Assessment of Paratuberculosis Vaccination Effect on In Vitro Formation of Neutrophil Extracellular Traps in a Sheep Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ovine Neutrophils’ Isolation and Culture
2.3. Bacteria Culture and Infection
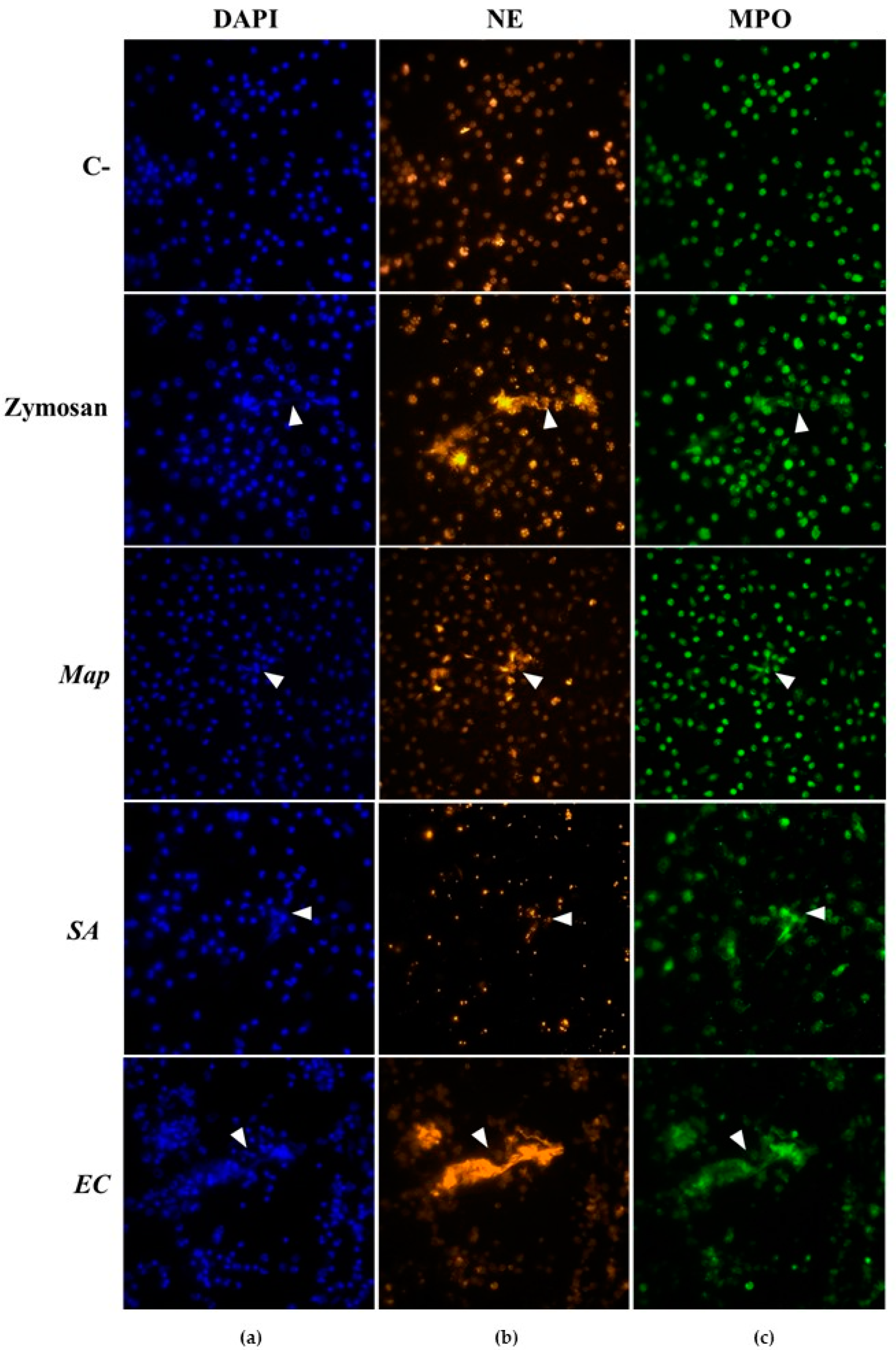
2.4. NETs Visualization
2.5. Quantification of Extracellular DNA Using PicoGreen®
2.6. Statistical Analysis
3. Results
3.1. NETs Visualization
3.2. Extracellular DNA Quantification
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rasmussen, P.; Barkema, H.W.; Mason, S.; Beaulieu, E.; Hall, D.C. Economic losses due to Johne’s disease (paratuberculosis) in dairy cattle. J. Dairy Sci. 2021, 104, 3123–3143. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Hearn, M.; Molina, E.; Geijo, M.; Vazquez, P.; Sevilla, I.A.; Garrido, J.M.; Juste, R.A. Immunization of adult dairy cattle with new heat-killed vaccine is associated with longer productive life prior to cows being sent to slaughter with suspected paratuberculosis. J. Dairy Sci. 2012, 95, 618–629. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P. Understanding the efficacy of vaccination in controlling ovine paratuberculosis. Small Rumin. Res. 2013, 110, 161–164. [Google Scholar] [CrossRef]
- Reddacliff, L.; Eppleston, J.; Windsor, P.; Whittington, R.; Jones, S. Efficacy of a killed vaccine for the control of paratuberculosis in Australian sheep flocks. Vet. Microbiol. 2006, 115, 77–90. [Google Scholar] [CrossRef] [PubMed]
- de Silva, K.; Plain, K.M.; Begg, D.J.; Purdie, A.C.; Whittington, R.J. CD4+ T-cells, γδ, T-cells and B-cells are associated with lack of vaccine protection in Mycobacterium avium subspecies paratuberculosis infection. Vaccine 2015, 33, 149–155. [Google Scholar] [CrossRef]
- Zurbrick, B.G.; Czuprynski, C.J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect. Immun. 1987, 55, 1588–1593. [Google Scholar] [CrossRef]
- Gollnick, N.S.; Mitchell, R.M.; Baumgart, M.; Janagama, H.K.; Sreevatsan, S.; Schukken, Y.H. Survival of Mycobacterium avium subsp. paratuberculosis in bovine monocyte-derived macrophages is not affected by host infection status but depends on the infecting bacterial genotype. Vet. Immunol. Immunopathol. 2007, 120, 93–105. [Google Scholar] [CrossRef]
- Taka, S.; Liandris, E.; Gazouli, M.; Sotirakoglou, K.; Theodoropoulos, G.; Bountouri, M.; Andreadou, M.; Ikonomopoulos, J. In vitro expression of the SLC11A1 gene in goat monocyte-derived macrophages challenged with Mycobacterium avium subsp paratuberculosis. Infect. Genet. Evol. 2013, 17, 8–15. [Google Scholar] [CrossRef]
- Johansen, M.D.; de Silva, K.; Plain, K.M.; Whittington, R.J.; Purdie, A.C. Mycobacterium avium subspecies paratuberculosis is able to manipulate host lipid metabolism and accumulate cholesterol within macrophages. Microb. Pathog. 2019, 130, 44–53. [Google Scholar] [CrossRef]
