Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Measurement of IgG Antibody against Nucleoprotein and Neutralizing Activity
2.3. Microbial Protein Microarray
2.4. Searching for Antibodies against Microbial Antigens Associated with COVID-19 Antibody Acquisition Using Machine Learning
2.5. Statistical Analysis
3. Results
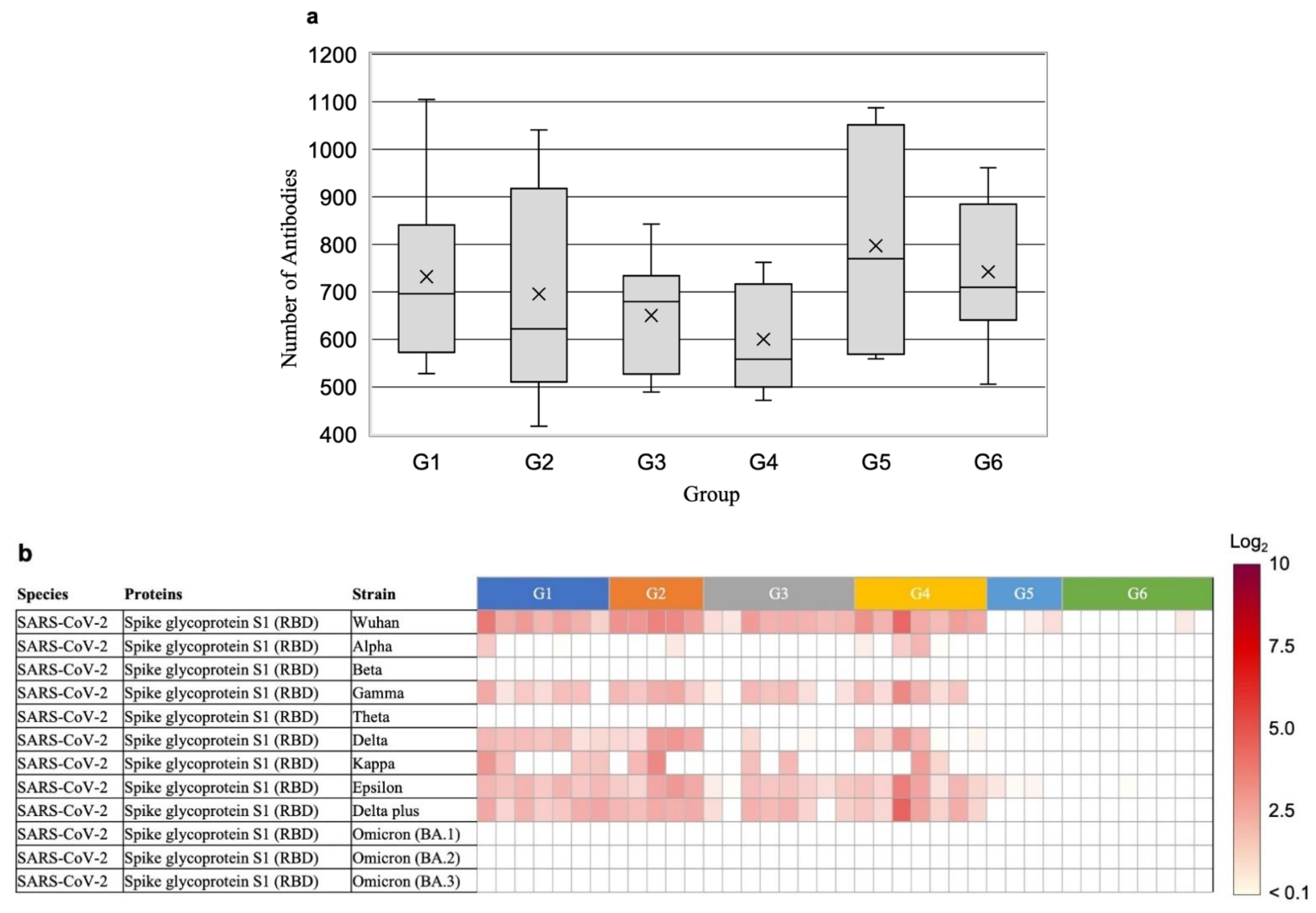
3.1. Effect of Vaccination on Antibody Profiles of Microbial Antigens
3.2. Relationship between Neutralizing Antibody Production and Antibody Profiles against Microbial Antigens after Vaccination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 5 September 2023).
