An Analysis of the Neutralizing Antibodies against the Main SARS-CoV-2 Variants in Healthcare Workers (HCWs) Vaccinated against or Infected by SARS-CoV-2
Abstract
:1. Introduction
2. Materials and Methods
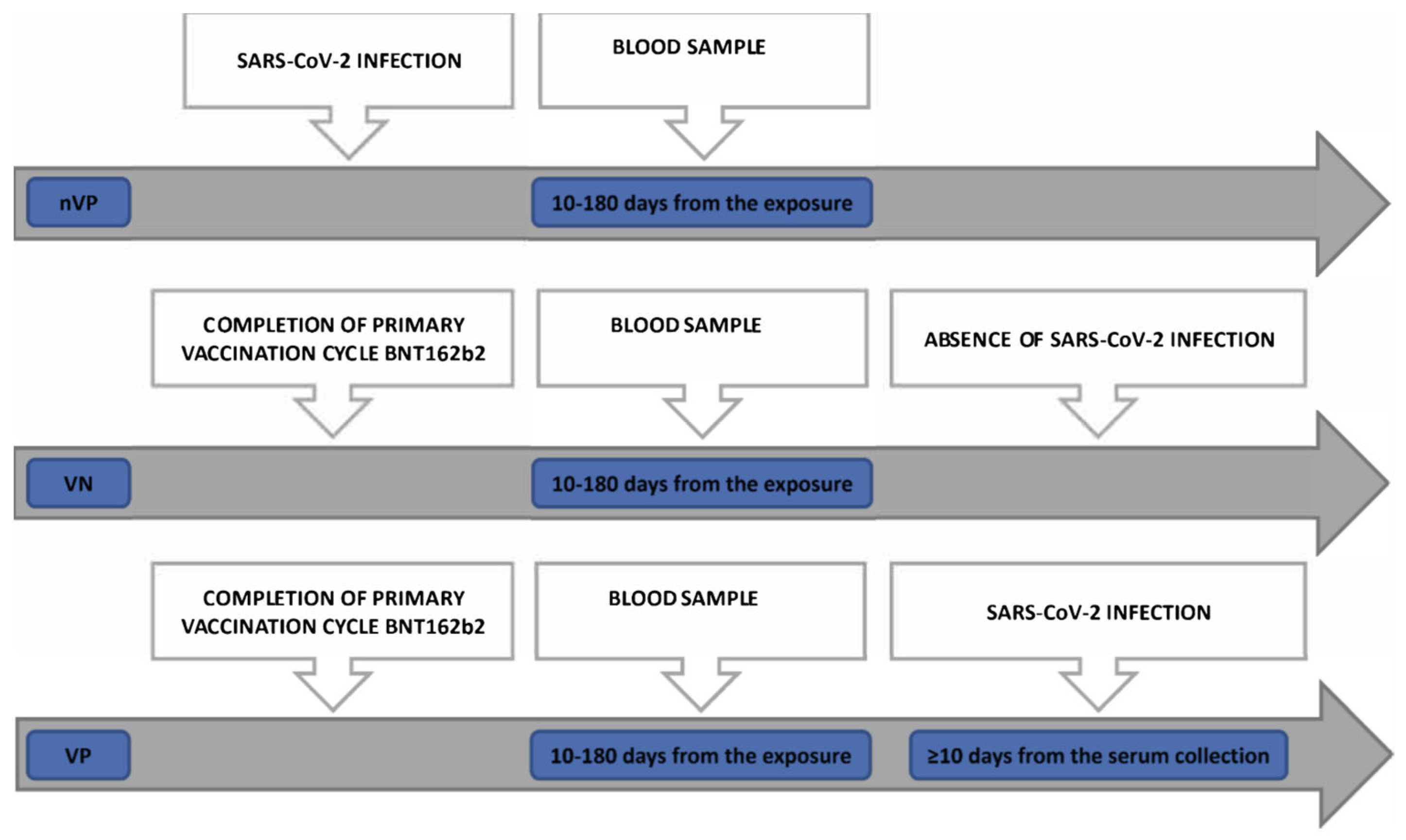
2.1. Population
- A non-vaccinated positive (nVP) cohort: unvaccinated subjects who were diagnosed as being positive for SARS-CoV-2 infection by molecular tests on nasopharyngeal swab (NPS) samples between August 2020 and March 2021 and whose sera were collected 10 to 180 days after the positive tests for COVID-19
- A vaccinated positive (VP) cohort: subjects who received 2 doses of BNT162b2 and were successively diagnosed as being positive for SARS-CoV-2 using RT-PCR molecular tests with NPS samples and whose sera were collected 10 to 180 days from administration of the second dose of vaccine, encompassing the humoral response peak and the decline of the humoral response [17,18], and at least 10 days before any diagnosis of infection (“positivization”) with the aim of avoiding evaluating the neutralization mediated by the antibodies elicited during an early asymptomatic phase of SARS-CoV-2 infection
- A vaccinated non-positive (VN) cohort: subjects who received 2 doses of BNT162b2, without evidence of post-vaccination SARS-CoV-2 infections, with sera collected 10 to 180 days after the second vaccine dose administration
2.2. Virus Neutralization Assay
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bakhiet, M.; Taurin, S. SARS-CoV-2: Targeted Managements and Vaccine Development. Cytokine Growth Factor Rev. 2021, 58, 16–29. [Google Scholar] [CrossRef]
- Chu, H.; Chan, J.F.; Yuen, T.T.; Shuai, H.; Yuan, S.; Wang, Y.; Hu, B.; Yip, C.C.; Tsang, J.O.; Huang, X.; et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: An observational study. Lancet Microbe 2020, 1, e14–e23. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef]
- Lu, X.; Zhang, L.; Du, H.; Zhang, J.; Li, Y.Y.; Qu, J.; Zhang, W.; Wang, Y.; Bao, S.; Li, Y.; et al. SARS-CoV-2 Infection in Children. N. Engl. J. Med. 2020, 382, 1663–1665. [Google Scholar] [CrossRef] [PubMed]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/Director-General/Speeches/Detail/Who-Director-General-s-Opening-Remarks-at-the-Media-Briefing-on-Covid-19---11-March-2020 (accessed on 20 March 2023).
