Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines
Abstract
:1. Introduction
2. Materials and Methods
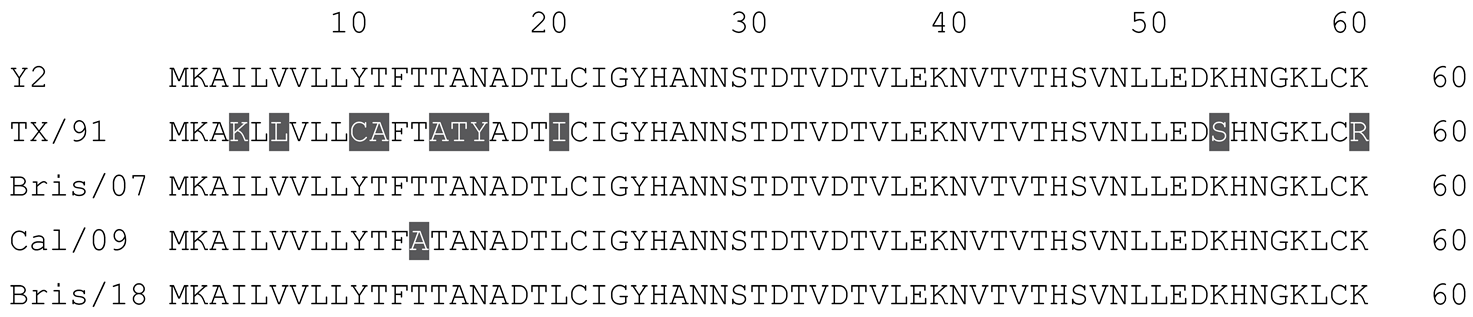
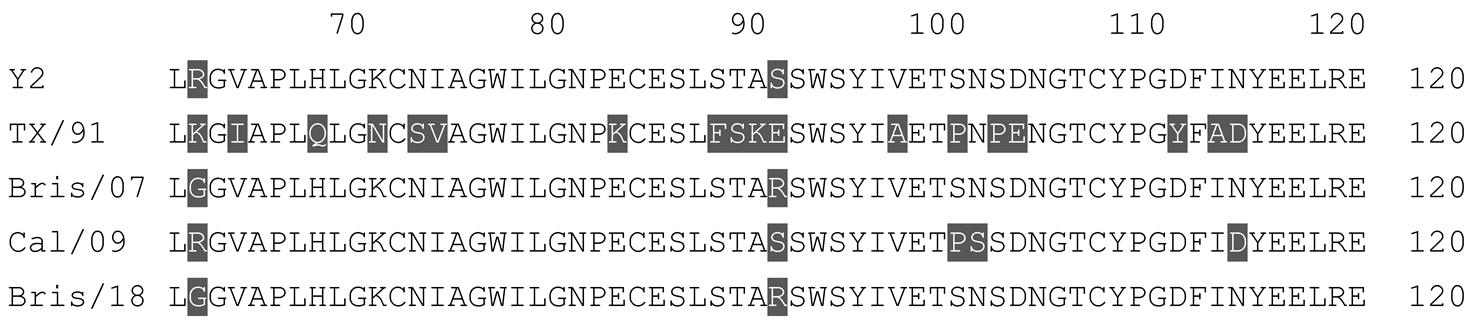
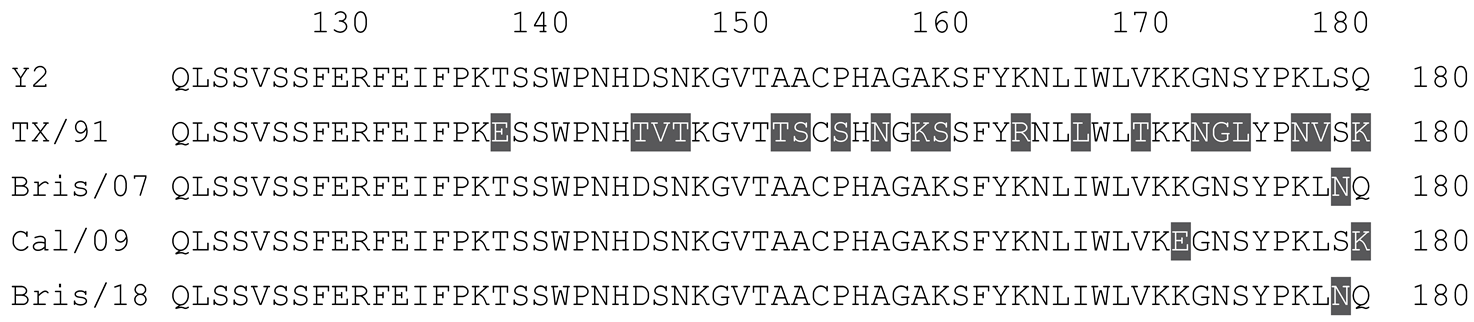
