Sustaining Vaccine Confidence in the 21st Century
Abstract
:1. Introduction
| Disease | Pre-vaccination (estimated annual average) | Post-vaccination (year) | ||
|---|---|---|---|---|
| Cases | Deaths | Cases | Deaths | |
| Diphtheria | 21,053 | 1,822 | 0 (2006) | 0 (2004) |
| Measles | 530,217 | 440 | 55 (2006) | 0 (2004) |
| Mumps | 162,344 | 39 | 6,584 (2006) | 0 (2004) |
| Pertussis | 200,752 | 4,034 | 15,632 (2006) | 27 (2004) |
| Poliomyelitis, acute | 19,794 | 1,393 | 0 (2006) | 0 (2004) |
| Poliomyelitis, paralytic | 16,316 | 1,879 | 0 (2006) | 0 (2004) |
| Rubella | 47,745 | 17 | 11 (2006) | 0 (2004) |
| Congenital rubella syndrome | 152 | Not available | 1 (2006) | 0 (2004) |
| Smallpox | 29,005 | 337 | 0 (2006) | 0 (2004) |
| Tetanus | 580 | 472 | 41 (2006) | 4 (2004) |
| Hepatitis A | 117,333 | 137 | 3,579 (2006) | 18 (2006) |
| Acute hepatitis B | 66,232 | 237 | 4,713 (2006) | 47 (2006) |
| Invasive Hib | 20,000 | 1,000 | 208 (2006) | <5 (2005) |
| IPD | 63,067 | 6,500 | 5,169 (2006) | 4,850 (2005) |
| Varicella | 4,085,120 | 105 | 48,445 (2006) | 19 (2004) |
2. Factors Associated with Improved Vaccine Confidence
2.1. Vaccine Recommendations and Health Policies
2.2. Vaccine Development and Manufacturing
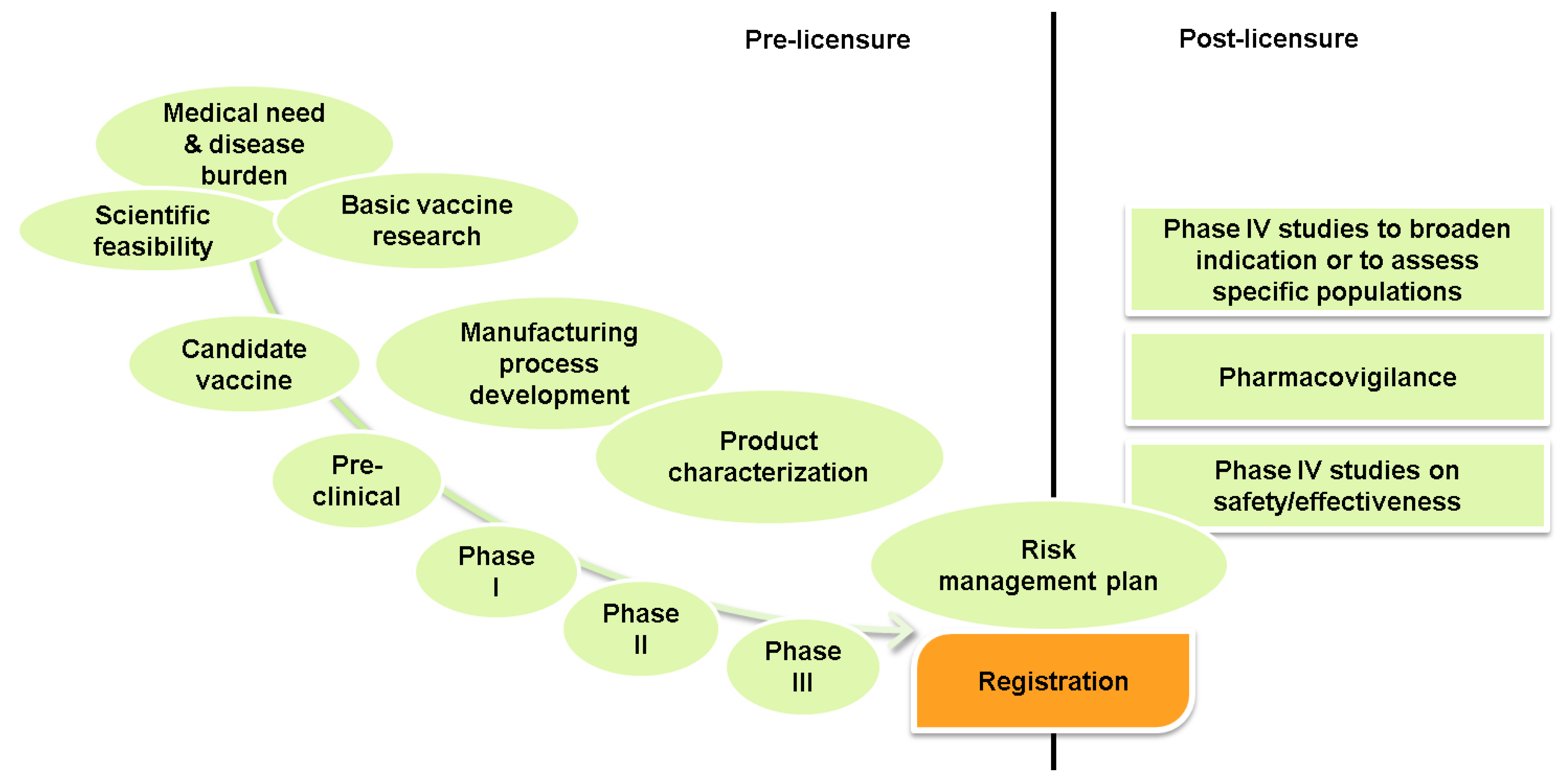
2.2.1. Rules and Regulations—Vaccine Registration and Evaluation
2.3. Vaccine Safety Surveillance: Pharmacovigilance
2.4. Disease Surveillance, Vaccine Impact and Uptake
2.5. Community Engagement
| MMR disease and complications | MMR vaccine and complications |
|---|---|
| Measles Measles virus causes rash, cough, runny nose, eye irritation and fever. Complications include:
| Children should receive 2 doses of MMR vaccine.