- Kosaka, M.; Hashimoto, T.; Ozaki, A.; Tanimoto, T.; Kami, M. Delayed COVID-19 vaccine roll-out in Japan. Lancet 2021, 397, 2334–2335. [Google Scholar] [CrossRef] [PubMed]
- COVID-19 Vaccines. Available online: https://japan.kantei.go.jp/ongoingtopics/vaccine.html (accessed on 5 September 2023).
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Bukhari, I.A.; Akram, J.; Meo, A.S.; Klonoff, D.C. COVID-19 vaccines: Comparison of biological, pharmacological characteristics and adverse effects of Pfizer/BioNTech and Moderna Vaccines. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1663–1669. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Wang, M.; Islam, M.S.; Liao, P.; Hu, Y.; Chen, X. Humoral and Cellular Immune Responses of COVID-19 vaccines against SARS-Cov-2 Omicron variant: A systemic review. Int. J. Biol. Sci. 2022, 18, 4629–4641. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, Y.; Kawamura, T.; Shimazu, Y.; Zhao, T.; Sugiyama, A.; Nakayama, A.; Kaneko, Y.; Nishikawa, Y.; Omata, F.; Takita, M.; et al. Humoral immunity after second dose of BNT162b2 vaccine in Japanese communities: An observational cross-sectional study, Fukushima Vaccination Community Survey. Sci. Rep. 2022, 12, 18929. [Google Scholar] [CrossRef]
- Zhao, T.; Nishi-Uchi, T.; Omata, F.; Takita, M.; Kawashima, M.; Nishikawa, Y.; Yamamoto, C.; Kobashi, Y.; Kawamura, T.; Shibuya, K.; et al. Humoral response to SARS-CoV-2 vaccination in haemodialysis patients and a matched cohort. BMJ Open 2022, 12, e065741. [Google Scholar] [CrossRef]
- Kawashima, M.; Saito, H.; Nishiuchi, T.; Yoshimura, H.; Wakui, M.; Tani, Y.; Nishikawa, Y.; Omata, F.; Takita, M.; Zhao, T.; et al. Antibody and T-Cell Responses against SARS-CoV-2 after Booster Vaccination in Patients on Dialysis: A Prospective Observational Study. Vaccines 2023, 11, 260. [Google Scholar] [CrossRef]
- Tani, Y.; Takita, M.; Kobashi, Y.; Wakui, M.; Zhao, T.; Yamamoto, C.; Saito, H.; Kawashima, M.; Sugiura, S.; Nishikawa, Y.; et al. Varying Cellular Immune Response against SARS-CoV-2 after the Booster Vaccination: A Cohort Study from Fukushima Vaccination Community Survey, Japan. Vaccines 2023, 11, 920. [Google Scholar] [CrossRef]
- Nakamura, N.; Kobashi, Y.; Kim, K.S.; Tani, Y.; Shimazu, Y.; Zhao, T.; Nishikawa, Y.; Omata, F.; Kawashima, M.; Yoshida, M.; et al. Stratifying elicited antibody dynamics after two doses of SARS-CoV-2 vaccine in a community-based cohort in Fukushima, Japan. medrxiv 2022, preprint. [Google Scholar] [CrossRef]
- Nakamura, N.; Park, H.; Kim, K.S.; Sato, Y.; Jeong, Y.D.; Iwanami, S.; Fujita, Y.; Zhao, T.; Tani, Y.; Nishikawa, Y.; et al. A personalized antibody score for predicting individual COVID-19 vaccine-elicited antibody levels from basic demographic and health information. medrxiv 2022, preprint. [Google Scholar] [CrossRef]
