Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Bacteria
2.3. rAMEV2 Cloning and Purification
2.4. AMEV2 Vaccination and A. baumannii Challenge
2.5. Organ Bacterial Burden Measurement
2.6. Determination of Antibody and Isotype Levels by ELISA
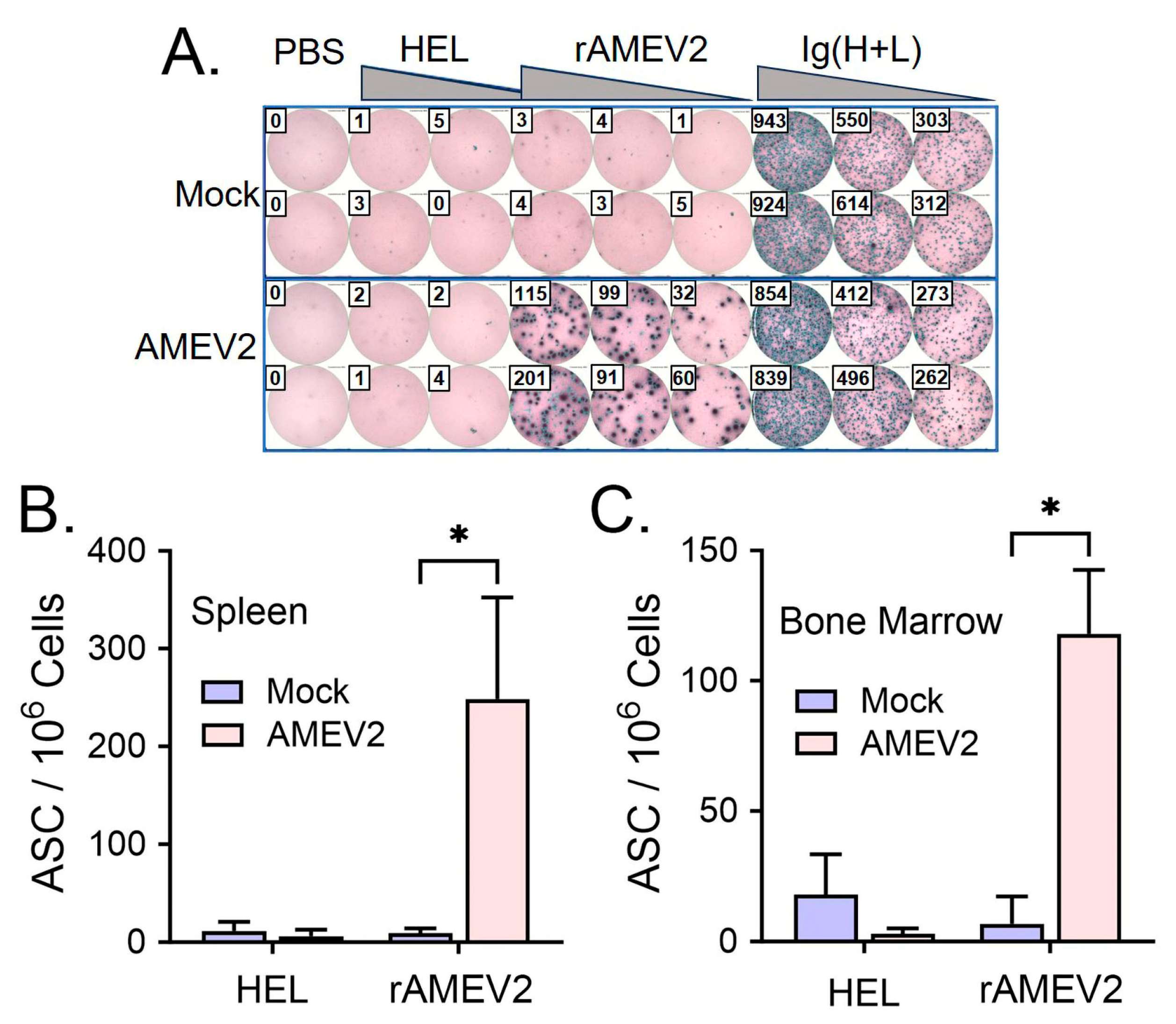
2.7. B-Cell ELISpot Assays
2.8. T-Cell ELISpot Assays
2.9. Statistical Analysis
3. Results
3.1. Immunoinformatics Based Peptide Selection
3.2. Generation of an Acinetobacter Multi-Epitope Vaccine, AMEV2
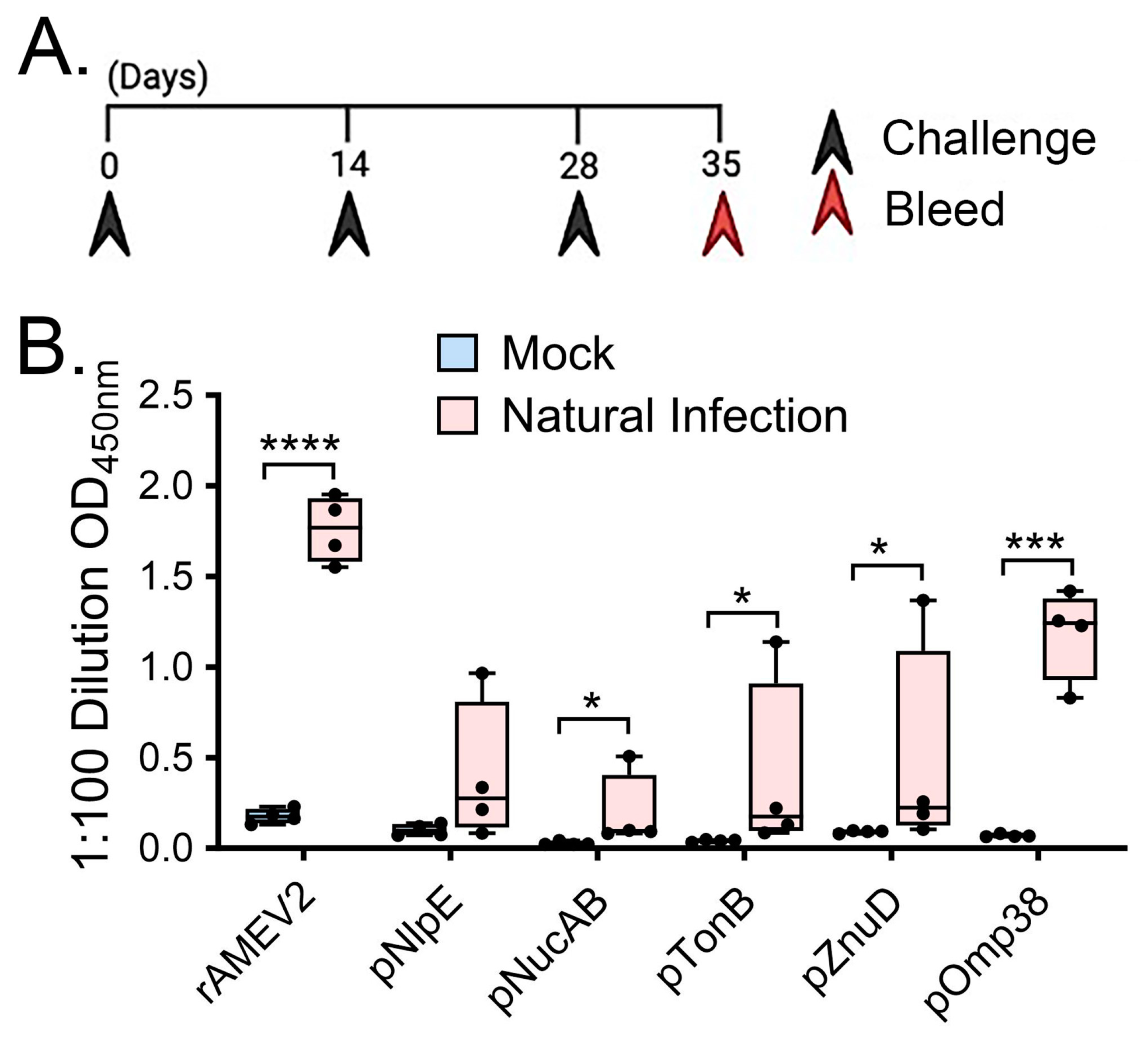
3.3. rAMEV2 Contains Epitopes Relevant to A. baumannii Natural Infection
3.4. AMEV2 Vaccination Generates Robust Humoral Immunity
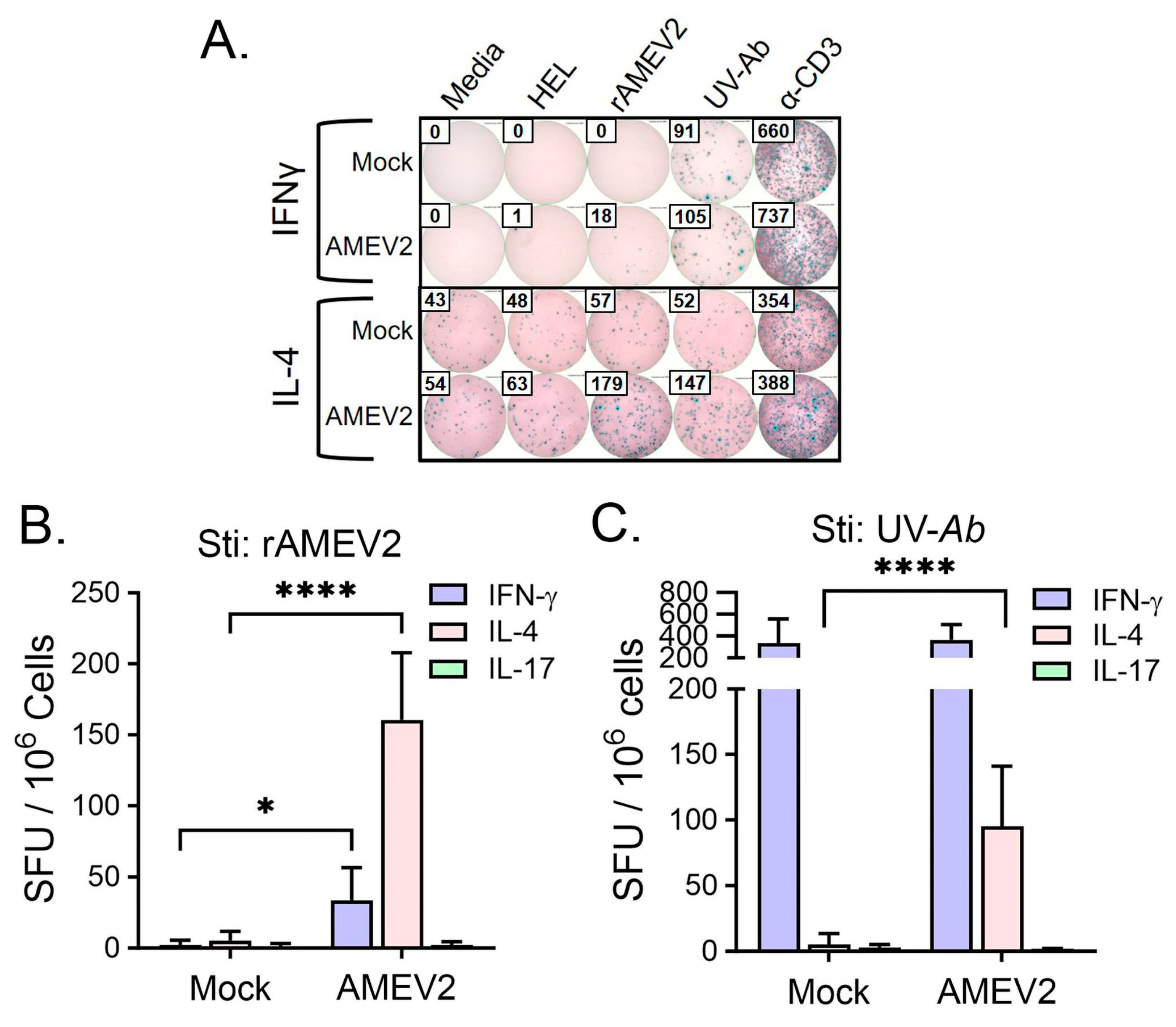
3.5. AMEV2 Vaccination Stimulates a Th2 Response
3.6. AMEV2 Affords Protection against Pulmonary Challenge
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Founou, R.C.; Founou, L.L.; Essack, S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0189621. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, P. Acinetobacter baumannii, the nosocomial pathogen par excellence. Pathol. Biol. 2004, 52, 301–303. [Google Scholar] [CrossRef]
- Joly-Guillou, M.L. Clinical impact and pathogenicity of Acinetobacter. Clin. Microbiol. Infect. 2005, 11, 868–873. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, M.K. Acinetobacter in modern warfare. Int. J. Antimicrob. Agents 2012, 39, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Morris, F.C.; Dexter, C.; Kostoulias, X.; Uddin, M.I.; Peleg, A.Y. The Mechanisms of Disease Caused by Acinetobacter baumannii. Front. Microbiol. 2019, 10, 1601. [Google Scholar] [CrossRef]
- Sievert, D.M.; Ricks, P.; Edwards, J.R.; Schneider, A.; Patel, J.; Srinivasan, A.; Kallen, A.; Limbago, B.; Fridkin, S.; National Healthcare Safety Network, T.; et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: Summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect. Control Hosp. Epidemiol. 2013, 34, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Bonomo, R.A. The deadly impact of extreme drug resistance in Acinetobacter baumannii. Crit. Care Med. 2014, 42, 1289–1291. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Garcia-Quintanilla, M.; Pulido, M.R.; Pachon, J.; McConnell, M.J. Immunization with lipopolysaccharide-deficient whole cells provides protective immunity in an experimental mouse model of Acinetobacter baumannii infection. PLoS ONE 2014, 9, e114410. [Google Scholar] [CrossRef]