Complications include: Mild problems
|
| Mumps Mumps virus causes fever, headache, muscle pain, loss of appetite and swollen glands. Complications include:
| |
| Rubella Rubella virus causes rash, arthritis (mostly in women) and mild fever. Complications include:
|
2.6. Crisis Management
2.7. Public Disclosure and Transparency
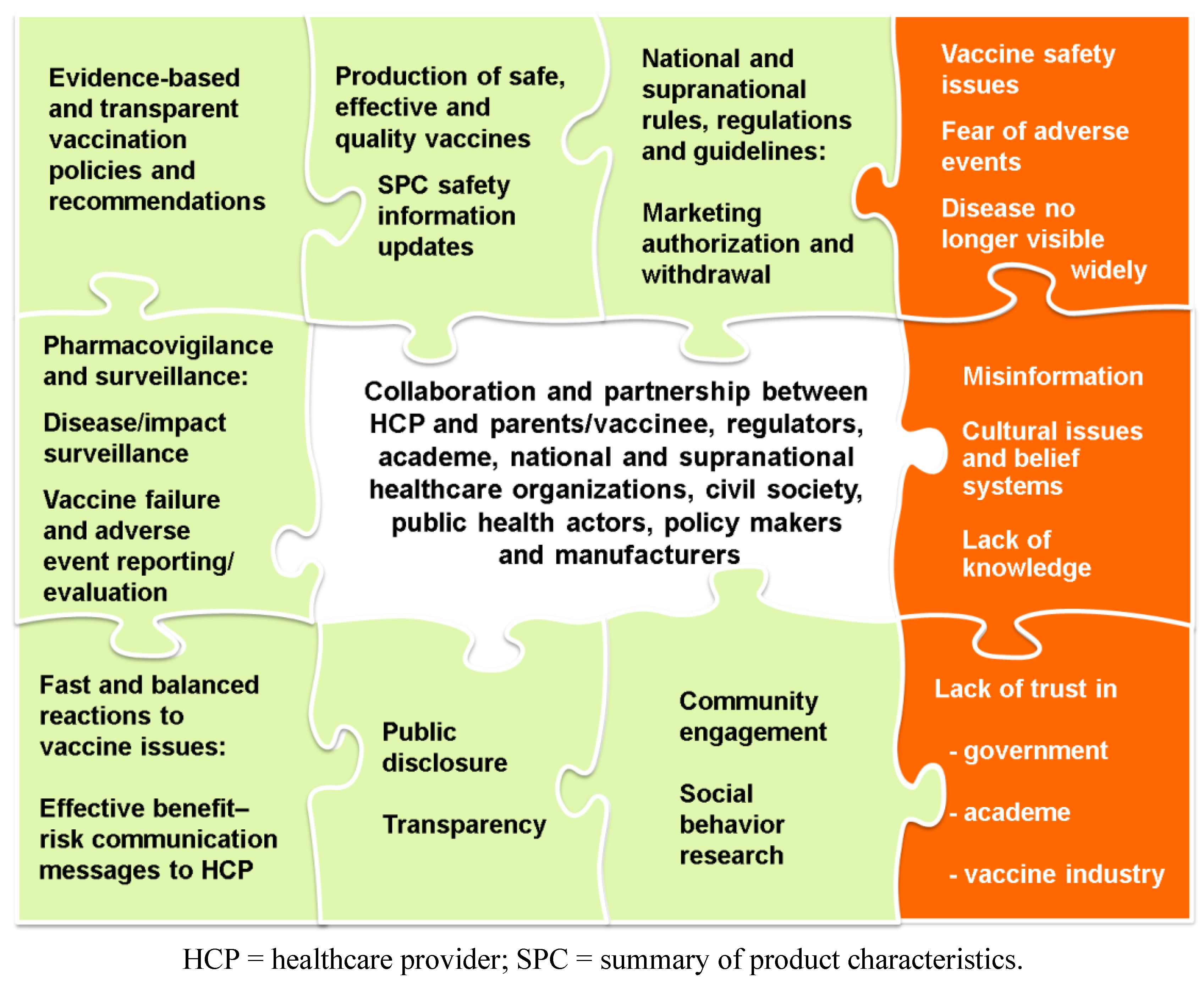
3. Conclusions: The Need for Partnerships
Acknowledgments
Conflict of Interest
Trademark Statement
References
- Duclos, P.; Okwo-Bele, J.M.; Gacic-Dobo, M.; Cherian, T. Global immunization: Status, progress, challenges and future. BMC Int. Health Hum. Rights 2009, 9, S2. [Google Scholar] [CrossRef]
- World Health Organization. Global routine vaccination coverage, 2010. Wkly. Epidemiol. Rec. 2011, 86, 509–513.