- Van Loveren, H.; Van Amsterdam, J.G.; Vandebriel, R.J.; Kimman, T.G.; Rumke, H.C.; Steerenberg, P.S.; Vos, J.G. Vaccine-induced antibody responses as parameters of the influence of endogenous and environmental factors. Environ. Health Perspect. 2001, 109, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Kostinov, P.M.; Zhuravlev, I.P.; Filatov, N.N.; Kostinova Mcapital A, C.; Polishchuk, B.V.; Shmitko, D.A.; Mashilov, V.C.; Vlasenko, E.A.; Ryzhov, A.A. Gender Differences in the Level of Antibodies to Measles Virus in Adults. Vaccines 2021, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Ovsyannikova, I.G.; Jacobson, R.M.; Vierkant, R.A.; Pankratz, V.S.; Poland, G.A. HLA supertypes and immune responses to measles-mumps-rubella viral vaccine: Findings and implications for vaccine design. Vaccine 2007, 25, 3090–3100. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Winter, A.K.; Zhan, Z.; Ajelli, M.; Trentini, F.; Wang, L.; Li, F.; Yang, J.; Xiang, X.; et al. Long-term measles antibody profiles following different vaccine schedules in China, a longitudinal study. Nat. Commun. 2023, 14, 1746. [Google Scholar] [CrossRef] [PubMed]
- Kotwal, G.J. Microorganisms and their interaction with the immune system. J. Leukoc. Biol. 1997, 62, 415–429. [Google Scholar] [CrossRef]
- Triki, H.; Abdallah, M.V.; Ben Aissa, R.; Bouratbine, A.; Ben Ali Kacem, M.; Bouraoui, S.; Koubaa, C.; Zouari, S.; Mohsni, E.; Crainic, R.; et al. Influence of host related factors on the antibody response to trivalent oral polio vaccine in Tunisian infants. Vaccine 1997, 15, 1123–1129. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. The influence of the intestinal microbiome on vaccine responses. Vaccine 2018, 36, 4433–4439. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Factors That Influence the Immune Response to Vaccination. Clin. Microbiol. Rev. 2019, 32, e00084-18. [Google Scholar] [CrossRef]
- Hamuro, K.; Saito, H.; Saito, T.; Kohda, N. Identification of antigens recognized by salivary IgA using microbial protein microarrays. Biosci. Microbiota Food Health 2022, 41, 177–184. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Lundberg, S.M.; Lee, S.-I. A unified approach to interpreting model predictions. Adv. Neural Inf. Process. Syst. 2017, 4768–4777. [Google Scholar]
- Fiorelli, D.; Caruso, V.; Belardi, R.; Bernardini, S.; Nuccetelli, M. Evaluation of autoantibody profile in healthy subjects after mRNA vaccination against COVID-19. Int. Immunopharmacol. 2023, 122, 110592. [Google Scholar] [CrossRef]
- Uversky, V.N.; Redwan, E.M.; Makis, W.; Rubio-Casillas, A. IgG4 Antibodies Induced by Repeated Vaccination May Generate Immune Tolerance to the SARS-CoV-2 Spike Protein. Vaccines 2023, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Jaycox, J.R.; Lucas, C.; Yildirim, I.; Dai, Y.; Wang, E.Y.; Monteiro, V.; Lord, S.; Carlin, J.; Kita, M.; Buckner, J.H.; et al. SARS-CoV-2 mRNA vaccines decouple anti-viral immunity from humoral autoimmunity. Nat. Commun. 2023, 14, 1299. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, K.M.; Schuler, C.F.T.; Chen, J.; Troost, J.P.; Wong, P.T.; Chen, K.; O’Shea, D.R.; Peng, W.; Gherasim, C.; Manthei, D.M.; et al. Wild-type SARS-CoV-2 neutralizing immunity decreases across variants and over time but correlates well with diagnostic testing. Front. Immunol. 2023, 14, 1055429. [Google Scholar] [CrossRef] [PubMed]
