SARS-CoV-2 Antibody Dynamics after COVID-19 Vaccination and Infection: A Real-World Cross-Sectional Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Serologic Assessment
2.3. Statistical Analysis
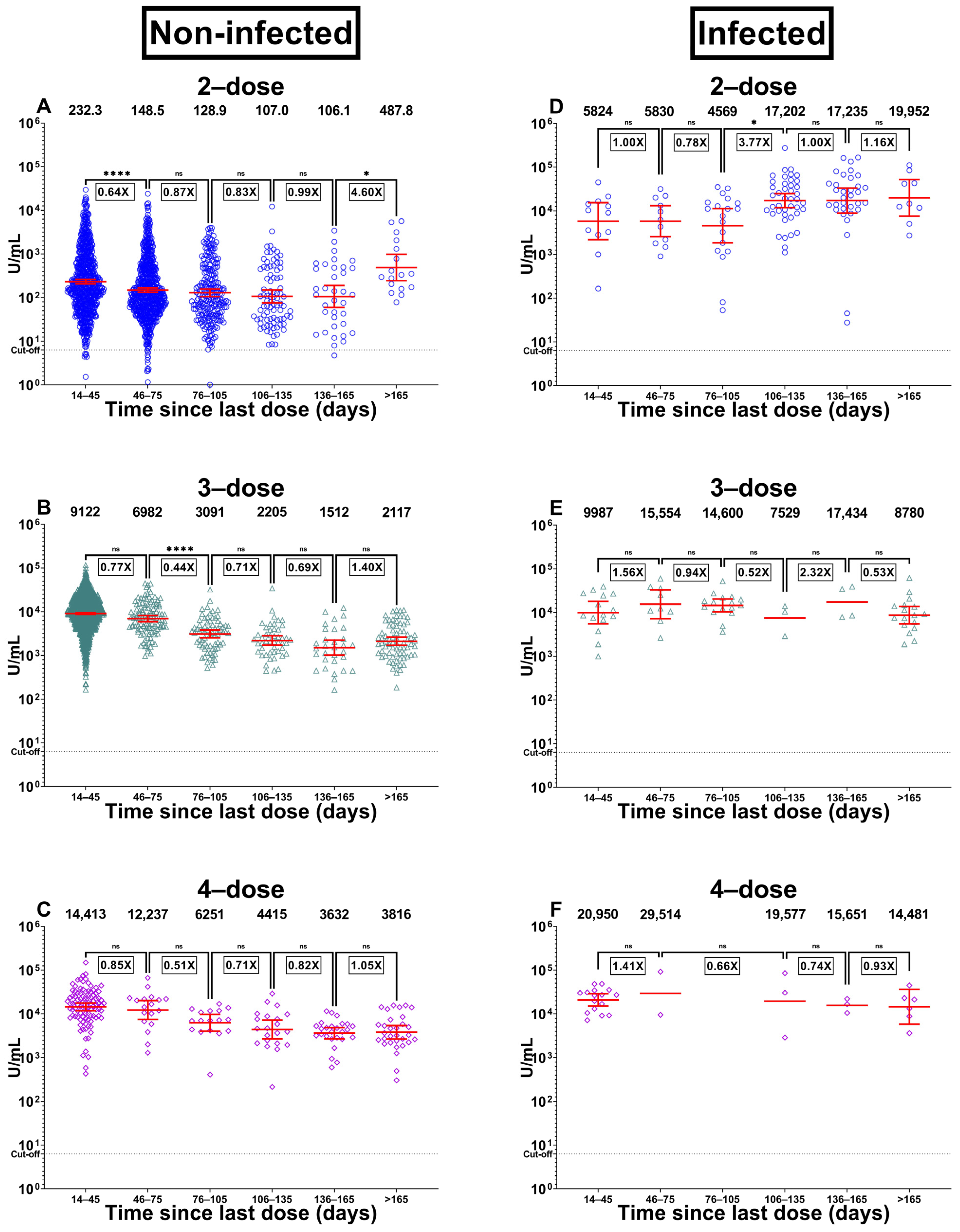
3. Results
3.1. Demographic Data of Study Participants
3.2. Immunity in Participants with No Previous Infection
3.3. Immunity in Participants with the Previous Infection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19. 11 March 2020. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 1 December 2022).