- Roush, S.W.; Murphy, T.V. Historical comparisons of morbidity and mortality for vaccine-preventable diseases in the United States. JAMA 2007, 298, 2155–2163. [Google Scholar] [CrossRef]
- WHO, UNICEF, World Bank; State of the World’s Vaccines and Immunization, 3rd ed.; World Health Organization: Geneva, Switzerland, 2009.
- Ozawa, S.; Mirelman, A.; Stack, M.L.; Walker, D.G.; Levine, O.S. Cost-effectiveness and economic benefits of vaccines in low- and middle-income countries: A systematic review. Vaccine 2012, 31, 96–108. [Google Scholar] [CrossRef]
- Stack, M.L.; Ozawa, S.; Bishai, D.M.; Mirelman, A.; Tam, Y.; Niessen, L.; Walker, D.G.; Levine, O.S. Estimated economic benefits during the “decade of vaccines” include treatment savings, gains in labor productivity. Health Aff. (Millwood) 2011, 30, 1021–1028. [Google Scholar]
- Bonanni, P.; Santos, J. Vaccine Evolution. In Understanding Modern Vaccines: Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Stanberry, L.R., Eds.; Elsevier B.V.: Amsterdam, the Netherlands, 2011; pp. 1–14. [Google Scholar]
- Blank, P.R.; Schwenkglenks, M.; Szucs, T.D. Disparities in influenza vaccination coverage rates by target group in five European countries: Trends over seven consecutive seasons. Infection 2009, 37, 390–400. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Global routine vaccination coverage, 2010. Morb. Mortal. Wkly. Rep. 2011, 60, 1520–1522.
- World Health Organization, Draft Global Vaccine Action Plan. Report by the Secretariat; World Health Organization: Geneva, Switzerland, 2012.
- Moxon, E.R.; Das, P.; Greenwood, B.; Heymann, D.L.; Horton, R.; Levine, O.S.; Plotkin, S.; Nossal, G. A call to action for the new decade of vaccines. Lancet 2011, 378, 298–302. [Google Scholar] [CrossRef]
- Bill & Melinda Gates Foundation. Bill and Melinda Gates pledge $10 billion in call for Decade of Vaccines. Increased vaccination could save more than 8 million children by 2020; significant funding gaps remain, others must join effort. Available online: http://www.gatesfoundation.org/ press-releases/Pages/decade-of-vaccines-wec-announcement-100129.aspx (accessed on 23 January 2013).
- Larson, H.J.; Cooper, L.Z.; Eskola, J.; Katz, S.L.; Ratzan, S. Addressing the vaccine confidence gap. Lancet 2011, 378, 526–535. [Google Scholar]
- Burnett, R.J.; Larson, H.J.; Moloi, M.H.; Tshatsinde, E.A.; Meheus, A.; Paterson, P.; Francois, G. Addressing public questioning and concerns about vaccination in South Africa: A guide for healthcare workers. Vaccine 2012, 30, C72–C78. [Google Scholar]
- Glanz, J.M.; Newcomer, S.R.; Narwaney, K.J.; Hambidge, S.J.; Daley, M.F.; Wagner, N.M.; McClure, D.L.; Xu, S.; Rowhani-Rahbar, A.; Lee, G.M.; et al. A population-based cohort study of undervaccination in 8 managed care organizations across the United States. JAMA 2013, 1–8. [Google Scholar]
- Kim, T.H.; Johnstone, J.; Loeb, M. Vaccine herd effect. Scand. J. Infect. Dis. 2011, 43, 683–689. [Google Scholar]
- World Health Organization, Global Vaccine Safety Blueprint; WHO/IVB/12.07; Quality, Safety and Standards unit of the Department of Immunization, Vaccines and Biologicals: Geneva, Switzerland, 2012.
- Pan American Health Organization, Immunization Safety. How to Address Events Allegedly Attributable to Vaccination or Immunization; PAHO/WHO: Washington, DC, USA, 2002.