- Huang, W.; Yao, Y.; Long, Q.; Yang, X.; Sun, W.; Liu, C.; Jin, X.; Li, Y.; Chu, X.; Chen, B.; et al. Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PLoS ONE 2014, 9, e100727. [Google Scholar] [CrossRef] [PubMed]
- KuoLee, R.; Harris, G.; Yan, H.; Xu, H.H.; Conlan, W.J.; Patel, G.B.; Chen, W. Intranasal immunization protects against Acinetobacter baumannii-associated pneumonia in mice. Vaccine 2015, 33, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.P.; Garcia, P.; Beceiro, A.; Rumbo, C.; Perez, A.; Moscoso, M.; Bou, G. Design of live attenuated bacterial vaccines based on D-glutamate auxotrophy. Nat. Commun. 2017, 8, 15480. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, S.; Yao, Y.; Xia, Y.; Yang, X.; Long, Q.; Sun, W.; Liu, C.; Li, Y.; Ma, Y. OmpW is a potential target for eliciting protective immunity against Acinetobacter baumannii infections. Vaccine 2015, 33, 4479–4485. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Yao, Y.; Wang, S.; Xia, Y.; Yang, X.; Long, Q.; Sun, W.; Liu, C.; Li, Y.; Chu, X.; et al. Immunization with a 22-kDa outer membrane protein elicits protective immunity to multidrug-resistant Acinetobacter baumannii. Sci. Rep. 2016, 6, 20724. [Google Scholar] [CrossRef] [PubMed]
- Skerniskyte, J.; Karazijaite, E.; Deschamps, J.; Krasauskas, R.; Armalyte, J.; Briandet, R.; Suziedeliene, E. Blp1 protein shows virulence-associated features and elicits protective immunity to Acinetobacter baumannii infection. BMC Microbiol. 2019, 19, 259. [Google Scholar] [CrossRef] [PubMed]
- Ud-Din, M.; Albutti, A.; Ullah, A.; Ismail, S.; Ahmad, S.; Naz, A.; Khurram, M.; Haq, M.U.; Afsheen, Z.; Bakri, Y.E.; et al. Vaccinomics to Design a Multi-Epitopes Vaccine for Acinetobacter baumannii. Int. J. Environ. Res. Public Health 2022, 19, 5568. [Google Scholar] [CrossRef] [PubMed]
- Tawfik, D.M.; Ahmad, T.A.; Sheweita, S.A.; Haroun, M.; El-Sayed, L.H. The detection of antigenic determinants of Acinetobacter baumannii. Immunol. Lett. 2017, 186, 59–67. [Google Scholar] [CrossRef]