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An MRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 MRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, P.J.M.; Caniels, T.G.; van der Straten, K.; Snitselaar, J.L.; Aldon, Y.; Bangaru, S.; Torres, J.L.; Okba, N.M.A.; Claireaux, M.; Kerster, G.; et al. Potent Neutralizing Antibodies from COVID-19 Patients Define Multiple Targets of Vulnerability. Science 2020, 369, 643–650. [Google Scholar] [CrossRef]
- Taylor, P.C.; Adams, A.C.; Hufford, M.M.; de la Torre, I.; Winthrop, K.; Gottlieb, R.L. Neutralizing Monoclonal Antibodies for Treatment of COVID-19. Nat. Rev. Immunol. 2021, 21, 382–393. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.W.X.; Chia, T.; Young, B.E. SARS-CoV-2 Variants of Concern and Vaccine Escape, from Alpha to Omicron and Beyond. Expert Rev. Respir. Med. 2022, 16, 499–502. [Google Scholar] [CrossRef]
- Amnesty Analysis Reveals over 7000 Health Workers Have Died from COVID-19 [Website]. London: Amnesty International; 3 September 2020. Available online: https://www.amnesty.org.uk/press-releases/More-7000-Health-Worker-Deaths-Covid-19-Globally-Uk-Deaths-Third-Highest (accessed on 20 March 2023).
- Gholami, M.; Khamis, A.H.; Ho, S.B. Response to “RE: COVID-19 and healthcare workers: A systematic review and meta-analysis”. Int. J. Infect Dis. 2021, 106, 140–141. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among Front-Line Health-Care Workers and the General Community: A Prospective Cohort Study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO)—Who Sage Roadmap for Prioritizing Uses of COVID-19 Vaccines in The Context of Limited Supply. An Approach to Inform Planning and Subsequent Recommendations Based upon Epidemiologic Setting and Vaccine Supply Scenarios, 13 November 2020. Available online: https://www.who.int/Docs/Default-Source/Immunization/Sage/Covid/Sage-Prioritization-Roadmap-Covid19-Vaccines.Pdf?Status=Temp&sfvrsn=bf227443_2 (accessed on 20 March 2023).