- Khare, S.; Nunes, J.S.; Figueiredo, J.F.; Lawhon, S.D.; Rossetti, C.A.; Gull, T.; Rice-Ficht, A.C.; Adams, L.G. Early phase morphological lesions and transcriptional responses of bovine ileum infected with Mycobacterium avium subsp. paratuberculosis. Vet. Pathol. 2009, 46, 717–728. [Google Scholar] [CrossRef] [Green Version]
- Bendixen, P. Application of the direct leukocyte-migration agarose test in cattle naturally infected with Mycobacterium paratuberculosis. Am. J. Vet. Res. 1977, 38, 1161–1162. [Google Scholar] [PubMed]
- Dotta, U.; Guglielmino, R.; Cagnasso, A.; D’Angelo, A.; Prato, S.; Bosso, M. Effects of subclinical bovine paratuberculosis on in-vitro polymorphonuclear neutrophil migration. J. Comp. Path. 1999, 121, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Ladero-Auñon, I.; Molina, E.; Holder, A.; Kolakowski, J.; Harris, H.; Urkitza, A.; Anguita, J.; Werling, D.; Elguezabal, N. Bovine neutrophils release extracellular traps and cooperate with macrophages in Mycobacterium avium subsp. paratuberculosis clearance In vitro. Front. Immunol. 2021, 12, 645304. [Google Scholar] [PubMed]
- Pooley, H.B.; Plain, K.M.; Purdie, A.C.; Begg, D.J.; Whittington, R.J.; de Silva, K. Integrated vaccine screening system: Using cellular functional capacity in vitro to assess genuine vaccine protectiveness in ruminants. Pathog. Dis. 2018, 76, fty029. [Google Scholar] [CrossRef]
- Arteche-Villasol, N.; Gutiérrez-Expósito, D.; Vallejo, R.; Espinosa, J.; Elguezabal, N.; Ladero-Auñon, I.; Royo, M.; Ferreras, M.C.; Benavides, J.; Pérez, V. Early response of monocyte-derived macrophages from vaccinated and non-vaccinated goats against in vitro infection with Mycobacterium avium subsp. paratuberculosis. Vet. Res. 2021, 52, 69. [Google Scholar]
- Ladero-Auñon, I.; Molina, E.; Oyanguren, M.; Barriales, D.; Fuertes, M.; Sevilla, I.A.; Luo, L.; Arrazuria, R.; De Buck, J.; Anguita, J.; et al. Oral vaccination stimulates neutrophil functionality and exerts protection in a Mycobacterium avium subsp. paratuberculosis infection model. NPJ Vaccines 2021, 6, 102. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.N. Trained immunity: A memory for innate host defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG vaccination induces long-term functional reprogramming of human neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef]
- Fernández, M.; Benavides, J.; Sevilla, I.A.; Fuertes, M.; Castaño, P.; Delgado, L.; García Marín, J.F.; Garrido, J.M.; Ferrera, M.C.; Pérez, V. Experimental infection of lambs with C and S-type strains of Mycobacterium avium subspecies paratuberculosis: Immunological and pathological findings. Vet. Res. 2014, 45, 5. [Google Scholar] [CrossRef]
- Arteche-Villasol, N.; Benavides, J.; Espinosa, J.; Vallejo, R.; Royo, M.; Ferreras, M.C.; Pérez, V.; Gutiérrez-Expósito, D. Optimized In vitro isolation of different subpopulation of immune cells from peripheral blood and comparative techniques for generation of monocyte-derived macrophages in small ruminants. Vet. Immunol. Immunopathol. 2020, 230, 110131. [Google Scholar] [CrossRef]