- Li, W.; Murthy, A.K.; Lanka, G.K.; Chetty, S.L.; Yu, J.J.; Chambers, J.P.; Zhong, G.; Forsthuber, T.G.; Guentzel, M.N.; Arulanandam, B.P. A T cell epitope-based vaccine protects against chlamydial infection in HLA-DR4 transgenic mice. Vaccine 2013, 31, 5722–5728. [Google Scholar] [CrossRef]
- Khan, M.S.; Khan, I.M.; Ahmad, S.U.; Rahman, I.; Khan, M.Z.; Khan, M.S.Z.; Abbas, Z.; Noreen, S.; Liu, Y. Immunoinformatics design of B and T-cell epitope-based SARS-CoV-2 peptide vaccination. Front. Immunol. 2022, 13, 1001430. [Google Scholar] [CrossRef]
- Umitaibatin, R.; Harisna, A.H.; Jauhar, M.M.; Syaifie, P.H.; Arda, A.G.; Nugroho, D.W.; Ramadhan, D.; Mardliyati, E.; Shalannanda, W.; Anshori, I. Immunoinformatics Study: Multi-Epitope Based Vaccine Design from SARS-CoV-2 Spike Glycoprotein. Vaccines 2023, 11, 399. [Google Scholar] [CrossRef]
- Ketter, P.; Guentzel, M.N.; Chambers, J.P.; Jorgensen, J.; Murray, C.K.; Cap, A.P.; Yu, J.J.; Eppinger, M.; Arulanandam, B.P. Genome Sequences of Four Acinetobacter baumannii-A. calcoaceticus Complex Isolates from Combat-Related Infections Sustained in the Middle East. Genome Announc. 2014, 2, e00026-14. [Google Scholar] [CrossRef]
- Ketter, P.M.; Guentzel, M.N.; Schaffer, B.; Herzig, M.; Wu, X.; Montgomery, R.K.; Parida, B.K.; Fedyk, C.G.; Yu, J.J.; Jorgensen, J.; et al. Severe Acinetobacter baumannii sepsis is associated with elevation of pentraxin 3. Infect. Immun. 2014, 82, 3910–3918. [Google Scholar] [CrossRef] [PubMed]
- Karulin, A.Y.; Hesse, M.D.; Tary-Lehmann, M.; Lehmann, P.V. Single-cytokine-producing CD4 memory cells predominate in type 1 and type 2 immunity. J. Immunol. 2000, 164, 1862–1872. [Google Scholar] [CrossRef]
- Kawamura, K.; McLaughlin, K.A.; Weissert, R.; Forsthuber, T.G. Myelin-reactive type B T cells and T cells specific for low-affinity MHC-binding myelin peptides escape tolerance in HLA-DR transgenic mice. J. Immunol. 2008, 181, 3202–3211. [Google Scholar] [CrossRef] [PubMed]
- Bremel, R.D.; Homan, E.J. An integrated approach to epitope analysis II: A system for proteomic-scale prediction of immunological characteristics. Immunome Res. 2010, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Bremel, R.D.; Homan, E.J. Recognition of higher order patterns in proteins: Immunologic kernels. PLoS ONE 2013, 8, e70115. [Google Scholar] [CrossRef] [PubMed]