- World Health Organization. WHO Coronavirus (COVID-19) Dashboard. 2023. Available online: https://covid19.who.int/ (accessed on 9 January 2023).
- Department of Disease Control, Thailand. DDC COVID-16 Interactive Dashboard. 2023. Available online: https://ddc.moph.go.th/covid19-dashboard (accessed on 9 January 2023).
- Chia, P.Y.; Ong, S.W.X.; Chiew, C.J.; Ang, L.W.; Chavatte, J.-M.; Mak, T.-M.; Cui, L.; Kalimuddin, S.; Ni Chia, W.; Tan, C.W.; et al. Virological and serological kinetics of SARS-CoV-2 Delta variant vaccine breakthrough infections: A multicentre cohort study. Clin. Microbiol. Infect. 2022, 28, 612.e1–612.e7. [Google Scholar] [CrossRef] [PubMed]
- Graña, C.; Ghosn, L.; Evrenoglou, T.; Jarde, A.; Minozzi, S.; Bergman, H.; Buckley, B.S.; Probyn, K.; Villanueva, G.; Henschke, N.; et al. Efficacy and safety of COVID-19 vaccines. Cochrane Database Syst. Rev. 2022, 12, CD015477. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Iketani, S.; Guo, Y.; Chan, J.F.-W.; Wang, M.; Liu, L.; Luo, Y.; Chu, H.; Huang, Y.; Nair, M.S.; et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature 2022, 602, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Madewell, Z.J.; Yang, Y.; Longini, I.M.; Halloran, M.E.; Dean, N.E. Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status. JAMA Netw. Open 2022, 5, e229317. [Google Scholar] [CrossRef] [PubMed]
- Nordström, P.; Ballin, M.; Nordström, A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: A retrospective, total population cohort study in Sweden. Lancet Infect. Dis. 2022, 22, 781–790, Erratum in Lancet Infect. Dis. 2022, 22, E159. [Google Scholar] [CrossRef]
- Andrews, N.; Stowe, J.; Kirsebom, F.; Toffa, S.; Sachdeva, R.; Gower, C.; Ramsay, M.; Bernal, J.L. Effectiveness of COVID-19 booster vaccines against COVID-19-related symptoms, hospitalization and death in England. Nat. Med. 2022, 28, 831–837. [Google Scholar] [CrossRef]
- Suntronwong, N.; Yorsaeng, R.; Puenpa, J.; Auphimai, C.; Thongmee, T.; Vichaiwattana, P.; Kanokudom, S.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. COVID-19 Breakthrough Infection after Inactivated Vaccine Induced Robust Antibody Responses and Cross-Neutralization of SARS-CoV-2 Variants, but Less Immunity against Omicron. Vaccines 2022, 10, 391. [Google Scholar] [CrossRef]
- Wanlapakorn, N.; Suntronwong, N.; Phowatthanasathian, H.; Yorsaeng, R.; Vichaiwattana, P.; Thongmee, T.; Auphimai, C.; Srimuan, D.; Thatsanatorn, T.; Assawakosri, S.; et al. Safety and immunogenicity of heterologous and homologous inactivated and adenoviral-vectored COVID-19 vaccine regimens in healthy adults: A prospective cohort study. Hum. Vaccines Immunother. 2022, 18, 2029111. [Google Scholar] [CrossRef]
- Niyomnaitham, S.; Toh, Z.Q.; Wongprompitak, P.; Jansarikit, L.; Srisutthisamphan, K.; Sapsutthipas, S.; Jantraphakorn, Y.; Mingngamsup, N.; Licciardi, P.V.; Chokephaibulkit, K. Immunogenicity and reactogenicity against the SARS-CoV-2 variants following heterologous primary series involving CoronaVac, ChAdox1 nCov-19 and BNT162b2 plus BNT162b2 booster vaccination: An open-label randomized study in healthy Thai adults. Hum. Vaccines Immunother. 2022, 18, 2091865. [Google Scholar] [CrossRef]