- Gholami, M.; Fawad, I.; Shadan, S.; Rowaiee, R.; Ghanem, H.; Khamis, A.H.; Ho, S.B. The COVID-19 Pandemic and Health and Care Workers: Findings From a Systematic Review and Meta Analysis (2020–2021). Int. J. Public Health 2023, 68, 1605421. [Google Scholar] [CrossRef]
- Grupel, D.; Gazit, S.; Schreiber, L.; Nadler, V.; Wolf, T.; Lazar, R.; Supino-Rosin, L.; Perez, G.; Peretz, A.; Ben Tov, A.; et al. Kinetics of SARS-CoV-2 Anti-S IgG after BNT162b2 Vaccination. Vaccine 2021, 39, 5337–5340. [Google Scholar] [CrossRef]
- Al-Sheboul, S.A.; Brown, B.; Shboul, Y.; Fricke, I.; Imarogbe, C.; Alzoubi, K.H. An Immunological Review of SARS-CoV-2 Infection and Vaccine Serology: Innate and Adaptive Responses to MRNA, Adenovirus, Inactivated and Protein Subunit Vaccines. Vaccines 2022, 11, 51. [Google Scholar] [CrossRef]
- Gandolfo, C.; Anichini, G.; Mugnaini, M.; Bocchia, M.; Terrosi, C.; Sicuranza, A.; Gori Savellini, G.; Gozzetti, A.; Franchi, F.; Cusi, M.G. Overview of Anti-SARS-CoV-2 Immune Response Six Months after BNT162b2 mRNA Vaccine. Vaccines 2022, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Bonura, F.; De Grazia, S.; Bonura, C.; Sanfilippo, G.L.; Giammanco, G.M.; Amodio, E.; Ferraro, D. Differing Kinetics of Anti-Spike Protein IgGs and Neutralizing Antibodies against SARS-CoV-2 after Comirnaty (BNT162b2) Immunization. J. Appl. Microbiol. 2022, 132, 3987–3994. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Laboratory Biosafety Guidance Related to Coronavirus Disease (COVID-19): Interim Guidance, 28 January 2021. Available online: https://www.who.int/Publications/i/Item/WHO-WPE-GIH-2021.1 (accessed on 20 March 2023).
- Reverberi, R. The Statistical Analysis of Immunohaematological Data. Blood Transfus. 2008, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2021. Available online: https://www.r-project.org/ (accessed on 20 March 2023).
- rStudio Team. rStudio: Integrated Development for R. rStudio, PBC, Boston, MA. 2020. Available online: http://www.rstudio.com/ (accessed on 20 March 2023).
- Istituto Superiore Di Sanità (ISS)—Prevalenza e Distribuzione Delle Varianti Del Virus SARS-CoV-2 Di Interesse per La Sanità Pubblica in Italia. Rapporto 19 Maggio 2021. Available online: https://www.epicentro.iss.it/Coronavirus/Pdf/Sars-Cov-2-Monitoraggio-Varianti-Rapporti-Periodici-19-Maggio-2021.Pdf (accessed on 20 March 2023).
- Istituto Superiore Di Sanità (ISS)—Prevalenza e Distribuzione Delle Varianti Del Virus SARS-CoV-2 Di Interesse per La Sanità Pubblica in Italia. Rapporto n. 2 Dell’11 Giugno 2021. Available online: https://www.epicentro.iss.it/Coronavirus/Pdf/Sars-Cov-2-Monitoraggio-Varianti-Rapporti-Periodici-11-Giugno-2021.Pdf (accessed on 20 March 2023).
- Istituto Superiore Di Sanità (ISS)—Prevalenza e Distribuzione Delle Varianti Di SARS-CoV-2 Di Interesse per La Sanità Pubblica in Italia. Rapporto n. 11 Del 15 Ottobre 2021. Available online: https://www.epicentro.iss.it/Coronavirus/Pdf/Sars-Cov-2-Monitoraggio-Varianti-Rapporti-Periodici-15-Ottobre-2021.Pdf (accessed on 20 March 2023).
- Istituto Superiore Di Sanità (ISS)—Prevalenza e Distribuzione Delle Varianti Di SARS-CoV-2 Di Interesse per La Sanità Pubblica in Italia. Rapporto n. 15 Del 10 Dicembre 2021. Available online: https://www.epicentro.iss.it/Coronavirus/Pdf/Sars-Cov-2-Monitoraggio-Varianti-Rapporti-Periodici-10-Dicembre-2021.Pdf (accessed on 20 March 2023).