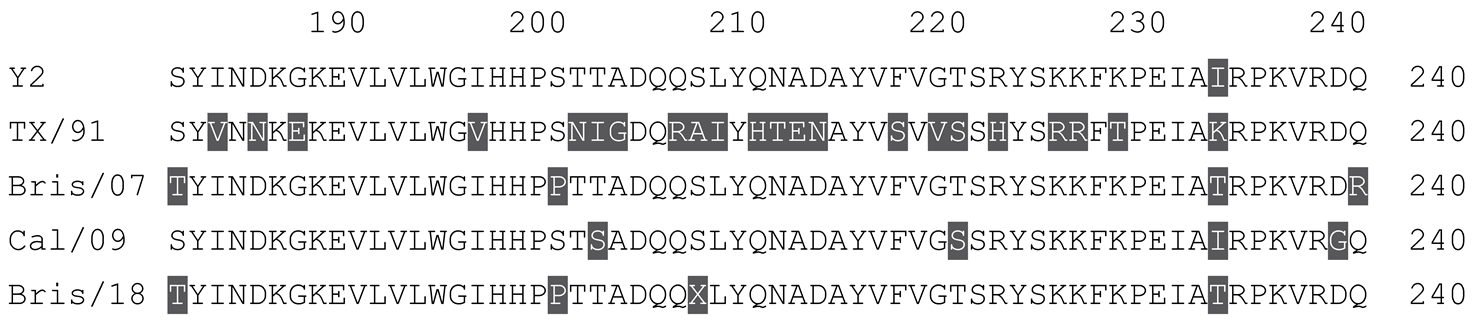
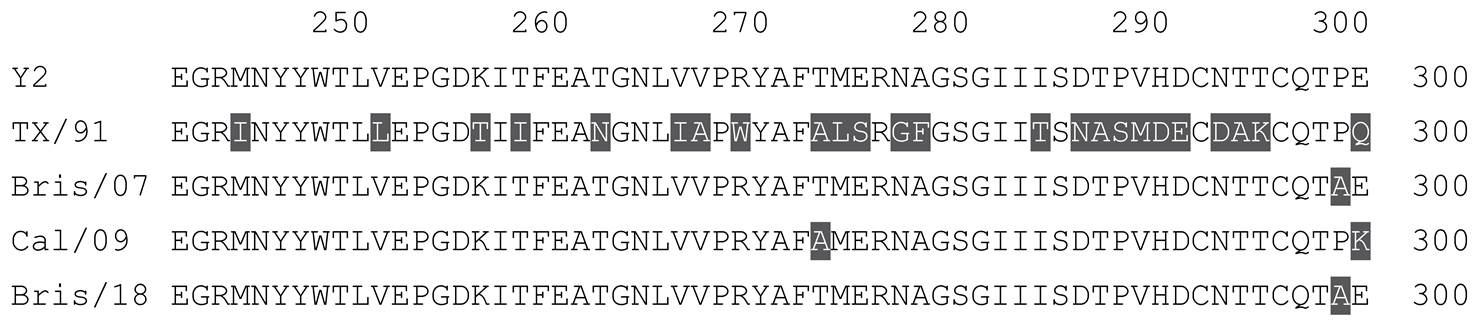
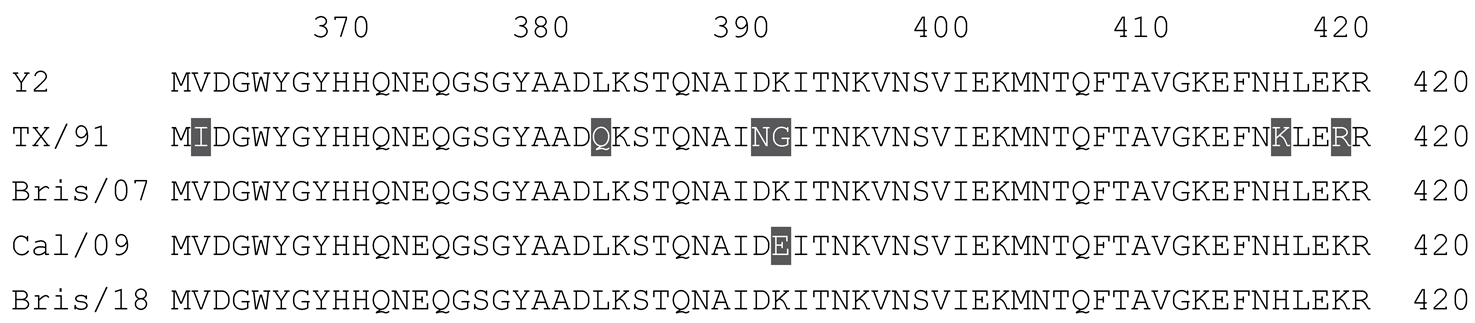