- Yu, N.Y.; Wagner, J.R.; Laird, M.R.; Melli, G.; Rey, S.; Lo, R.; Dao, P.; Sahinalp, S.C.; Ester, M.; Foster, L.J.; et al. PSORTb 3.0: Improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 2010, 26, 1608–1615. [Google Scholar] [CrossRef]
- Emini, E.A.; Hughes, J.V.; Perlow, D.S.; Boger, J. Induction of hepatitis A virus-neutralizing antibody by a virus-specific synthetic peptide. J. Virol. 1985, 55, 836–839. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- May, H.C.; Yu, J.J.; Zhang, H.; Wang, Y.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Thioredoxin-A is a virulence factor and mediator of the type IV pilus system in Acinetobacter baumannii. PLoS ONE 2019, 14, e0218505. [Google Scholar] [CrossRef]
- Ketter, P.M.; Yu, J.J.; Guentzel, M.N.; May, H.C.; Gupta, R.; Eppinger, M.; Klose, K.E.; Seshu, J.; Chambers, J.P.; Cap, A.P.; et al. Acinetobacter baumannii Gastrointestinal Colonization Is Facilitated by Secretory IgA Which Is Reductively Dissociated by Bacterial Thioredoxin A. mBio 2018, 9, e01298-18. [Google Scholar] [CrossRef] [PubMed]
- Correddu, D.; Montano Lopez, J.J.; Vadakkedath, P.G.; Lai, A.; Pernes, J.I.; Watson, P.R.; Leung, I.K.H. An improved method for the heterologous production of soluble human ribosomal proteins in Escherichia coli. Sci. Rep. 2019, 9, 8884. [Google Scholar] [CrossRef] [PubMed]
- McNiff, M.L.; Haynes, E.P.; Dixit, N.; Gao, F.P.; Laurence, J.S. Thioredoxin fusion construct enables high-yield production of soluble, active matrix metalloproteinase-8 (MMP-8) in Escherichia coli. Protein Expr. Purif. 2016, 122, 64–71. [Google Scholar] [CrossRef]
- Livingston, B.; Crimi, C.; Newman, M.; Higashimoto, Y.; Appella, E.; Sidney, J.; Sette, A. A rational strategy to design multiepitope immunogens based on multiple Th lymphocyte epitopes. J. Immunol. 2002, 168, 5499–5506. [Google Scholar] [CrossRef]