- Kelly, J.D.; Leonard, S.; Hoggatt, K.J.; Boscardin, W.J.; Lum, E.N.; Moss-Vazquez, T.A.; Andino, R.; Wong, J.K.; Byers, A.; Bravata, D.M.; et al. Incidence of Severe COVID-19 Illness Following Vaccination and Booster With BNT162b2, mRNA-1273, and Ad26.COV2.S Vaccines. JAMA 2022, 328, 1427–1437. [Google Scholar] [CrossRef]
- Akkiz, H. The Biological Functions and Clinical Significance of SARS-CoV-2 Variants of Corcern. Front. Med. 2022, 9, 849217. [Google Scholar] [CrossRef]
- Lyke, K.E.; Atmar, R.L.; Islas, C.D.; Posavad, C.M.; Szydlo, D.; Paul Chourdhury, R.; Deming, M.E.; Eaton, A.; Jackson, L.A.; Branche, A.R.; et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep. Med. 2022, 3, 100679. [Google Scholar] [CrossRef]
- Jacobsen, H.; Strengert, M.; Maass, H.; Ynga Durand, M.A.; Katzmarzyk, M.; Kessel, B.; Harries, M.; Rand, U.; Abassi, L.; Kim, Y.; et al. Diminished neutralization responses towards SARS-CoV-2 Omicron VoC after mRNA or vector-based COVID-19 vaccinations. Sci. Rep. 2022, 12, 19858. [Google Scholar] [CrossRef]
- Song, Y.C.; Liu, S.J.; Lee, H.J.; Liao, H.C.; Liu, C.T.; Wu, M.Y.; Yen, H.R. Humoral and cellular immunity in three different types of COVID-19 vaccines against SARS-CoV-2 variants in a real-world data analysis. J. Microbiol. Immunol. Infect. 2023, 56, 705–717. [Google Scholar] [CrossRef]
- Seidel, A.; Zanoni, M.; Gross, R.; Krnavek, D.; Erdemci-Evin, S.; von Maltitz, P.; Albers, D.P.J.; Conzelmann, C.; Liu, S.; Weil, T.; et al. BNT162b2 booster after heterologous prime-boost vaccination induces potent neutralizing antibodies and T cell reactivity against SARS-CoV-2 Omicron BA.1 in young adults. Front. Immunol. 2022, 13, 882918. [Google Scholar] [CrossRef] [PubMed]
- Nakaharai, K.; Nakazawa, Y.; Mishima, Y.; Saito, M.; Shinozaki, Y.; Yoshida, M. Association between a low response to rubella vaccination and reduced anti-severe acute respiratory syndrome coronavirus 2 immune response after vaccination with BNT162b2: A cross-sectional study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2023, 29, 253.e1–253.e5. [Google Scholar] [CrossRef] [PubMed]
- Rooijakkers, S.H.; Milder, F.J.; Bardoel, B.W.; Ruyken, M.; van Strijp, J.A.; Gros, P. Staphylococcal complement inhibitor: Structure and active sites. J. Immunol. 2007, 179, 2989–2998. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Minardi, L.M.; Kuenstner, J.T.; Zekan, S.M.; Kruzelock, R. Anti-microbial Antibodies, Host Immunity, and Autoimmune Disease. Front. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Shenoy, S. Gut microbiome, Vitamin D, ACE2 interactions are critical factors in immune-senescence and inflammaging: Key for vaccine response and severity of COVID-19 infection. Inflamm. Res. 2022, 71, 13–26. [Google Scholar] [CrossRef]