- Lantos, J.D.; Jackson, M.A.; Opel, D.J.; Marcuse, E.K.; Myers, A.L.; Connelly, B.L. Controversies in vaccine mandates. Curr. Probl. Pediatr. Adolesc. Health Care 2010, 40, 38–58. [Google Scholar]
- Stanton, B.F. Assessment of relevant cultural considerations is essential for the success of a vaccine. J. Health Popul. Nutr. 2004, 22, 286–292. [Google Scholar]
- Lau, C.Y.; Stansbury, J.P.; Gust, D.A.; Kafaar, Z. Social and behavioral science in HIV vaccine trials: A gap assessment of the literature. Expert Rev. Vaccines 2009, 8, 179–190. [Google Scholar]
- Funk, S.; Salathé, M.; Jansen, V.A. Modelling the influence of human behaviour on the spread of infectious diseases: A review. J. R. Soc. Interface 2010, 7, 1247–1256. [Google Scholar] [CrossRef]
- Myers, L.B.; Goodwin, R. Determinants of adults’ intention to vaccinate against pandemic swine flu. BMC Public Health 2011, 11, 15. [Google Scholar]
- Connolly, T.; Reb, J. Toward interactive, Internet-based decision aid for vaccination decisions: Better information alone is not enough. Vaccine 2012, 30, 3813–3818. [Google Scholar]
- MacDonald, N.E.; Smith, J.; Appleton, M. Risk perception, risk management and safety assessment: What can governments do to increase public confidence in their vaccine system? Biologicals 2012, 40, 384–388. [Google Scholar]
- Muscat, M. Who gets measles in Europe? J. Infect. Dis. 2011, 204, S353–S365. [Google Scholar]
- Jones, A.M.; Omer, S.B.; Bednarczyk, R.A.; Halsey, N.A.; Moulton, L.H.; Salmon, D.A. Parents’ source of vaccine information and impact on vaccine attitudes, beliefs, and nonmedical exemptions. Adv. Prev. Med. 2012, 2012, 932741. [Google Scholar]
- Centers for Disease Control and Prevention, Some common misconceptions about vaccination and how to respond to them. Available online: http://www.cdc.gov/print.do?url=http://www.cdc.gov/vaccines/vac-gen/6mishome.htm/ (accessed on 2 January 2013).
- Betsch, C.; Brewer, N.T.; Brocard, P.; Davies, P.; Gaissmaier, W.; Haase, N.; Leask, J.; Renkewitz, F.; Renner, B.; Reyna, V.F.; et al. Opportunities and challenges of Web 2.0 for vaccination decisions. Vaccine 2012, 30, 3727–3733. [Google Scholar]
- Betsch, C.; Sachse, K. Dr. Jekyll or Mr. Hyde? (How) the Internet influences vaccination decisions: Recent evidence and tentative guidelines for online vaccine communication. Vaccine 2012, 30, 3723–3726. [Google Scholar]
- Kata, A. Anti-vaccine activists, Web 2.0, and the postmodern paradigm—An overview of tactics and tropes used online by the anti-vaccination movement. Vaccine 2012, 30, 3778–3789. [Google Scholar] [CrossRef]
- Poland, G.A.; Jacobson, R.M. The clinician’s guide to the anti-vaccinationists’ galaxy. Hum. Immunol. 2012, 73, 859–866. [Google Scholar] [CrossRef]
- Carrillo-Santisteve, P.; Lopalco, P.L. Measles still spreads in Europe: Who is responsible for the failure to vaccinate? Clin. Microbiol. Infect. 2012, 18, 50–56. [Google Scholar] [CrossRef]
- Muscat, M.; Bang, H.; Glismann, S. Measles is still a cause for concern in Europe. Eur. Surveill. 2008, 13. [Google Scholar]
- Jegede, A.S. What led to the Nigerian boycott of the polio vaccination campaign? PLoS Med. 2007, 4, e73. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Progress toward poliomyelitis eradication—Nigeria, January 2011–September 2012. MMWR Morb. Mortal. Wkly. Rep. 2012, 61, 899–904.
- Taylor, K.; Nguyen, A.; Stéphenne, J. The need for new vaccines. Vaccine 2009, 27, G3–G8. [Google Scholar] [CrossRef]
- Kennedy, A.; Lavail, K.; Nowak, G.; Basket, M.; Landry, S. Confidence about vaccines in the United States: Understanding parents’ perceptions. Health Aff. (Millwood) 2011, 30, 1151–1159. [Google Scholar]
- Blecher, M.S.; Meheus, F.; Kollipara, A.; Hecht, R.; Cameron, N.A.; Pillay, Y.; Hanna, L. Financing vaccinations—The South African experience. Vaccine 2012, 30, C79–C86. [Google Scholar] [CrossRef]
- Shen, A.K.; Spinner, J.R.; Salmon, D.A.; Gellin, B.G. Strengthening the U.S. vaccine and immunization enterprise: The role of the National Vaccine Advisory Committee. Public Health Rep. 2011, 126, 4–8. [Google Scholar]
- Duclos, P.; Ortynsky, S.; Abeysinghe, N.; Cakmak, N.; Janusz, C.B.; Jauregui, B.; Mihigo, R.; Mosina, L.; Sadr-Azodi, N.; Takashima, Y.; et al. Monitoring of progress in the establishment and strengthening of national immunization technical advisory groups. Vaccine 2012, 30, 7147–7152. [Google Scholar] [CrossRef]
- Bridges, C.B.; Woods, L.; Coyne-Beasley, T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. Morb. Mortal. Wkly. Rep. 2013, 62, 9–19. [Google Scholar]
- Akinsanya-Beysolow, I.; Jenkins, R.; Meissner, H.C. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for persons aged 0 through 18 years—United States, 2013. Morb. Mortal. Wkly. Rep. 2013, 62, 2–8. [Google Scholar]
- Marshall, V.; Baylor, N.W. Food and Drug Administration regulation and evaluation of vaccines. Pediatrics 2011, 127, S23–S30. [Google Scholar] [CrossRef]
- Lebron, J.A.; Wolf, J.J.; Kaplanski, C.V.; Ledwith, B.J. Ensuring the quality, potency and safety of vaccines during preclinical development. Expert Rev. Vaccines 2005, 4, 855–866. [Google Scholar] [CrossRef]
- European Medicines Agency, Guideline on the Conduct of Pharmacovigilance for Vaccines for Pre- and Post-exposure Prophylaxis against Infectious Diseases; European Medicines Agency: London, UK, 2009; EMEA/CHMP/PhVWP/503449/2007. Available online: http://www.ema. europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500011272.pdf (accessed on 30 May 2013).