- Yorsaeng, R.; Suntronwong, N.; Phowatthanasathian, H.; Assawakosri, S.; Kanokudom, S.; Thongmee, T.; Vichaiwattana, P.; Auphimai, C.; Wongsrisang, L.; Srimuan, D.; et al. Immunogenicity of a third dose viral-vectored COVID-19 vaccine after receiving two-dose inactivated vaccines in healthy adults. Vaccine 2022, 40, 524–530. [Google Scholar] [CrossRef]
- Nantanee, R.; Aikphaibul, P.; Jaru-Ampornpan, P.; Sodsai, P.; Himananto, O.; Theerawit, T.; Sophonphan, J.; Tovichayathamrong, P.; Manothummetha, K.; Laohasereekul, T.; et al. Immunogenicity and reactogenicity after booster dose with AZD1222 via intradermal route among adult who had received CoronaVac. Vaccine 2022, 40, 3320–3329. [Google Scholar] [CrossRef]
- Mahasirimongkol, S.; Khunphon, A.; Kwangsukstid, O.; Sapsutthipas, S.; Wichaidit, M.; Rojanawiwat, A.; Wichuckchinda, N.; Puangtubtim, W.; Pimpapai, W.; Soonthorncharttrawat, S.; et al. The Pilot Study of Immunogenicity and Adverse Events of a COVID-19 Vaccine Regimen: Priming with Inactivated Whole SARS-CoV-2 Vaccine (CoronaVac) and Boosting with the Adenoviral Vector (ChAdOx1 nCoV-19) Vaccine. Vaccines 2022, 10, 536. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Abolhassani, H.; Du, L.; Piralla, A.; Bertoglio, F.; de Campos-Mata, L.; Wan, H.; Schubert, M.; Cassaniti, I.; Wang, Y.; et al. Heterologous immunization with inactivated vaccine followed by mRNA-booster elicits strong immunity against SARS-CoV-2 Omicron variant. Nat. Commun. 2022, 13, 2670. [Google Scholar] [CrossRef] [PubMed]
- Gruell, H.; Vanshylla, K.; Tober-Lau, P.; Hillus, D.; Schommers, P.; Lehmann, C.; Kurth, F.; Sander, L.E.; Klein, F. mRNA booster immunization elicits potent neutralizing serum activity against the SARS-CoV-2 Omicron variant. Nat. Med. 2022, 28, 477–480. [Google Scholar] [CrossRef]
- Zhang, B.; Huo, J.; Huang, Y.; Teo, S.Y.; Duan, K.; Li, Y.; Toh, L.K.; Lam, K.P.; Xu, S. mRNA Booster Vaccination Enhances Antibody Responses against SARS-CoV2 Omicron Variant in Individuals Primed with mRNA or Inactivated Virus Vaccines. Vaccines 2022, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Suntronwong, N.; Kanokudom, S.; Auphimai, C.; Assawakosri, S.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; Chansaenroj, J.; et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. J. Med. Virol. 2022, 94, 5713–5722. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Menni, C.; May, A.; Polidori, L.; Louca, P.; Wolf, J.; Capdevila, J.; Hu, C.; Ourselin, S.; Steves, C.J.; Valdes, A.M.; et al. COVID-19 vaccine waning and effectiveness and side-effects of boosters: A prospective community study from the ZOE COVID Study. Lancet Infect. Dis. 2022, 22, 1002–1010. [Google Scholar] [CrossRef]
- Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Assawakosri, S.; Yorsaeng, R.; Nilyanimit, P.; Aeemjinda, R.; Khanarat, N.; Vichaiwattana, P.; Klinfueng, S.; et al. The Fourth Dose of mRNA COVID-19 Vaccine Following 12 Different Three-Dose Regimens: Safety and Immunogenicity to Omicron BA.4/BA.5. Vaccines 2023, 11, 570. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Suntronwong, N.; Kanokudom, S.; Assawakosri, S.; Vichaiwattana, P.; Klinfueng, S.; Wongsrisang, L.; Thongmee, T.; Aeemjinda, R.; Khanarat, N.; et al. Seroprevalence of SARS-CoV-2 anti-nucleocapsid total Ig, anti-RBD IgG antibodies, and infection in Thailand: A cross-sectional survey from October 2022 to January 2023. Res. Sq. 2023, 1–12. [Google Scholar] [CrossRef]
- Goldberg, Y.; Mandel, M.; Bar-On, Y.M.; Bodenheimer, O.; Freedman, L.S.; Ash, N.; Alroy-Preis, S.; Huppert, A.; Milo, R. Protection and Waning of Natural and Hybrid Immunity to SARS-CoV-2. N. Engl. J. Med. 2022, 386, 2201–2212. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Alós, L.; Armenteros, J.J.A.; Madsen, J.R.; Hansen, C.B.; Jarlhelt, I.; Hamm, S.R.; Heftdal, L.D.; Pries-Heje, M.M.; Møller, D.L.; Fogh, K.; et al. Modeling of waning immunity after SARS-CoV-2 vaccination and influencing factors. Nat. Commun. 2022, 13, 1614. [Google Scholar] [CrossRef]
- Tré-Hardy, M.; Cupaiolo, R.; Wilmet, A.; Beukinga, I.; Blairon, L. Waning antibodies in SARS-CoV-2 naïve vaccinees: Results of a three-month interim analysis of ongoing immunogenicity and efficacy surveillance of the mRNA-1273 vaccine in healthcare workers. J. Infect. 2021, 83, 381–412. [Google Scholar] [CrossRef] [PubMed]
- Althaus, T.; Landier, J.; Zhu, F.; Raps, H.; Dejoux, O.; Costantini, A.; Lavagna, C.; Rampal, P.; Mattiuzzo, G.; Xu, S.; et al. The Impact of Severe Acute Respiratory Syndrome Coronavirus 2 Vaccination and Infection on Neutralizing Antibodies: A Nation-wide Cross-sectional Analysis. J. Infect. Dis. 2023, 227, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.B.; Dvoncova, K.; Pérez-Alós, L.; Fogh, K.; Madsen, J.R.; Garred, C.H.; Jarlhelt, I.; Nielsen, P.B.; Petersen, S.S.; Fjordager, C.G.; et al. SARS-CoV-2 antibody dynamics over time and risk factors associated with infection and long COVID-19 symptoms in large working environments. J. Intern. Med. 2023, 293, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Owusu, J.T.; Prapasiri, P.; Ditsungnoen, D.; Leetongin, G.; Yoocharoen, P.; Rattanayot, J.; Olsen, S.J.; Muangchana, C. Seasonal influenza vaccine coverage among high-risk populations in Thailand, 2010–2012. Vaccine 2015, 33, 742–747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suntronwong, N.; Vichaiwattana, P.; Klinfueng, S.; Korkong, S.; Thongmee, T.; Vongpunsawad, S.; Poovorawan, Y. Climate factors influence seasonal influenza activity in Bangkok, Thailand. PLoS ONE 2020, 15, e0239729. [Google Scholar] [CrossRef]


| 2–Dose | 3–Dose | 4–Dose | p-Value | |
|---|---|---|---|---|
| No history of infection (n, 3906) | ||||
| n | 1836 | 1859 | 211 | |
| Male (%) | 562 (30.6) | 482 (25.9) | 83 (39.3) | <0.001 * |
| Age, mean (SD) | 41.49 (±14.01) | 41.68 (±13.04) | 47.73 (±15.79) | <0.001 † |