- Creus-Cuadros, A.; Tabusi, M.M.; Carpio-Arias, V.; Finlay, B.B. Gut microbiota, malnutrition, and immunity: COVID-19′s confounding triad. Cell Host Microbe 2023, 31, 851–855. [Google Scholar] [CrossRef]
- Grigg, J.B.; Sonnenberg, G.F. Host-Microbiota Interactions Shape Local and Systemic Inflammatory Diseases. J. Immunol. 2017, 198, 564–571. [Google Scholar] [CrossRef]
- Jiao, J.; Shen, Y.; Wang, P.; Zuo, K.; Yang, X.; Chen, M.; Dong, Y.; Li, J. Characterization of the Intestinal Microbiome in Healthy Adults over Sars-Cov-2 Vaccination. Front. Biosci. Landmark Ed. 2022, 27, 280. [Google Scholar] [CrossRef]



| ID a | Sex | Age (Years) | Date of Blood Sampling (d) | CoV-2IgG(N) d | |||
|---|---|---|---|---|---|---|---|
| 1st b | 2nd b | 3rd b | 4th c | ||||
| 1 | Male | 52 | 21 | 127 | 207 | 58 | ND |
| 2 | Female | 32 | 21 | 127 | 207 | 79 | ND |
| 3 | Male | 63 | 21 | 131 | 213 | 79 | ND |
| 4 | Male | 29 | 21 | 127 | 207 | 79 | ND |
| 5 | Male | 52 | 21 | 128 | 211 | 76 | ND |
| 6 | Female | 56 | 22 | 127 | 208 | 72 | ND |
| 7 | Male | 60 | 21 | 130 | 210 | 76 | ND |
| 8 | Female | 33 | 21 | 131 | 208 | 76 | ND |
| G1 | G2 | G3 | G4 | G5 | G6 | |
|---|---|---|---|---|---|---|
| Number of subjects (male/female) | 7 (5/2) | 5 (3/2) | 8 (3/5) | 7 (1/6) | 4 (3/1) | 8 (4/4) |
| Age (years), mean ± sd | 42.8 ± 15.5 | 53.9 ± 13.0 | 48.1 ± 12.7 | 50.0 ± 13.9 | 58.5 ± 22.2 | 70.3 ± 13.6 |
| Days since second vaccination (d), mean ± sd | 89.1 ± 16.0 | 99.8 ± 7.2 | 80.4 ± 3.0 | 77.9 ± 5.1 | 88.8 ± 5.1 | 92.9 ± 5.4 |
| CoV-2IgG(N) * | ND † | ND | ND | ND | ND | ND |
| CoV-2Nab ‡, (AU/mL) mean ± sd | 1299.2 ± 1286.7 | 659.8 ± 280.4 | 284.7 ± 191.5 | 666.2 ± 261.8 | 37.5 ± 2.6 | 15.8 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, H.; Yoshimura, H.; Yoshida, M.; Tani, Y.; Kawashima, M.; Uchiyama, T.; Zhao, T.; Yamamoto, C.; Kobashi, Y.; Sawano, T.; et al. Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays. Vaccines 2023, 11, 1694. https://doi.org/10.3390/vaccines11111694
Saito H, Yoshimura H, Yoshida M, Tani Y, Kawashima M, Uchiyama T, Zhao T, Yamamoto C, Kobashi Y, Sawano T, et al. Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays. Vaccines. 2023; 11(11):1694. https://doi.org/10.3390/vaccines11111694
Chicago/Turabian StyleSaito, Hiroaki, Hiroki Yoshimura, Makoto Yoshida, Yuta Tani, Moe Kawashima, Taiga Uchiyama, Tianchen Zhao, Chika Yamamoto, Yurie Kobashi, Toyoaki Sawano, and et al. 2023. "Antibody Profiling of Microbial Antigens in the Blood of COVID-19 mRNA Vaccine Recipients Using Microbial Protein Microarrays" Vaccines 11, no. 11: 1694. https://doi.org/10.3390/vaccines11111694