- Leroux-Roels, G.; Bonanni, P.; Tantawichien, T.; Zepp, F. Vaccine Development. In Understanding Modern Vaccines: Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Stanberry, L.R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 115–150. [Google Scholar]
- Bonhoeffer, J.; Black, S.; Izurieta, H.; Zuber, P.; Sturkenboom, M. Current status and future directions of post-marketing vaccine safety monitoring with focus on USA and Europe. Biologicals 2012, 40, 393–397. [Google Scholar] [CrossRef]
- World Health Organization, Vaccine Introduction Guidelines. Adding a Vaccine to a National Immunization Program: Decision and Implementation; World Health Organization: Geneva, Switzerland, 2005; WHO/IVB/05.18.
- The National Vaccine Advisory Committee, A report of the National Vaccine Advisory Committee. Strengthening the supply of routinely recommended vaccines in the United States. Available online: http://www.hhs.gov/nvpo/nvac/nvac-vsr.html#problems/ (accessed on 3 January 2013).
- Strugnell, R.; Zepp, F.; Cunningham, A.L.; Tantawichien, T. Vaccine Antigens. In Understanding Modern Vaccines: Perspectives in Vaccinology; Garçon, N., Stern, P.L., Cunningham, A.L., Stanberry, L.R., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2011; pp. 61–88. [Google Scholar]
- Minor, P. Considerations for setting the specifications of vaccines. Expert Rev. Vaccines 2012, 11, 579–585. [Google Scholar] [CrossRef]
- Dellepiane, N.; Griffiths, E.; Milstien, J.B. New challenges in assuring vaccine quality. Bull. World Health Organ. 2000, 78, 155–162. [Google Scholar]
- Wilkie, D. The Chiron case: Good Manufacturing Practice gone bad. Available online: http://www.the-scientist.com/?articles.view/articleNo/16290/title/The-Chiron-Case--Good-Manufacturing-Practice-Gone-Bad/ (accessed on 23 January 2013).
- MacDonald, G. GSK pulls vaccine batch in Canada over contamination concerns. Available online: http://www.in-pharmatechnologist.com/Regulatory-Safety/GSK-pulls-vaccine-batch-in-Canada-over-contamination-concerns/ (accessed on 23 January 2013).
- Taylor, N. India plans $37m investment in vaccine cGMP compliance. Available online: http://www.in-pharmatechnologist.com/Processing/India-plans-37m-investment-in-vaccine-cGMP-compliance/ (accessed on 3 January 2013).
- Centers for Disease Control and Prevention, Recalled vaccines. Available online: http://www.cdc.gov/vaccines/recs/recalls/default.htm/ (accessed on 8 January 2013).
- World Health Organization, The Global Vaccine Safety Initiative (GVSI). Available online: http://www.who.int/vaccine_safety/initiative/en/ (accessed on 7 January 2013).
- ICH. ICH Harmonised Tripartite Guideline; ICH: Geneva, Switzerland, 2004. Available online: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E2E/Step4/E2E_Guideline.pdf (accessed on 30 May 2013).
- European Medicines Agency, Guideline on Good Pharmacovigilance Practices (GVP). Module VII—Periodic Safety Update Report; European Medicines Agency: London, UK, 2012; EMA/816292/2011.
- Chen, R.T.; Rastogi, S.C.; Mullen, J.R.; Hayes, S.W.; Cochi, S.L.; Donlon, J.A.; Wassilak, S.G. The Vaccine Adverse Event Reporting System (VAERS). Vaccine 1994, 12, 542–550. [Google Scholar] [CrossRef]
- European Medicines Agency. EudraVigilance. Available online: http://eudravigilance.ema. europa.eu/human/index.asp (accessed on 3 December 2012).
