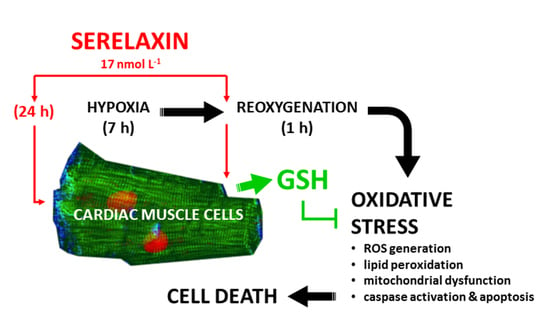
Human Relaxin-2 (Serelaxin) Attenuates Oxidative Stress in Cardiac Muscle Cells Exposed In Vitro to Hypoxia–Reoxygenation. Evidence for the Involvement of Reduced Glutathione Up-Regulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Intracellular ROS and Mitochondrial O2•−
2.3. Evaluation of Lipid Peroxidation
2.4. Mitochondrial Activity
2.5. Mitochondrial Membrane Potential (Δψ)
2.6. Mitochondrial Transition Pore Opening (mPTP)
2.7. Caspase Activity
2.8. Cell Death Assay
2.9. Superoxide Dismutase (SOD) Activity
2.10. Glutathione Levels
2.11. Assay of RLX Antioxidant Properties
2.12. Statistical Analysis
3. Results
3.1. RLX Protects from HR-Induced Cellular Oxidative Stress
3.2. RLX Improves Cell Mitochondrial Activity Impaired by HR
3.3. RLX Protects from HR-Induced Cell Apoptosis
3.4. Possible Mechanisms of the RLX’s Antioxidant Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dschietzig, T.; Stangl, K. Relaxin: A pregnancy hormone as central player of body fluid and circulation homeostasis. Cell Mol. Life Sci. 2003, 60, 688–700. [Google Scholar] [PubMed]
- Nistri, S.; Bigazzi, M.; Bani, D. Relaxin as a cardiovascular hormone: Physiology; pathophysiology and therapeutic promises. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Romero, G.; Salama, G. Cardioprotective actions of relaxin. Mol. Cell. Endocrinol. 2019, 487, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Bani, D.; Masini, E.; Bello, M.G.; Bigazzi, M.; Sacchi, T.B. Relaxin protects against myocardial injury caused by ischemia and reperfusion in rat heart. Am. J. Pathol. 1998, 152, 1367–1376. [Google Scholar]
- Masini, E.; Cuzzocrea, S.; Mazzon, E.; Muià, C.; Vannacci, A.; Fabrizi, F.; Bani, D. Protective effects of relaxin in ischemia/reperfusion-induced intestinal injury due to splanchnic artery occlusion. Br. J. Pharm. 2006, 148, 1124–1132. [Google Scholar] [CrossRef] [Green Version]
- Collino, M.; Rogazzo, M.; Pini, A.; Benetti, E.; Rosa, A.C.; Chiazza, F.; Fantozzi, R.; Bani, D.; Masini, E. Acute treatment with relaxin protects the kidney against ischaemia/reperfusion injury. J. Cell Mol. Med. 2013, 17, 1494–1505. [Google Scholar] [CrossRef]
- Perna, A.M.; Masini, E.; Nistri, S.; Briganti, V.; Chiappini, L.; Stefano, P.; Bigazzi, M.; Pieroni, C.; Sacchi, T.B.; Bani, D. Novel drug development opportunity for relaxin in acute myocardial infarction: Evidences from a swine model. Faseb. J. 2005, 19, 1525–1527. [Google Scholar] [CrossRef] [Green Version]
- Raleigh, J.V.; Mauro, A.G.; Devarakonda, T.; Marchetti, C.; He, J.; Kim, E.; Filippone, S.; Das, A.; Toldo, S.; Abbate, A.; et al. Reperfusion therapy with recombinant human relaxin-2 (Serelaxin) attenuates myocardial infarct size and NLRP3 inflammasome following ischemia/reperfusion injury via eNOS-dependent mechanism. Cardiovasc. Res. 2017, 113, 609–619. [Google Scholar] [CrossRef] [Green Version]
- Masini, E.; Bani, D.; Bello, M.G.; Bigazzi, M.; Mannaioni, P.F.; Sacchi, T.B. Relaxin counteracts myocardial damage induced by ischemia-reperfusion in isolated guinea pig hearts: Evidence for an involvement of nitric oxide. Endocrinology 1997, 138, 4713–4720. [Google Scholar] [CrossRef]
- Alexiou, K.; Matschke, K.; Westphal, A.; Stangl, K.; Dschietzig, T. Relaxin is a candidate drug for lung preservation: Relaxin-induced protection of rat lungs from ischemia-reperfusion injury. J. Heart Lung Transpl. 2010, 29, 454–460. [Google Scholar] [CrossRef]
