2 | 9.6 | 7.9 | 9.2 | 2.4 | 9.1 |
| Ethanol | nd | nd | nd | nd | nd | 70.9 | 59.0 | ||
| Ethyl butanoate | 1040 | 1036 | 8.1 | 8.9 | 7.9 | 8.3 | 8.8 | nd | nd |
| 2-hydroxy propanamide | 1114 | * | - | - | 2.6 | - | 4.4 | - | - |
| Methyl hexanoate | 1182 | 1187 | 6.9 | 2.1 | 3.5 | - | - | - | - |
| Ethyl hexanoate | 1234 | 1238 | 1.7 | 3.4 | 0.4 | - | - | - | - |
| Sum | 99.9 | 99.8 | 99.9 | 94.6 | 100.0 | 100.0 | 100.0 |
| Components 1 | LRI 2 | LRI 3 | MP | UP | PM | PU | BMP | BUP | PMd | PUd |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetaldehyde | 650 | 655 | - | 1.9 | 1.3 | - | - | - | 6.0 | 2.3 |
| Acetone | 838 | 842 | 57.4 | 8.6 | 0.4 | 26.9 | 53.1 | 76.4 | - | 0.8 |
| Ethyl acetate | 880 | 885 | - | 61.4 | 61.4 | 2.9 | 46.9 | - | 33.9 | 16.2 |
| Ethanol | 940 | 938 | - | 25.3 | 36.9 | 70.2 | - | - | 60.1 | 80.7 |
| Methyl butanoate | 986 | 985 | 42.6 | 2.8 | - | - | - | 23.6 | - | - |
| Sum | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| M | U | BM | BU | BMP | BUP | PM | PU | MP | UP | WM | WU | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Catechin | - | 253.7 | - | 108.9 | 98.1 | 135.1 | 177.5 | 210.8 | 165.3 | 96.6 | 153.4 | 178.4 |
| Epicatechin | 236.4 | 251.4 | 181.2 | 195.6 | 237.6 | 288.8 | 184.4 | 316.4 | 168.5 | 229.5 | 195.9 | 220.8 |
| Caffeic acid | 11.8 | 10.8 | 11.7 | 12.2 | 10.0 | 18.2 | 8.4 | 18.5 | 7.2 | 14.4 | 17.8 | 48.9 |
| p–Coumaric acid | 5.6 | 5.9 | - | 7.6 | 13.1 | 17.1 | 4.8 | 15.1 | 11.2 | 16.5 | 8.2 | 13.4 |
| Ferulic acid and derivatives * | 75.8 | 134.4 | 73.7 | 141.6 | 140.6 | 180.7 | 56.2 | 197.3 | 103.9 | 173.2 | 133.1 | 200.6 |
| Rutin | - | 4.8 | 1.1 | 3.4 | 6.1 | 5.4 | 6.5 | 13.5 | 9.8 | 10.8 | 7.1 | 7.1 |
| Flavonols ** | 19.3 | 18.2 | 1.6 | 12.2 | 10.8 | 15.4 | 8.4 | 8.5 | 28.4 | 14.1 | 12.8 | 13.2 |
| Anthocyanins *** | 216.6 | 219.1 | 123.5 | 131.4 | 131.7 | 144.6 | 237.9 | 258.5 | 227.2 | 222.6 | 223.5 | 151.7 |
| Sum | 565.5 | 898.3 | 398.8 | 612.9 | 648.0 | 805.3 | 684.1 | 1038.6 | 721.5 | 777.7 | 751.8 | 834.1 |
| Md | Ud | BMd | BUd | BMPd | BUPd | PMd | PUd | MPd | UPd | WMd | WUd | |
| Epicatechin | 202.3 | 194.8 | 200.7 | 73.9 | 133.5 | 141.4 | 157.8 | 126.5 | 222.6 | 192.7 | 148.6 | 290.2 |
| Caffeic acid | 38.6 | 77.2 | 20.0 | 5.8 | 31.7 | 5.7 | 31.0 | 88.7 | 47.9 | 54.5 | nd | nd |
| p-Coumaric acid | 20.4 | 31.7 | 22.8 | 26.2 | 25.3 | 29.9 | 23.2 | 34.3 | 19.7 | 62.2 | 21.3 | 31.8 |
| Ferulic acid and derivatives * | 216.2 | 242.5 | 167.2 | 83.2 | 92.7 | 90.4 | 134.4 | 207.8 | 227.9 | 120.9 | 122.9 | 178.8 |
| Flavonols ** | 5.3 | 21.7 | 5.9 | 6.9 | 8.6 | 9.0 | 8.3 | 13.4 | 10.5 | 28.1 | 10.3 | 5.4 |
| Anthocyanins *** | 64.9 | 158.3 | 85.2 | 37.9 | 50.6 | 36.8 | 69.4 | 129.7 | 76.8 | 30.4 | 82.6 | 58.8 |
| Sum | 547.7 | 726.2 | 501.8 | 233.8 | 342.4 | 313.2 | 424.1 | 600.4 | 605.4 | 488.8 | 385.7 | 565.0 |
| Samples | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|
| BUP | 14.63 ± 0.22 d | 0.83 ± 0.05 f |
| BMP | 12.66 ± 0.13 f | 1.14 ± 0.04 d |