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing Antibody Levels Are Highly Predictive of Immune Protection from Symptomatic SARS-CoV-2 Infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Cromer, D.; Steain, M.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Kent, S.J.; Triccas, J.A.; Khoury, D.S.; Davenport, M.P. Neutralising Antibody Titres as Predictors of Protection against SARS-CoV-2 Variants and the Impact of Boosting: A Meta-Analysis. Lancet Microbe 2022, 3, e52–e61. [Google Scholar] [CrossRef]
- Planas, D.; Saunders, N.; Maes, P.; Guivel-Benhassine, F.; Planchais, C.; Buchrieser, J.; Bolland, W.-H.; Porrot, F.; Staropoli, I.; Lemoine, F.; et al. Considerable Escape of SARS-CoV-2 Omicron to Antibody Neutralization. Nature 2022, 602, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nair, M.S.; Liu, L.; Iketani, S.; Luo, Y.; Guo, Y.; Wang, M.; Yu, J.; Zhang, B.; Kwong, P.D.; et al. Antibody Resistance of SARS-CoV-2 Variants B.1.351 and B.1.1.7. Nature 2021, 593, 130–135. [Google Scholar] [CrossRef] [PubMed]
- McCallum, M.; Bassi, J.; De Marco, A.; Chen, A.; Walls, A.C.; Di Iulio, J.; Tortorici, M.A.; Navarro, M.-J.; Silacci-Fregni, C.; Saliba, C.; et al. SARS-CoV-2 Immune Evasion by the B.1.427/B.1.429 Variant of Concern. Science 2021, 373, 648–654. [Google Scholar] [CrossRef]
- Noor, R. Host Protective Immunity against Severe Acute Respiratory Coronavirus 2 (SARS-CoV-2) and the COVID-19 Vaccine-Induced Immunity against SARS-CoV-2 and Its Variants. Viruses 2022, 14, 2541. [Google Scholar] [CrossRef] [PubMed]
- Kotaki, R.; Adachi, Y.; Moriyama, S.; Onodera, T.; Fukushi, S.; Nagakura, T.; Tonouchi, K.; Terahara, K.; Sun, L.; Takano, T.; et al. SARS-CoV-2 Omicron-Neutralizing Memory B Cells Are Elicited by Two Doses of BNT162b2 MRNA Vaccine. Sci. Immunol. 2022, 7, eabn8590. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Azman, A.S.; Sun, R.; Lu, W.; Zheng, N.; Zhou, J.; Wu, Q.; Deng, X.; Zhao, Z.; et al. Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis. Clin. Infect. Dis. 2022, 74, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, A.K.; Juno, J.A.; Wang, J.J.; Selva, K.J.; Reynaldi, A.; Tan, H.-X.; Lee, W.S.; Wragg, K.M.; Kelly, H.G.; Esterbauer, R.; et al. Evolution of Immune Responses to SARS-CoV-2 in Mild-Moderate COVID-19. Nat. Commun. 2021, 12, 1162. [Google Scholar] [CrossRef] [PubMed]
- Amodio, E.; Genovese, D.; Fallucca, A.; Ferro, P.; Sparacia, B.; D’Azzo, L.; Fertitta, A.; Maida, C.M.; Vitale, F. Clinical Severity in Different Waves of SARS-CoV-2 Infection in Sicily: A Model of Smith’s “Law of Declining Virulence” from Real-World Data. Viruses 2022, 15, 125. [Google Scholar] [CrossRef]