- Gu, Y.; Sun, X.; Li, B.; Huang, J.; Zhan, B.; Zhu, X. Vaccination with a Paramyosin-Based Multi-Epitope Vaccine Elicits Significant Protective Immunity against Trichinella spiralis Infection in Mice. Front. Microbiol. 2017, 8, 1475. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guo, L.; Kong, M.; Su, X.; Yang, D.; Zou, M.; Liu, Y.; Lu, L. Design and Evaluation of a Multi-Epitope Peptide of Human Metapneumovirus. Intervirology 2015, 58, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zaro, J.L.; Shen, W.C. Fusion protein linkers: Property, design and functionality. Adv. Drug Deliv. Rev. 2013, 65, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Snapper, C.M.; Paul, W.E. Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 1987, 236, 944–947. [Google Scholar] [CrossRef] [PubMed]
- Fattahian, Y.; Rasooli, I.; Mousavi Gargari, S.L.; Rahbar, M.R.; Darvish Alipour Astaneh, S.; Amani, J. Protection against Acinetobacter baumannii infection via its functional deprivation of biofilm associated protein (Bap). Microb. Pathog. 2011, 51, 402–406. [Google Scholar] [CrossRef]
- Rasooli, I.; Abdolhamidi, R.; Jahangiri, A.; Darvish Alipour Astaneh, S. Outer Membrane Protein, Oma87 Prevents Acinetobacter baumannii Infection. Int. J. Pept. Res. Ther. 2020, 26, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Xue, J.; Jiang, M.; Lin, S.; Huang, Y.; Deng, K.; Shu, L.; Xu, H.; Li, Z.; Yao, J.; et al. A Multiepitope Peptide, rOmp22, Encapsulated in Chitosan-PLGA Nanoparticles as a Candidate Vaccine Against Acinetobacter baumannii Infection. Int. J. Nanomed. 2021, 16, 1819–1836. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Guan, L.; Dong, Y.; Wang, C.; Feng, L.; Xie, Y. Design and evaluation of a multi-epitope assembly peptide vaccine against Acinetobacter baumannii infection in mice. Swiss Med. Wkly. 2019, 149, w20052. [Google Scholar] [CrossRef] [PubMed]
- May, H.C.; Yu, J.J.; Shrihari, S.; Seshu, J.; Klose, K.E.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Thioredoxin Modulates Cell Surface Hydrophobicity in Acinetobacter baumannii. Front. Microbiol. 2019, 10, 2849. [Google Scholar] [CrossRef] [PubMed]
- LaVallie, E.R.; DiBlasio, E.A.; Kovacic, S.; Grant, K.L.; Schendel, P.F.; McCoy, J.M. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology 1993, 11, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Siroy, A.; Cosette, P.; Seyer, D.; Lemaitre-Guillier, C.; Vallenet, D.; Van Dorsselaer, A.; Boyer-Mariotte, S.; Jouenne, T.; De, E. Global comparison of the membrane subproteomes between a multidrug-resistant Acinetobacter baumannii strain and a reference strain. J. Proteome Res. 2006, 5, 3385–3398. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.L.; Neu, H.M.; Alamneh, Y.A.; Reddinger, R.M.; Jacobs, A.C.; Singh, S.; Abu-Taleb, R.; Michel, S.L.J.; Zurawski, D.V.; Merrell, D.S. Characterization of Acinetobacter baumannii Copper Resistance Reveals a Role in Virulence. Front. Microbiol. 2020, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Garg, N.; Singh, R.; Shukla, G.; Capalash, N.; Sharma, P. Immunoprotective potential of in silico predicted Acinetobacter baumannii outer membrane nuclease, NucAb. Int. J. Med. Microbiol. 2016, 306, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zimbler, D.L.; Arivett, B.A.; Beckett, A.C.; Menke, S.M.; Actis, L.A. Functional features of TonB energy transduction systems of Acinetobacter baumannii. Infect. Immun. 2013, 81, 3382–3394. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Rasooli, I.; Oskouei, R.H.; Pishgahi, M.; Jahangir, A.; Andisi, V.F.; Schryvers, A.B. Hybrid antigens expressing surface loops of BauA from Acinetobacter baumannii are capable of inducing protection against infection. Front. Immunol. 2022, 13, 933445. [Google Scholar] [CrossRef]
- Hesse, L.E.; Lonergan, Z.R.; Beavers, W.N.; Skaar, E.P. The Acinetobacter baumannii Znu System Overcomes Host-Imposed Nutrient Zinc Limitation. Infect. Immun. 2019, 87, e00746-19. [Google Scholar] [CrossRef]
- Qamsari, M.M.; Rasooli, I.; Chaudhuri, S.; Astaneh, S.D.A.; Schryvers, A.B. Hybrid Antigens Expressing Surface Loops of ZnuD From Acinetobacter baumannii Is Capable of Inducing Protection Against Infection. Front. Immunol. 2020, 11, 158. [Google Scholar] [CrossRef]
- Nie, D.; Hu, Y.; Chen, Z.; Li, M.; Hou, Z.; Luo, X.; Mao, X.; Xue, X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020, 27, 26. [Google Scholar] [CrossRef]
- Choi, C.H.; Lee, E.Y.; Lee, Y.C.; Park, T.I.; Kim, H.J.; Hyun, S.H.; Kim, S.A.; Lee, S.K.; Lee, J.C. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell. Microbiol. 2005, 7, 1127–1138. [Google Scholar] [CrossRef]