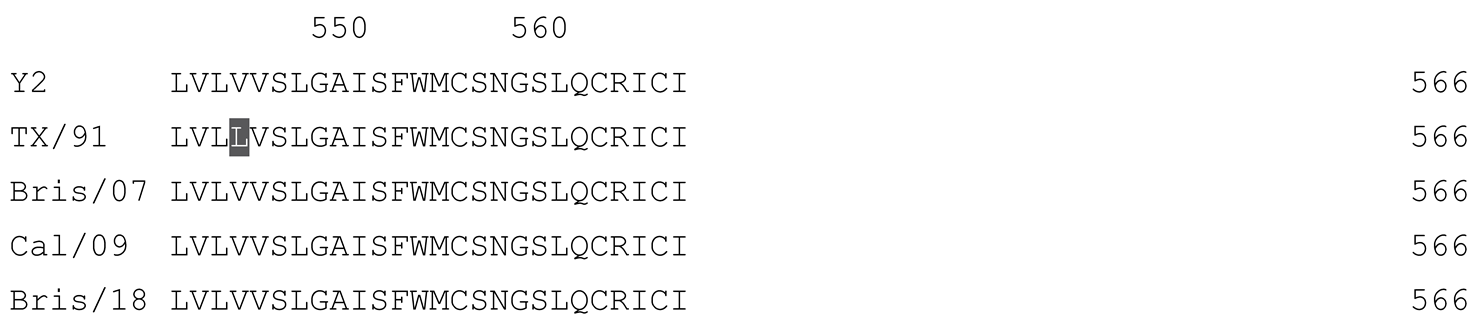
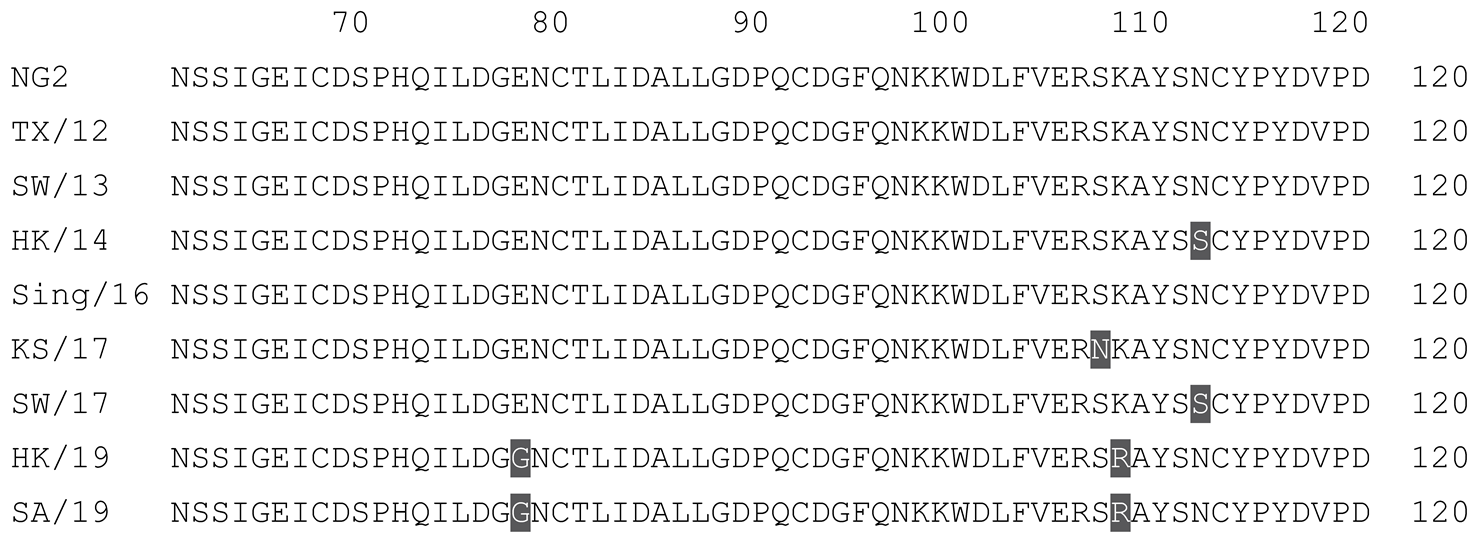