- Lieu, T.A.; Kulldorff, M.; Davis, R.L.; Lewis, E.M.; Weintraub, E.; Yih, K.; Yin, R.; Brown, J.S.; Platt, R. Real-time vaccine safety surveillance for the early detection of adverse events. Med. Care 2007, 45, S89–S95. [Google Scholar] [CrossRef]
- Baggs, J.; Gee, J.; Lewis, E.; Fowler, G.; Benson, P.; Lieu, T.; Naleway, A.; Klein, N.P.; Baxter, R.; Belongia, E.; et al. The Vaccine Safety Datalink: A model for monitoring immunization safety. Pediatrics 2011, 127, S45–S53. [Google Scholar] [CrossRef]
- Eurosurveillance Editorial Team. ECDC in collaboration with the VAESCO consortium to develop a complementary tool for vaccine safety monitoring in Europe. Euro Surveill. 2009, 14, 19345.
- Laverty, H.; Gunn, M.; Goldman, M. Improving R&D productivity of pharmaceutical companies through public-private partnership: Experiences from the Innovative Medicines Initiative. Expert Rev. Pharmacoecon. Outcomes Res. 2012, 12, 545–548. [Google Scholar] [CrossRef]
- World Health Organization. Global Advisory Committee on Vaccine Safety, 3–4 December 2009. Wkly. Epidemiol. Rec. 2010, 85, 29–33.
- Graham, J.E.; Borda-Rodriguez, A.; Huzair, F.; Zinck, E. Capacity for a global vaccine safety system: The perspective of national regulatory authorities. Vaccine 2012, 30, 4953–4959. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Intussusception among recipients of rotavirus vaccine—United States, 1998–1999. Morb. Mortal. Wkly. Rep. 1999, 48, 577–581.
- Centers for Disease Control and Prevention. Withdrawal of rotavirus vaccine recommendation. Morb. Mortal. Wkly. Rep. 1999, 48, 1007.
- Velázquez, F.R.; Colindres, R.E.; Grajales, C.; Hernández, M.T.; Mercadillo, M.G.; Torres, F.J.; Cervantes-Apolinar, M.; DeAntonio-Suarez, R.; Ortega-Barria, E.; Blum, M.; et al. Postmarketing surveillance of intussusception following mass introduction of the attenuated human rotavirus vaccine in Mexico. Pediatr. Infect. Dis. J. 2012, 31, 736–744. [Google Scholar] [CrossRef]
- Shui, I.M.; Baggs, J.; Patel, M.; Parashar, U.D.; Rett, M.; Belongia, E.A.; Hambidge, S.J.; Glanz, J.M.; Klein, N.P.; Weintraub, E. Risk of intussusception following administration of a pentavalent rotavirus vaccine in U.S. infants. JAMA 2012, 307, 598–604. [Google Scholar] [CrossRef]
- Buttery, J.P.; Danchin, M.H.; Lee, K.J.; Carlin, J.B.; McIntyre, P.B.; Elliott, E.J.; Booy, R.; Bines, J.E. Intussusception following rotavirus vaccine administration: Post-marketing surveillance in the National Immunization Program in Australia. Vaccine 2011, 29, 3061–3066. [Google Scholar] [CrossRef]
- Patel, M.M.; López-Collada, V.R.; Bulhões, M.M.; de Oliveira, L.H.; Bautista Márquez, A.; Flannery, B.; Esparza-Aguilar, M.; Montenegro Renoiner, E.I.; Luna-Cruz, M.E.; Sato, H.K.; et al. Intussusception risk and health benefits of rotavirus vaccination in Mexico and Brazil. N. Engl. J. Med. 2011, 364, 2283–2292. [Google Scholar] [CrossRef]
- Loughlin, J.; Mast, T.C.; Doherty, M.C.; Wang, F.T.; Wong, J.; Seeger, J.D. Postmarketing evaluation of the short-term safety of the pentavalent rotavirus vaccine. Pediatr. Infect. Dis. J. 2012, 31, 292–296. [Google Scholar] [CrossRef]
- World Health Organization. Rotavirus vaccines. WHO position paper—January 2013. Wkly. Epidemiol. Rec. 2013, 88, 49–64.
- Centers for Disease Control and Prevention. Progress in the introduction of rotavirus vaccine—Latin America and the Caribbean, 2006–2010. Morb. Mortal. Wkly. Rep. 2011, 60, 1611–1614.
- Tate, J.E.; Patel, M.M.; Cortese, M.M.; Lopman, B.A.; Gentsch, J.R.; Fleming, J.; Steele, A.D.; Parashar, U.D. Remaining issues and challenges for rotavirus vaccine in preventing global childhood diarrheal morbidity and mortality. Expert Rev. Vaccines 2012, 11, 211–220. [Google Scholar] [CrossRef]
- Gastañaduy, P.A.; Sánchez-Uribe, E.; Esparza-Aguilar, M.; Desai, R.; Parashar, U.D.; Patel, M.; Richardson, V. Effect of rotavirus vaccine on diarrhea mortality in different socioeconomic regions of Mexico. Pediatrics 2013, 131, e1115–e1120. [Google Scholar] [CrossRef]
- World Health Organization. Immunization surveillance, assessment and monitoring. WHO/UNICEF joint reporting process. Available online: http://www.who.int/immunization_monitoring/routine/joint_reporting/en/index.html#/ (accessed on 28 Janyary 2013).