- Carreño, J.M.; Alshammary, H.; Tcheou, J.; Singh, G.; Raskin, A.J.; Kawabata, H.; Sominsky, L.A.; Clark, J.J.; Adelsberg, D.C.; Bielak, D.A.; et al. Activity of Convalescent and Vaccine Serum against SARS-CoV-2 Omicron. Nature 2022, 602, 682–688. [Google Scholar] [CrossRef]
- An, K.; Zhu, X.; Yan, J.; Xu, P.; Hu, L.; Bai, C. A Systematic Study on the Binding Affinity of SARS-CoV-2 Spike Protein to Antibodies. AIMS Microbiol. 2022, 8, 595–611. [Google Scholar] [CrossRef]
- Chen, R.E.; Zhang, X.; Case, J.B.; Winkler, E.S.; Liu, Y.; VanBlargan, L.A.; Liu, J.; Errico, J.M.; Xie, X.; Suryadevara, N.; et al. Resistance of SARS-CoV-2 Variants to Neutralization by Monoclonal and Serum-Derived Polyclonal Antibodies. Nat. Med. 2021, 27, 717–726. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhou, J.; Tian, M.; Huang, M.; Liu, S.; Xie, Y.; Han, P.; Bai, C.; Han, P.; Zheng, A.; et al. Omicron SARS-CoV-2 Mutations Stabilize Spike up-RBD Conformation and Lead to a Non-RBM-Binding Monoclonal Antibody Escape. Nat. Commun. 2022, 13, 4958. [Google Scholar] [CrossRef] [PubMed]
- Alidjinou, E.K.; Demaret, J.; Corroyer-Simovic, B.; Vuotto, F.; Miczek, S.; Labreuche, J.; Goffard, A.; Trauet, J.; Lupau, D.; Dendooven, A.; et al. Serum Neutralization of SARS Coronavirus 2 Omicron Sublineages BA.1 and BA.2 and Cellular Immune Responses 3 Months after Booster Vaccination. Clin. Microbiol. Infect. 2023, 29, 258.e1–258.e4. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Rickeard, T.; Gallagher, E.; Gower, C.; Kall, M.; Groves, N.; O’Connell, A.-M.; et al. COVID-19 Vaccine Effectiveness against the Omicron (B.1.1.529) Variant. N. Engl. J. Med. 2022, 386, 1532–1546. [Google Scholar] [CrossRef] [PubMed]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, S.N.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 Omicron Neutralization by Therapeutic Antibodies, Convalescent Sera, and Post-MRNA Vaccine Booster. BioRxiv 2021. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Chenchula, S.; Karunakaran, P.; Sharma, S.; Chavan, M. Current evidence on efficacy of COVID-19 booster dose vaccination against the Omicron variant: A systematic review. J. Med. Virol. 2022, 94, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Alu, A.; Lei, H.; Yang, J.; Hong, W.; Song, X.; Li, J.; Yang, L.; Wang, W.; Shen, G.; et al. A recombinant spike-XBB.1.5 protein vaccine induces broad-spectrum immune responses against XBB.1.5-included Omicron variants of SARS-CoV-2. MedComm 2023, 4, e263. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Ciesla, A.A.; Roper, L.E.; Scobie, H.M.; Ali, A.R.; Miller, J.D.; Wiegand, R.E.; Accorsi, E.K.; Verani, J.R.; Shang, N.; et al. Early Estimates of Bivalent mRNA Booster Dose Vaccine Effectiveness in Preventing Symptomatic SARS-CoV-2 Infection Attributable to Omicron BA.5- and XBB/XBB.1.5-Related Sublineages Among Immunocompetent Adults—Increasing Community Access to Testing Program, United States, December 2022–January 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 119–124. [Google Scholar] [CrossRef]