| U | 12.99 ± 0.10 f | 0.91 ± 0.04 f |
| M | 13.69 ± 0.12 e | 1.54 ± 0.06 bc |
| UP | 14.39 ± 0.15 d | 1.17 ± 0.03 d |
| MP | 19.28 ± 0.06 a | 2.53 ± 0.03 a |
| PU | 16.38 ± 0.13 c | 1.06 ± 0.07 de |
| PM | 17.27 ± 0.21 b | 1.59 ± 0.05 b |
| WU | 11.34 ± 0.15 h | 1.19 ± 0.03 d |
| WM | 13.83 ± 0.11 e | 1.19 ± 0.05 d |
| BU | 12.84 ± 0.03 f | 0.96 ± 0.05 ef |
| BM | 11.97 ± 0.10 g | 1.08 ± 0.05 de |
| Ud | 12.16 ± 0.08 g | 1.16 ± 0.06 d |
| Md | 13.67 ± 0.15 e | 1.40 ± 0.05 c |
| Samples | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg/TE) | MCA (mg EDTAE/g) | PHD (mmol TE/g) |
|---|---|---|---|---|---|---|
| BUP | 27.92±0.53 b | 38.19 ± 0.19 c | 56.45 ± 0.33 c | 38.81 ± 0.57 c | 7.73 ± 0.09 a | 1.22 ± 0.13 abc |
| BMP | 23.65 ± 0.32 c | 33.50 ± 0.13 e | 49.55 ± 0.24 ef | 33.58 ± 0.19 ef | 7.06 ± 0.06 ab | 1.09 ± 0.03 bc |
| U | 21.73 ± 0.24 de | 31.47 ± 0.41 f | 48.57 ± 0.40 f | 32.52 ± 0.38 f | 4.57 ± 0.78 ef | 1.06 ± 0.07 bc |
| M | 21.11 ± 0.35 e | 31.73 ± 0.53 f | 48.85 ± 0.18 ef | 33.22 ± 0.29 ef | 3.89 ± 0.32 f | 1.13 ± 0.14 abc |
| UP | 27.17 ± 0.50 b | 36.35 ± 0.93 d | 54.92 ± 0.31 c | 37.47 ± 0.61 d | 3.65 ± 0.20 f | 1.10 ± 0.04 abc |
| MP | 33.20 ± 0.96 a | 45.41 ± 0.35 a | 72.26 ± 0.42 a | 48.11 ± 0.50 a | 6.87 ± 0.21 abc | 1.27 ± 0.09 ab |
| PU | 26.79 ± 0.26 b | 38.19 ± 0.28 c | 61.02 ± 0.67 b | 39.15 ± 0.16 c | 6.09 ± 0.12 bcd | 1.13 ± 0.03 abc |
| PM | 27.29 ± 0.61 b | 40.98 ± 0.85 b | 61.76 ± 1.05 b | 42.15 ± 0.34 b | 6.98 ± 0.48 abc | 1.17 ± 0.08 abc |
| WU | 18.47 ± 0.51 f | 25.52 ± 0.66 i | 35.30 ± 0.18 g | 26.90 ± 0.44 h | 1.91 ± 0.14 g | 0.95 ± 0.07 c |
| WM | 21.70 ± 0.50 de | 32.52 ± 0.09 ef | 50.38 ± 1.31 e | 36.31 ± 0.17 d | 2.18 ± 0.44 g | 1.03 ± 0.04 bc |
| BU | 21.64 ± 0.09 de | 28.49 ± 0.45 gh | 52.79 ± 0.72 d | 34.41 ± 0.50 e | 5.98 ± 0.25 cd | 1.23 ± 0.11 ab |
| BM | 20.95 ± 0.50 e | 29.87 ± 0.17 g | 47.98 ± 0.61 f | 30.59 ± 0.47 g | 6.92 ± 0.30 abc | 1.26 ± 0.16 ab |
| Ud | 21.47 ± 0.24 de | 28.16 ± 0.81 h | 48.33 ± 0.25 f | 32.40 ± 0.24 f | 1.64 ± 0.24 g | 1.38 ± 0.12 a |
| Md | 22.73 ± 0.09 cd | 32.25 ± 0.32 ef | 52.42 ± 0.39 d | 34.26 ± 0.55 e | 5.57 ± 0.57 de | 1.30 ± 0.07 ab |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzoli, S.; Cairone, F.; Carradori, S.; Mocan, A.; Menghini, L.; Paolicelli, P.; Ak, G.; Zengin, G.; Cesa, S. Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries. Antioxidants 2020, 9, 632. https://doi.org/10.3390/antiox9070632
Garzoli S, Cairone F, Carradori S, Mocan A, Menghini L, Paolicelli P, Ak G, Zengin G, Cesa S. Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries. Antioxidants. 2020; 9(7):632. https://doi.org/10.3390/antiox9070632
Chicago/Turabian StyleGarzoli, Stefania, Francesco Cairone, Simone Carradori, Andrei Mocan, Luigi Menghini, Patrizia Paolicelli, Gunes Ak, Gokhan Zengin, and Stefania Cesa. 2020. "Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries" Antioxidants 9, no. 7: 632. https://doi.org/10.3390/antiox9070632