- Kageyama, S.; Nakamura, K.; Kupiec-Weglinski, J.W. Relaxin in liver transplantation: A personal perspective. Mol. Cell Endocrinol. 2019, 482, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Jakubauskiene, L.; Jakubauskas, M.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Relaxin positively influences ischemia-reperfusion injury in solid organ transplantation: A comprehensive review. Int. J. Mol. Sci. 2020, 21, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccalini, G.; Sassoli, C.; Formigli, L.; Bani, D.; Nistri, S. Relaxin protects cardiac muscle cells from hypoxia/reoxygenation injury: Involvement of the Notch-1 pathway. Faseb J. 2015, 29, 239–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunleye, O.; Campo, B.; Herrera, D.; Uiterweer, E.D.P.; Conrad, K.P. Relaxin confers cytotrophoblast protection from hypoxia-reoxygenation injury through the phosphatidylinositol 3-kinase-Akt/protein kinase B cell survival pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R559–R568. [Google Scholar] [CrossRef]
- Poyton, R.O.; Ball, K.A.; Castello, P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009, 20, 332–340. [Google Scholar] [CrossRef]
- Castello, P.R.; Woo, D.K.; Ball, K.; Wojcik, J.; Liu, L.; Poyton, R.O. Oxygen-regulated isoforms of cytochrome c oxidase have differential effects on its nitric oxide production and on hypoxic signaling. Proc. Natl. Acad. Sci. USA 2008, 105, 8203–8208. [Google Scholar] [CrossRef] [Green Version]
- Galkin, A.; Higgs, A.; Moncada, S. Nitric oxide and hypoxia. Essays Biochem. 2007, 3, 29–42. [Google Scholar]
- Xie, Y.W.; Kaminski, P.M.; Wolin, M.S. Inhibition of rat cardiac muscle contraction and mitochondrial respiration by endogenous peroxynitrite formation during posthypoxic reoxygenation. Circ. Res. 1998, 82, 891–897. [Google Scholar] [CrossRef]
- Kokura, S.; Yoshida, N.; Yoshikawa, T. Anoxia/reoxygenation-induced leukocyte-endothelial cell interactions. Free Radic Biol Med. 2002, 33, 427–432. [Google Scholar] [CrossRef]
- Ferrer-Sueta, G.; Radi, R. Chemical biology of peroxynitrite: Kinetics, diffusion, and radicals. Acs Chem. Biol. 2009, 4, 161–177. [Google Scholar] [CrossRef]
- Becatti, M.; Fiorillo, C.; Barygina, V.; Cecchi, C.; Lotti, T.; Prignano, F.; Silvestro, A.; Nassi, P.; Taddei, N. SIRT1 regulates MAPK pathways in vitiligo skin: Insight into the molecular pathways of cell survival. J. Cell Mol. Med. 2014, 18, 514–529. [Google Scholar] [CrossRef] [PubMed]
- Becatti, M.; Barygina, V.; Mannucci, A.; Emmi, G.; Prisco, D.; Lotti, T.; Fiorillo, C.; Taddei, N. Sirt1 protects against oxidative stress-induced apoptosis in fibroblasts from psoriatic patients: A new insight into the pathogenetic mechanisms of psoriasis. Int. J. Mol. Sci. 2018, 19, 1572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barygina, V.; Becatti, M.; Lotti, T.; Moretti, S.; Taddei, N.; Fiorillo, C. Treatment with low-dose cytokines reduces oxidative-mediated injury in perilesional keratinocytes from vitiligo skin. J. Derm. Sci. 2015, 79, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Pini, A.; Boccalini, G.; Baccari, M.C.; Becatti, M.; Garella, R.; Fiorillo, C.; Calosi, L.; Bani, D.; Nistri, S. Protection from cigarette smoke-induced vascular injury by recombinant human relaxin-2 (serelaxin). J. Cell Mol. Med. 2016, 20, 891–902. [Google Scholar] [CrossRef]
- Becatti, M.; Bencini, A.; Nistri, S.; Conti, L.; Fabbrini, M.G.; Lucarini, L.; Ghini, V.; Severi, M.; Fiorillo, C.; Giorgi, C.; et al. Different antioxidant efficacy of two MnII-containing superoxide anion scavengers on hypoxia/reoxygenation-exposed cardiac muscle cells. Sci. Rep. 2019, 9, 10320. [Google Scholar] [CrossRef] [Green Version]