- Kim, S.W.; Choi, C.H.; Moon, D.C.; Jin, J.S.; Lee, J.H.; Shin, J.H.; Kim, J.M.; Lee, Y.C.; Seol, S.Y.; Cho, D.T.; et al. Serum resistance of Acinetobacter baumannii through the binding of factor H to outer membrane proteins. FEMS Microbiol. Lett. 2009, 301, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Lin, L.; Ibrahim, A.S.; Baquir, B.; Pantapalangkoor, P.; Bonomo, R.A.; Doi, Y.; Adams, M.D.; Russo, T.A.; Spellberg, B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE 2012, 7, e29446. [Google Scholar] [CrossRef]
- Bentancor, L.V.; Routray, A.; Bozkurt-Guzel, C.; Camacho-Peiro, A.; Pier, G.B.; Maira-Litran, T. Evaluation of the trimeric autotransporter Ata as a vaccine candidate against Acinetobacter baumannii infections. Infect. Immun. 2012, 80, 3381–3388. [Google Scholar] [CrossRef] [PubMed]
- McConnell, M.J.; Pachon, J. Active and passive immunization against Acinetobacter baumannii using an inactivated whole cell vaccine. Vaccine 2010, 29, 1–5. [Google Scholar] [CrossRef]
- Russo, T.A.; Beanan, J.M.; Olson, R.; MacDonald, U.; Cox, A.D.; St Michael, F.; Vinogradov, E.V.; Spellberg, B.; Luke-Marshall, N.R.; Campagnari, A.A. The K1 capsular polysaccharide from Acinetobacter baumannii is a potential therapeutic target via passive immunization. Infect. Immun. 2013, 81, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, S.; Ketter, P.M.; Yu, J.J.; Grimm, R.C.; May, H.C.; Cap, A.P.; Chambers, J.P.; Guentzel, M.N.; Arulanandam, B.P. Vaccination with a live attenuated Acinetobacter baumannii deficient in thioredoxin provides protection against systemic Acinetobacter infection. Vaccine 2017, 35, 3387–3394. [Google Scholar] [CrossRef]
- Nielsen, T.B.; Yan, J.; Slarve, M.; Lu, P.; Li, R.; Ruiz, J.; Lee, B.; Burk, E.; Talyansky, Y.; Oelschlaeger, P.; et al. Monoclonal Antibody Therapy against Acinetobacter baumannii. Infect. Immun. 2021, 89, e0016221. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Kesavan, D.K.; Cheng, J.; Vasudevan, A.; Wang, H.; Wan, J.; Abdelaziz, M.H.; Su, Z.; Wang, S.; Xu, H. Vesicle-Mediated Dendritic Cell Activation in Acinetobacter baumannii Clinical Isolate, which Contributes to Th2 Response. J. Immunol. Res. 2019, 2019, 2835256. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2017, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, K.W.; Pantapalangkoor, P.; Nielsen, T.; Tan, B.; Junus, J.; Hujer, K.M.; Wright, M.S.; Bonomo, R.A.; Adams, M.D.; Chen, W.; et al. Host fate is rapidly determined by innate effector-microbial interactions during Acinetobacter baumannii bacteremia. J. Infect. Dis. 2015, 211, 1296–1305. [Google Scholar] [CrossRef]