| Ig anti-RBD, GMT (95%CI) U/mL | 173.4 (160.9, 186.9) | 7599 (7249, 7967) | 8199 (7025, 9568) | <0.001 ¶ |
| Time since last dose vaccination (days), median (IQR) | 41.0 (31.0, 68.0) | 29.0 (23.0, 35.0) | 60.0 (30.0, 144.0) | <0.001 § |
| 14–45 days, median (IQR) | 31.0 (29.0, 33.0) | 28.0 (21.0, 31.0) | 29.5 (28.0, 32.3) | <0.001 § |
| 46–75 days, median (IQR) | 49.0 (42.0, 59.0) | 53.0 (49.0, 62.0) | 61.5 (54.8, 66.0) | <0.001 § |
| 76–105 days, median (IQR) | 90.0 (83.0, 98.0) | 95.0 (90.0, 103.3) | 89.0 (81.0, 99.0) | <0.001 § |
| 106–135 days, median (IQR) | 118.0 (112.5, 125.0) | 115.5 (110.3, 126.8) | 120.0 (115.5, 129.5) | 0.226 § |
| 136–165 days, median (IQR) | 139.0 (138.0, 149.0) | 150.0 (139.0, 154.0) | 152.5 (145.0, 159.0) | <0.001 § |
| >165 days, median (IQR) | 190.0 (176.0, 224.0) | 189.0 (182.0, 212.3) | 183.0 (173.0, 207.0) | 0.341 § |
| Had history of infection (n, 220) | ||||
| n | 126 | 64 | 30 | |
| Male (%) | 125 (32.5) | 11 (17.2) | 5 (16.7) | 0.035 * |
| Age, mean (SD) | 37.33 (±12.75) | 39.79 (±12.96) | 39.31 (±13.12) | 0.821 † |
| Ig anti-RBD, GMT (95%CI) U/mL | 11,689 (8787, 15,549) | 11,541 (9225, 14,438) | 19,205 (14,145, 26,075) | 0.172 ¶ |
| Time since last dose vaccination (days), median (IQR) | 125.0 (89.75, 145.3) | 80.50 (51.50, 171.0) | 36.0 (35.0, 148.0) | 0.036 § |
| 14–45 days, median (IQR) | 24.0 (14.0, 33.5) | 34.0 (29.0, 37.0) | 35.0 (35.0, 35.0) | 0.003 § |
| 46–75 days, median (IQR) | 49.0 (39.0, 57.0) | 56.0 (54.5, 67.5) | 55.5 (52.0, 59.0) | 0.058 § |
| 76–105 days, median (IQR) | 91.0 (84.0, 99.0) | 80.5 (79.3, 81.0) | N/A | <0.001 § |
| 106–135 days, median (IQR) | 125.0 (120.0, 126.0) | 112.0 (106.0, 130.0) | 114.0 (108.0, 130.0) | 0.320 § |
| 136–165 days, median (IQR) | 151.0 (142.0, 153.0) | 156.5 (140.5, 162.8) | 148.0 (147.0, 148.0) | 0.336 § |
| >165 days, median (IQR) | 202.0 (175.0, 237.0) | 216.0 (186.0, 230.0) | 215.0 (182.8, 235.5) | 0.551 § |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yorsaeng, R.; Atsawawaranunt, K.; Suntronwong, N.; Kanokudom, S.; Chansaenroj, J.; Assawakosri, S.; Nilyanimit, P.; Aeemjinda, R.; Khanarat, N.; Wongsrisang, L.; et al. SARS-CoV-2 Antibody Dynamics after COVID-19 Vaccination and Infection: A Real-World Cross-Sectional Analysis. Vaccines 2023, 11, 1184. https://doi.org/10.3390/vaccines11071184
Yorsaeng R, Atsawawaranunt K, Suntronwong N, Kanokudom S, Chansaenroj J, Assawakosri S, Nilyanimit P, Aeemjinda R, Khanarat N, Wongsrisang L, et al. SARS-CoV-2 Antibody Dynamics after COVID-19 Vaccination and Infection: A Real-World Cross-Sectional Analysis. Vaccines. 2023; 11(7):1184. https://doi.org/10.3390/vaccines11071184
Chicago/Turabian StyleYorsaeng, Ritthideach, Kamolthip Atsawawaranunt, Nungruthai Suntronwong, Sitthichai Kanokudom, Jira Chansaenroj, Suvichada Assawakosri, Pornjarim Nilyanimit, Ratchadawan Aeemjinda, Nongkanok Khanarat, Lakkhana Wongsrisang, and et al. 2023. "SARS-CoV-2 Antibody Dynamics after COVID-19 Vaccination and Infection: A Real-World Cross-Sectional Analysis" Vaccines 11, no. 7: 1184. https://doi.org/10.3390/vaccines11071184