- Fiorillo, C.; Becatti, M.; Pensalfini, A.; Cecchi, C.; Lanzilao, L.; Donzelli, G.; Nassi, N.; Giannini, L.; Borchi, E.; Nassi, P. Curcumin protects cardiac cells against ischemia-reperfusion injury: Effects on oxidative stress, NF-kappaB, and JNK pathways. Free Radic. Biol. Med. 2008, 15, 839–846. [Google Scholar] [CrossRef]
- Whittaker, A.; Dinu, M.; Cesari, F.; Gori, A.M.; Fiorillo, C.; Becatti, M.; Casini, A.; Marcucci, R.; Benedettelli, S.; Sofi, F. A khorasan wheat-based replacement diet improves risk profile of patients with type 2 diabetes mellitus (T2DM): A randomized crossover trial. Eur. J. Nutr. 2017, 56, 1191–1200. [Google Scholar] [CrossRef] [Green Version]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-E-Silva, O. Ischemia/reperfusion injury revisited: An overview of the latest pharmacological strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef] [Green Version]
- Moore, X.L.; Tan, S.L.; Lo, C.Y.; Fang, L.; Su, Y.D.; Gao, X.M.; Woodcock, E.A.; Summers, R.J.; Tregear, G.W.; Bathgate, R.A.; et al. Relaxin antagonizes hypertrophy and apoptosis in neonatal rat cardiomyocytes. Endocrinology 2007, 148, 1582–1589. [Google Scholar] [CrossRef] [Green Version]
- Willcox, J.M.; Summerlee, A.J. Relaxin protects astrocytes from hypoxia in vitro. PLoS ONE 2014, 9, e90864. [Google Scholar] [CrossRef] [Green Version]
- Waza, A.A.; Hamid, Z.; Bhat, S.A.; Shah, N.U.D.; Bhat, M.; Ganai, B. Relaxin protects cardiomyocytes against hypoxia-induced damage in in-vitro conditions: Involvement of Nrf2/HO-1 signaling pathway. Life Sci. 2018, 213, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Johnson, F.; Giulivi, C. Superoxide dismutases and their impact upon human health. Mol. Asp. Med. 2005, 26, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yang, Y.; Jiang, Y.J.; Lei, J.M.; Guo, J.W.; Xiao, H. Relaxin ameliorates high glucose-induced cardiomyocyte hypertrophy and apoptosis via the Notch1 pathway. Exp. Med. 2018, 15, 691–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patil, N.A.; Rosengren, K.J.; Separovic, F.; Wade, J.D.; Bathgate, R.A.D.; Hossain, M.A. Relaxin family peptides: Structure-activity relationship studies. Br. J. Pharm. 2017, 174, 950–961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becatti, M.; Taddei, N.; Cecchi, C.; Nassi, N.; Nassi, P.A.; Fiorillo, C. SIRT1 modulates MAPK pathways in ischemic-reperfused cardiomyocytes. Cell Mol. Life Sci. 2012, 69, 2245–2260. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistri, S.; Fiorillo, C.; Becatti, M.; Bani, D. Human Relaxin-2 (Serelaxin) Attenuates Oxidative Stress in Cardiac Muscle Cells Exposed In Vitro to Hypoxia–Reoxygenation. Evidence for the Involvement of Reduced Glutathione Up-Regulation. Antioxidants 2020, 9, 774. https://doi.org/10.3390/antiox9090774
Nistri S, Fiorillo C, Becatti M, Bani D. Human Relaxin-2 (Serelaxin) Attenuates Oxidative Stress in Cardiac Muscle Cells Exposed In Vitro to Hypoxia–Reoxygenation. Evidence for the Involvement of Reduced Glutathione Up-Regulation. Antioxidants. 2020; 9(9):774. https://doi.org/10.3390/antiox9090774
Chicago/Turabian StyleNistri, Silvia, Claudia Fiorillo, Matteo Becatti, and Daniele Bani. 2020. "Human Relaxin-2 (Serelaxin) Attenuates Oxidative Stress in Cardiac Muscle Cells Exposed In Vitro to Hypoxia–Reoxygenation. Evidence for the Involvement of Reduced Glutathione Up-Regulation" Antioxidants 9, no. 9: 774. https://doi.org/10.3390/antiox9090774