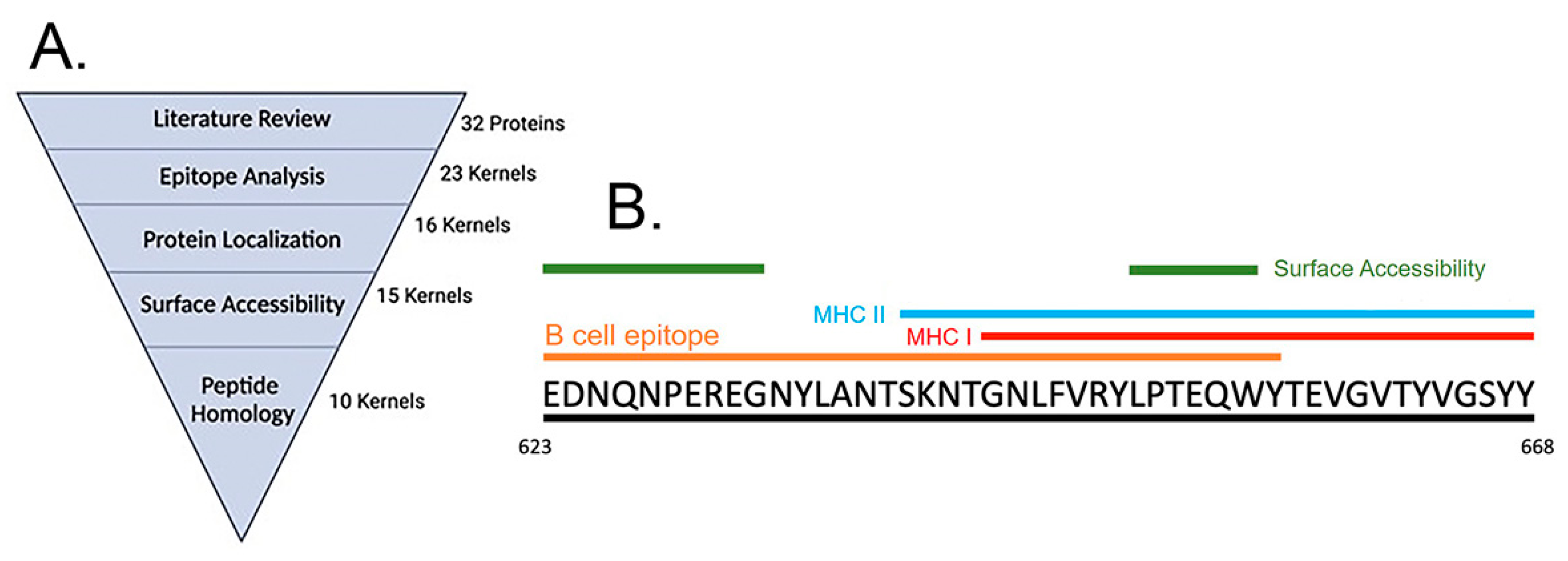
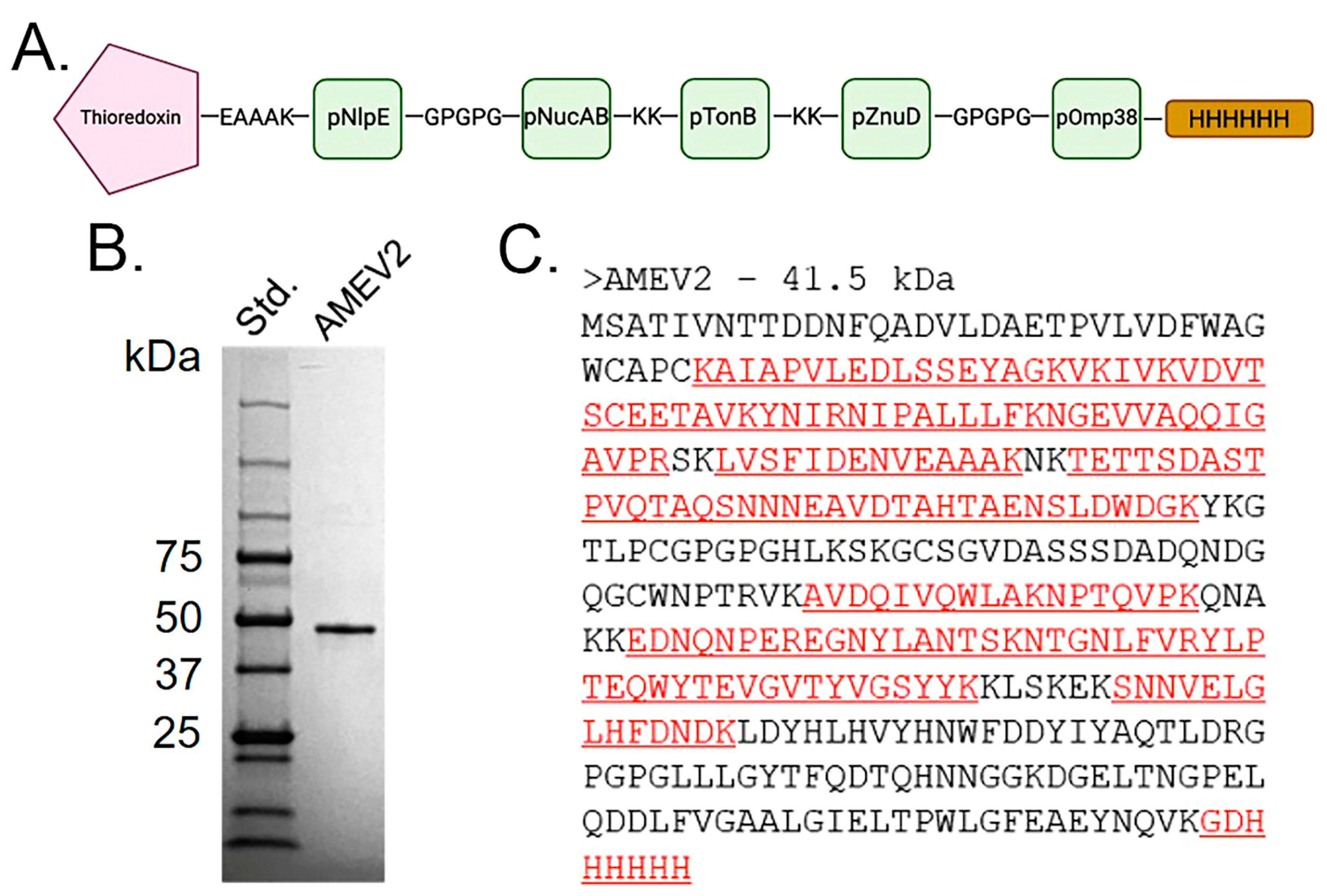


| Peptide Antigen | Derived from Protein Name | NCBI Reference Sequence | Peptide Range | Peptide Size | Peptide Amino Acid Sequence |
|---|---|---|---|---|---|
| pNlpE | Copper resistance protein NlpE | WP_000749178.1 | 23–68 | 46 | NKTETTSDASTPVQTAQSNNNEAVDTAHTAENSLDWDGKYKGTLPC |
| pNucAB | ExeM/NucH family extracellular endonuclease | WP_000847239.1 | 604–656 | 53 | HLKSKGCSGVDASSSDADQNDGQGCWNPTRVKAVDQIVQWLAKNPTQVPKQNA |
| pOmp38 | OmpA family protein | WP_000777885.1 | 29–85 | 57 | LLLGYTFQDTQHNNGGKDGELTNGPELQDDLFVGAALGIELTPWLGFEAEYNQVKGD |
| pTonB | TonB-dependent siderophore receptor | WP_001998816.1 | 623–668 | 46 | EDNQNPEREGNYLANTSKNTGNLFVRYLPTEQWYTEVGVTYVGSYY |
| pZnuD | Zinc piracy TonB-dependent receptor ZnuD | WP_000899872.1 | 497–538 | 42 | LSKEKSNNVELGLHFDNDKLDYHLHVYHNWFDDYIYAQTLDR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeffreys, S.; Tompkins, M.P.; Aki, J.; Papp, S.B.; Chambers, J.P.; Guentzel, M.N.; Hung, C.-Y.; Yu, J.-J.; Arulanandam, B.P. Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection. Vaccines 2024, 12, 358. https://doi.org/10.3390/vaccines12040358
Jeffreys S, Tompkins MP, Aki J, Papp SB, Chambers JP, Guentzel MN, Hung C-Y, Yu J-J, Arulanandam BP. Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection. Vaccines. 2024; 12(4):358. https://doi.org/10.3390/vaccines12040358
Chicago/Turabian StyleJeffreys, Sean, Megan P. Tompkins, Jadelynn Aki, Sara B. Papp, James P. Chambers, M. Neal Guentzel, Chiung-Yu Hung, Jieh-Juen Yu, and Bernard P. Arulanandam. 2024. "Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection" Vaccines 12, no. 4: 358. https://doi.org/10.3390/vaccines12040358
APA StyleJeffreys, S., Tompkins, M. P., Aki, J., Papp, S. B., Chambers, J. P., Guentzel, M. N., Hung, C.-Y., Yu, J.-J., & Arulanandam, B. P. (2024). Development and Evaluation of an Immunoinformatics-Based Multi-Peptide Vaccine against Acinetobacter baumannii Infection. Vaccines, 12(4), 358. https://doi.org/10.3390/vaccines12040358