- Villagra-Blanco, R.; Silva, L.M.R.; Muñoz-Caro, T.; Yang, Z.; Li, J.; Gärtner, U.; Taubert, A.; Zhang, X.; Hermosilla, C. Bovine polymorphonuclear neutrophils cast neutrophil extracellular traps against the abortive parasite Neospora caninum. Front. Immunol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Caro, T.; Hermosilla, C.; Silva, L.M.R.; Cortes, H.; Taubert, A. Neutrophil extracellular traps as innate immune reaction against the emerging apicomplexan parasite Besnoitia besnoiti. PLoS ONE 2014, 9, e91415. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Newton, S.M.; Wilkinson, K.A.; Kampmann, B.; Hall, B.M.; Nawroly, N.; Packe, G.E.; Davidson, R.N.; Griffiths, C.J.; Wilkinson, R.J. Neutrophil-mediated innate immune resistance to mycobacteria. J. Clin. Investig. 2007, 117, 1988–1994. [Google Scholar] [CrossRef] [PubMed]
- Speziale, P.; Pietrocola, G. Staphylococcus aureus induces neutrophil extracellular traps (NETs) and neutralizes their bactericidal potential. Comput. Struct. Biotechnol. J. 2021, 19, 3451–3457. [Google Scholar] [CrossRef] [PubMed]
- Petretto, A.; Bruschi, M.; Pratesi, F.; Croia, C.; Candiano, G.; Ghiggeri, G.; Migliorini, P. Neutrophil extracellular traps (NET) induced by different stimuli: A comparative proteomic analysis. PLoS ONE 2019, 14, e0218946. [Google Scholar]
- Pilsczek, F.H.; Salina, D.; Karen, K.H.P.; Fahey, C.; Yipp, B.G.; Sibley, C.D.; Robbins, S.M.; Green, F.H.Y.; Surette, M.G.; Sugai, M.; et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J. Immunol. 2010, 185, 7413–7425. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Ramos-Kichik, V.; Mondragón-Flores, R.; Mondragón-Castelán, M.; Gonzalez-Pozos, S.; Muñiz-Hernandez, S.; Rojas-Espinosa, O.; Chacón-Salinas, R.; Estrada-Parra, S.; Estrada-García, I. Neutrophil extracellular traps are induced by Mycobacterium tuberculosis. Tuberculosis 2009, 89, 29–37. [Google Scholar] [CrossRef]
- Trentini, M.M.; de Oliveira, F.M.; Kipnis, A.; Junqueira-Kipnis, A.P. The role of neutrophils in the induction of specific Th1 and Th17 during vaccination against tuberculosis. Front. Microbiol. 2016, 7, 898. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arteche-Villasol, N.; Gutiérrez-Expósito, D.; Criado, M.; Benavides, J.; Pérez, V. Assessment of Paratuberculosis Vaccination Effect on In Vitro Formation of Neutrophil Extracellular Traps in a Sheep Model. Vaccines 2022, 10, 1403. https://doi.org/10.3390/vaccines10091403
Arteche-Villasol N, Gutiérrez-Expósito D, Criado M, Benavides J, Pérez V. Assessment of Paratuberculosis Vaccination Effect on In Vitro Formation of Neutrophil Extracellular Traps in a Sheep Model. Vaccines. 2022; 10(9):1403. https://doi.org/10.3390/vaccines10091403
Chicago/Turabian StyleArteche-Villasol, Noive, Daniel Gutiérrez-Expósito, Miguel Criado, Julio Benavides, and Valentín Pérez. 2022. "Assessment of Paratuberculosis Vaccination Effect on In Vitro Formation of Neutrophil Extracellular Traps in a Sheep Model" Vaccines 10, no. 9: 1403. https://doi.org/10.3390/vaccines10091403