| nVP | VP | VN | p-Value | |
|---|---|---|---|---|
| No. of Subjects | 91 | 102 | 100 | |
| Sex, N (%) | ||||
| 56 (61.5%) | 59 (57.8%) | 62 (62%) | 0.804 |
| 35 (38.5%) | 43 (42.2%) | 38 (38%) | |
| Age in years, median (IQR) | 36 (29–51.5) | 32 (27–51) | 33.5 (28–51) | 0.485 |
| Days to serum collection from exposure, median (IQR) | 61 (31.5–102.5) | 51 (18.3–995) | 57.5 (18.8–104) | 0.140 |
| nVP | VP | VN | |
|---|---|---|---|
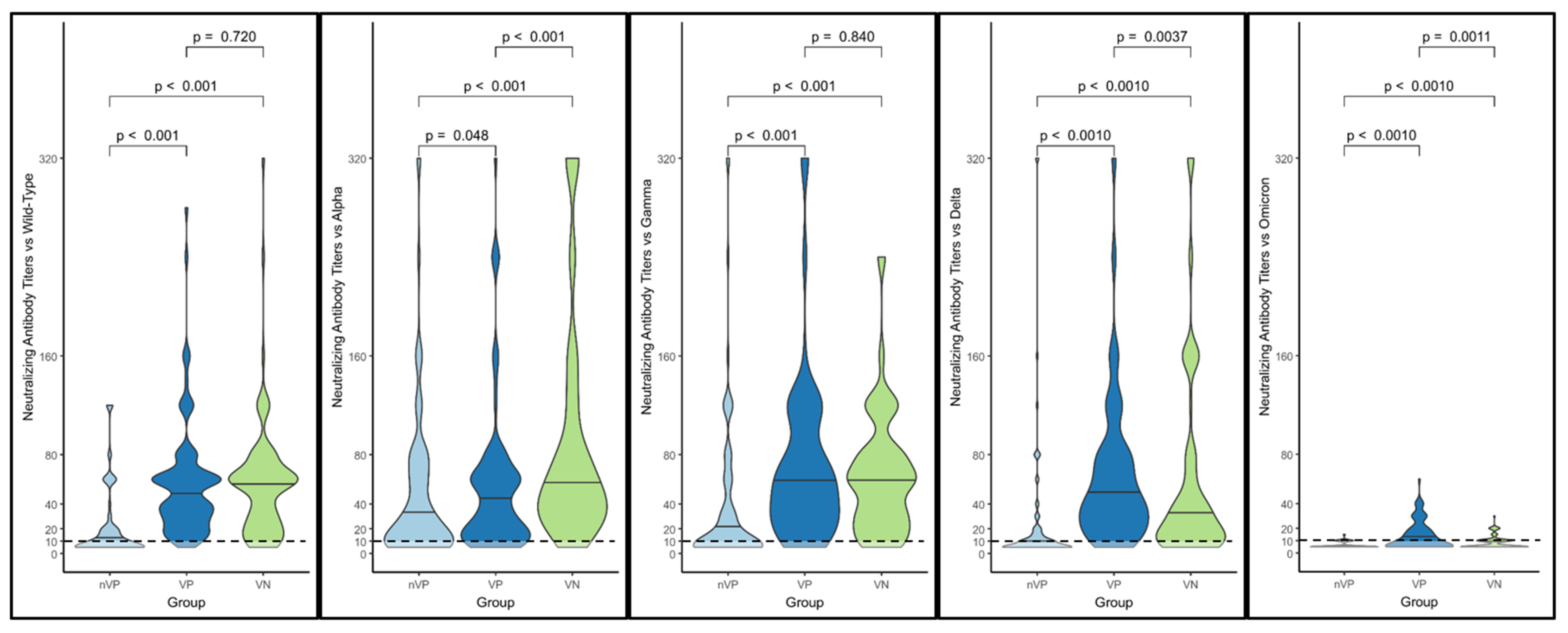
| Neutralizing antibody titers against the variants (reciprocal value of the sample dilution), geometric mean (±GSD) | |||
| - Wild-type | 11.68 (±2.72) | 43.92 (±2.11) | 41.10 (±2.42) |
| - Alpha | 24.11 (±3.40) | 33.25 (±2.71) | 60.90 (±2.67) |
| - Delta | 10.55 (±2.77) | 43.27 (±2.52) | 29.02 (±3.24) |
| - Gamma | 15.88 (±3.62) | 48.45 (±2.79) | 45.66 (±2.52) |
| - Omicron | 5.92 (±3.92) | 10.50 (±2.10) | 7.45 (±1.64) |
| Absence of neutralizing antibodies against the variants, N (%) | |||
| - Wild-type | 42 (46.15%) | 1 (0.98%) | 6 (6%) |
| - Alpha | 20 (21.74%) | 6 (5.88%) | 2 (2%) |
| - Delta | 45 (49.45%) | 4 (3.92%) | 12 (12%) |
| - Gamma | 40 (43.96%) | 7 (6.86%) | 6 (6%) |
| - Omicron | 70 (76.92%) | 43 (42.16%) | 56 (56%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Immordino, P.; Pisciotta, V.; Amodio, E.; Bonura, C.; Bonura, F.; Cacioppo, F.; Calamusa, G.; Capra, G.; Casuccio, A.; De Grazia, S.; et al. An Analysis of the Neutralizing Antibodies against the Main SARS-CoV-2 Variants in Healthcare Workers (HCWs) Vaccinated against or Infected by SARS-CoV-2. Vaccines 2023, 11, 1702. https://doi.org/10.3390/vaccines11111702
Immordino P, Pisciotta V, Amodio E, Bonura C, Bonura F, Cacioppo F, Calamusa G, Capra G, Casuccio A, De Grazia S, et al. An Analysis of the Neutralizing Antibodies against the Main SARS-CoV-2 Variants in Healthcare Workers (HCWs) Vaccinated against or Infected by SARS-CoV-2. Vaccines. 2023; 11(11):1702. https://doi.org/10.3390/vaccines11111702
Chicago/Turabian StyleImmordino, Palmira, Vincenzo Pisciotta, Emanuele Amodio, Celestino Bonura, Floriana Bonura, Federica Cacioppo, Giuseppe Calamusa, Giuseppina Capra, Alessandra Casuccio, Simona De Grazia, and et al. 2023. "An Analysis of the Neutralizing Antibodies against the Main SARS-CoV-2 Variants in Healthcare Workers (HCWs) Vaccinated against or Infected by SARS-CoV-2" Vaccines 11, no. 11: 1702. https://doi.org/10.3390/vaccines11111702