- World Health Organization, WHO-recommended Standards for Surveillance of Selected Vaccine-preventable Diseases; World Health Organization: Geneva, Switzerland, 2003.
- VENICE II Consortium. Vaccination coverage assessment in EU/EEA, 2011. Available online: http://venice.cineca.org/Final_Vaccination_Coverage_Assesment_Survey_2011_1.pdf (accessed on 23 January 2013).
- Bonanni, P.; Levi, M.; Latham, N.B.; Bechini, A.; Tiscione, E.; Lai, P.; Panatto, D.; Gasparini, R.; Boccalini, S. An overview on the implementation of HPV vaccination in Europe. Hum. Vaccin. 2011, 7, S128–S135. [Google Scholar] [CrossRef]
- World Health Organization. Human papillomavirus vaccines. WHO position paper. Wkly. Epidemiol. Rec. 2009, 84, 118–131.
- Noakes, K.; Yarwood, J.; Salisbury, D. Parental response to the introduction of a vaccine against human papilloma virus. Hum. Vaccin. 2006, 2, 243–248. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Tsu, V.; Deeks, S.L.; Cubie, H.; Wang, S.A.; Vicari, A.S.; Brotherton, J.M. Human papillomavirus vaccine introduction—The first five years. Vaccine 2012, 30, F139–F148. [Google Scholar] [CrossRef]
- Kane, M.A.; Serrano, B.; de Sanjose, S.; Wittet, S. Implementation of human papillomavirus immunization in the developing world. Vaccine 2012, 30, F192–F200. [Google Scholar] [CrossRef]
- Watson-Jones, D.; Tomlin, K.; Remes, P.; Baisley, K.; Ponsiano, R.; Soteli, S.; de Sanjose, S.; Changalucha, J.; Kapiga, S.; Hayes, R.J. Reasons for receiving or not receiving HPV vaccination in primary schoolgirls in Tanzania: A case control study. PLoS One 2012, 7, e45231. [Google Scholar] [CrossRef]
- Leask, J.; Kinnersley, P.; Jackson, C.; Cheater, F.; Bedford, H.; Rowles, G. Communicating with parents about vaccination: A framework for health professionals. BMC Pediatr. 2012, 12, 154. [Google Scholar] [CrossRef]
- Simone, B.; Carrillo-Santisteve, P.; Lopalco, P.L. Healthcare workers role in keeping MMR vaccination uptake high in Europe: A review of evidence. Euro Surveill. 2012, 17, 20206. [Google Scholar]
- Steben, M.; Jeronimo, J.; Wittet, S.; Lamontagne, D.S.; Ogilvie, G.; Jensen, C.; Smith, J.; Franceschi, S. Upgrading public health programs for human papillomavirus prevention and control is possible in low- and middle-income countries. Vaccine 2012, 30, F183–F191. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention, MMR Vaccine. What You Need to Know. Available online: http://www.cdc.gov/vaccines/pubs/vis/downloads/vis-mmr.pdf (accessed on 15 April 2013).
- Centers for Disease Control and Prevention, Measles; Mumps; Rubella. In Epidemiology and Prevention of Vaccine-preventable Diseases, 12th ed.; Public Health Foundation: Washington DC, USA, 2012; pp. 173–192, 205–214, 275–290.
- World Health Organization. 3rd Global meeting on implementing new and under-utilized vaccines, 16–18 June 2009. Workgroup 8. Training of health staff and review of the Global Immunization Training Framework. Available online: http://www.who.int/nuvi/2009_meeting_ summary_training/en/ (accessed on 22 February 2013).
- Wakefield, A.J.; Murch, S.H.; Anthony, A.; Linnell, J.; Casson, D.M.; Malik, M.; Berelowitz, M.; Dhillon, A.P.; Thomson, M.A.; Harvey, P.; et al. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet 1998, 351, 637–641. [Google Scholar] [CrossRef]
- Hall, A.; Kane, M.; Roure, C.; Meheus, A. Multiple sclerosis and hepatitis B vaccine? Vaccine 1999, 17, 2473–2475. [Google Scholar]
- Gangarosa, E.J.; Galazka, A.M.; Wolfe, C.R.; Phillips, L.M.; Gangarosa, R.E.; Miller, E.; Chen, R.T. Impact of anti-vaccine movements on pertussis control: The untold story. Lancet 1998, 351, 356–361. [Google Scholar] [CrossRef]
- Ascherio, A.; Zhang, S.; Hernan, M.; Olek, M.; Coplan, P.; Brodovicz, K. Hepatitis B vaccination and the risk of multiple sclerosis: Case-control studies. Gastroenterol. Clin. Biol. 2001, 25, 927–929. [Google Scholar]
- Baleta, A.F.; van den Heever, J.; Burnett, R.J. Meeting the need for advocacy, social mobilisation and communication in the introduction of three new vaccines in South Africa—Successes and challenges. Vaccine 2012, 30, C66–C71. [Google Scholar] [CrossRef]
- World Health Organization, Communication for Behavioural Impact (COMBI). A Toolkit for Behavioural and Social Communication in Outbreak Response; World Health Organization: Geneva, Switzerland, 2012; WHO/HSE/GCR/2012.13.
- Wallace, C.; Leask, J.; Trevena, L.J. Effects of a web based decision aid on parental attitudes to MMR vaccination: A before and after study. BMJ 2006, 332, 146–149. [Google Scholar] [CrossRef]
- London School of Hygiene & Tropical Medicine. The Vaccine Confidence Project. About the VCI. Available online: http://www.vaccineconfidence.org/VCI.html/ (accessed on 24 January 2013).
- Osterhaus, A.D.; Vanlangendonck, C. About courageous scientists, responsible policy makers, bridge-builders and preparedness for the next influenza pandemic. Vaccine 2012, 30, 7437–7438. [Google Scholar] [CrossRef]
- Lexchin, J.; Bero, L.A.; Djulbegovic, B.; Clark, O. Pharmaceutical industry sponsorship and research outcome and quality: Systematic review. BMJ 2003, 326, 1167–1170. [Google Scholar] [CrossRef]
- Smith, R. Medical journals and pharmaceutical companies: Uneasy bedfellows. BMJ 2003, 326, 1202–1205. [Google Scholar] [CrossRef]
- Melander, H.; Ahlqvist-Rastad, J.; Meijer, G.; Beermann, B. Evidence b(i)ased medicine—Selective reporting from studies sponsored by pharmaceutical industry: Review of studies in new drug applications. BMJ 2003, 326, 1171–1173. [Google Scholar] [CrossRef]
- Godlee, F. Doctors, patients, and the drug industry. BMJ 2009, 338, b463. [Google Scholar] [CrossRef]
- Lundh, A.; Sismondo, S.; Lexchin, J.; Busuioc, O.A.; Bero, L. Industry sponsorship and research outcome. Cochrane Database Syst. Rev. 2012, 12, MR000033. [Google Scholar]
- Herxheimer, A. Relationships between the pharmaceutical industry and patients’ organisations. BMJ 2003, 326, 1208–1210. [Google Scholar] [CrossRef]
- Abbasi, K.; Smith, R. No more free lunches. BMJ 2003, 326, 1155–1156. [Google Scholar] [CrossRef]
- Moynihan, R. Is the relationship between pharma and medical education on the rocks? BMJ 2008, 337, a925. [Google Scholar] [CrossRef]
- Moynihan, R. Key opinion leaders: Independent experts or drug representatives in disguise? BMJ 2008, 336, 1402–1403. [Google Scholar]
- Coombes, R. Drug industry’s new code criticised for lacking teeth. BMJ 2005, 331, 1225. [Google Scholar] [CrossRef]
- Maeda, Y.; Miyahara, M. Determinants of trust in industry, government, and citizen’s groups in Japan. Risk Anal. 2003, 23, 303–310. [Google Scholar] [CrossRef]
- DeAngelis, C.D.; Drazen, J.M.; Frizelle, F.A.; Haug, C.; Hoey, J.; Horton, R.; Kotzin, S.; Laine, C.; Marusic, A.; Overbeke, A.J.; et al. Clinical trial registration: A statement from the International Committee of Medical Journal Editors. JAMA 2004, 292, 1363–1364. [Google Scholar] [CrossRef]
- ClinicalTrials.gov, a service of the U.S. National Institutes of Health. Available online: http://www.clinicaltrials.gov/ (accessed on 30 May 2013).
- Godlee, F.; Groves, T. The new BMJ policy on sharing data from drug and device trials. BMJ 2012, 345, e7888. [Google Scholar] [CrossRef]
- Anonymous. Toward clinical transparency. Nat. Med. 2012, 18, 1593. [Google Scholar] [CrossRef]
- Godlee, F. Clinical trial data for all drugs in current use. BMJ 2012, 345, e7304. [Google Scholar] [CrossRef]
- Coombes, R. Andrew Witty: The acceptable face of big pharma? BMJ 2013, 346, f1458. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hardt, K.; Schmidt-Ott, R.; Glismann, S.; Adegbola, R.A.; Meurice, F.P. Sustaining Vaccine Confidence in the 21st Century. Vaccines 2013, 1, 204-224. https://doi.org/10.3390/vaccines1030204
Hardt K, Schmidt-Ott R, Glismann S, Adegbola RA, Meurice FP. Sustaining Vaccine Confidence in the 21st Century. Vaccines. 2013; 1(3):204-224. https://doi.org/10.3390/vaccines1030204
Chicago/Turabian StyleHardt, Karin, Ruprecht Schmidt-Ott, Steffen Glismann, Richard A. Adegbola, and François P. Meurice. 2013. "Sustaining Vaccine Confidence in the 21st Century" Vaccines 1, no. 3: 204-224. https://doi.org/10.3390/vaccines1030204