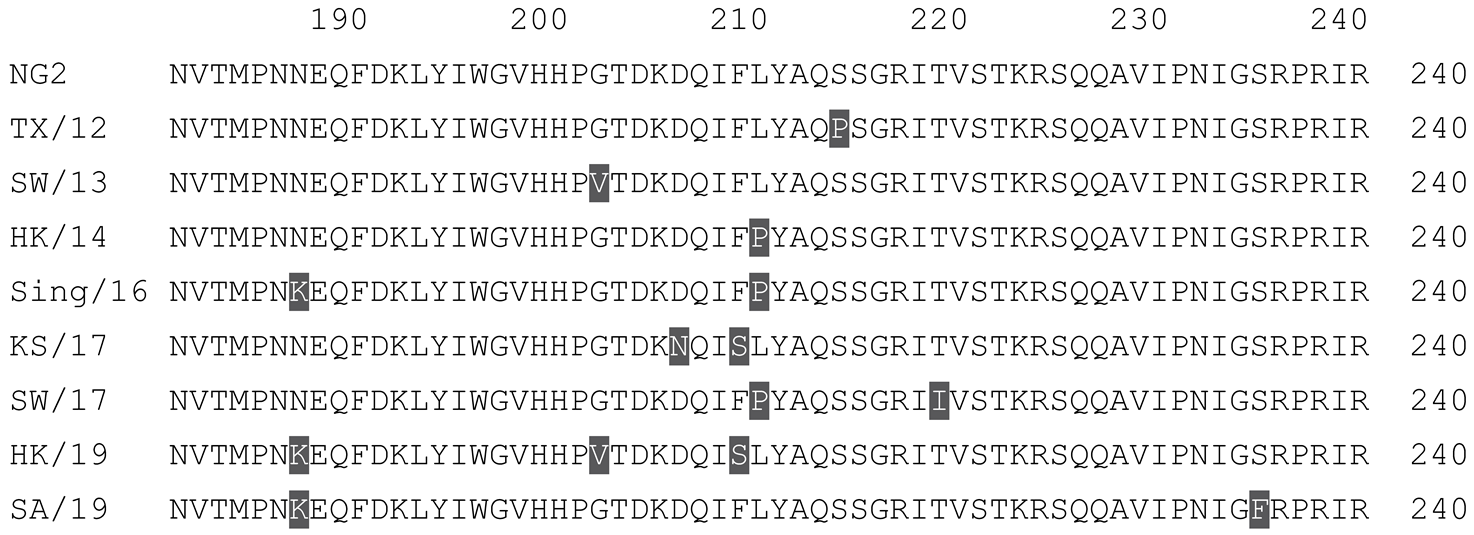
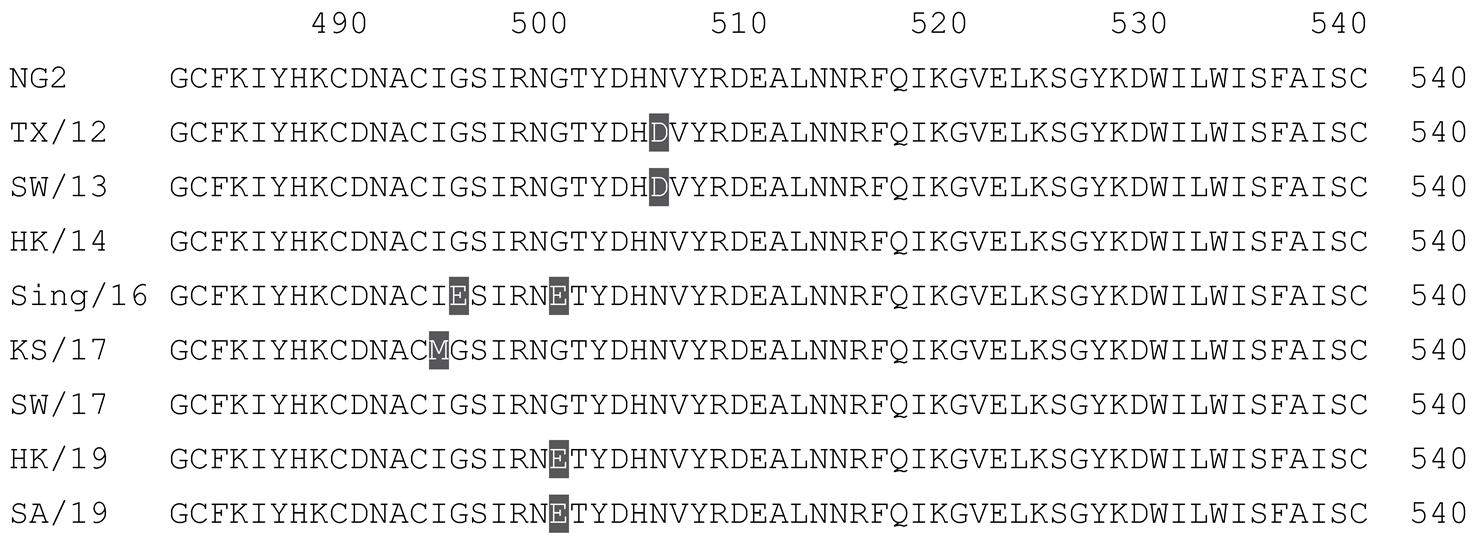
2.1. COBRA HA Designs and Protein Synthesis
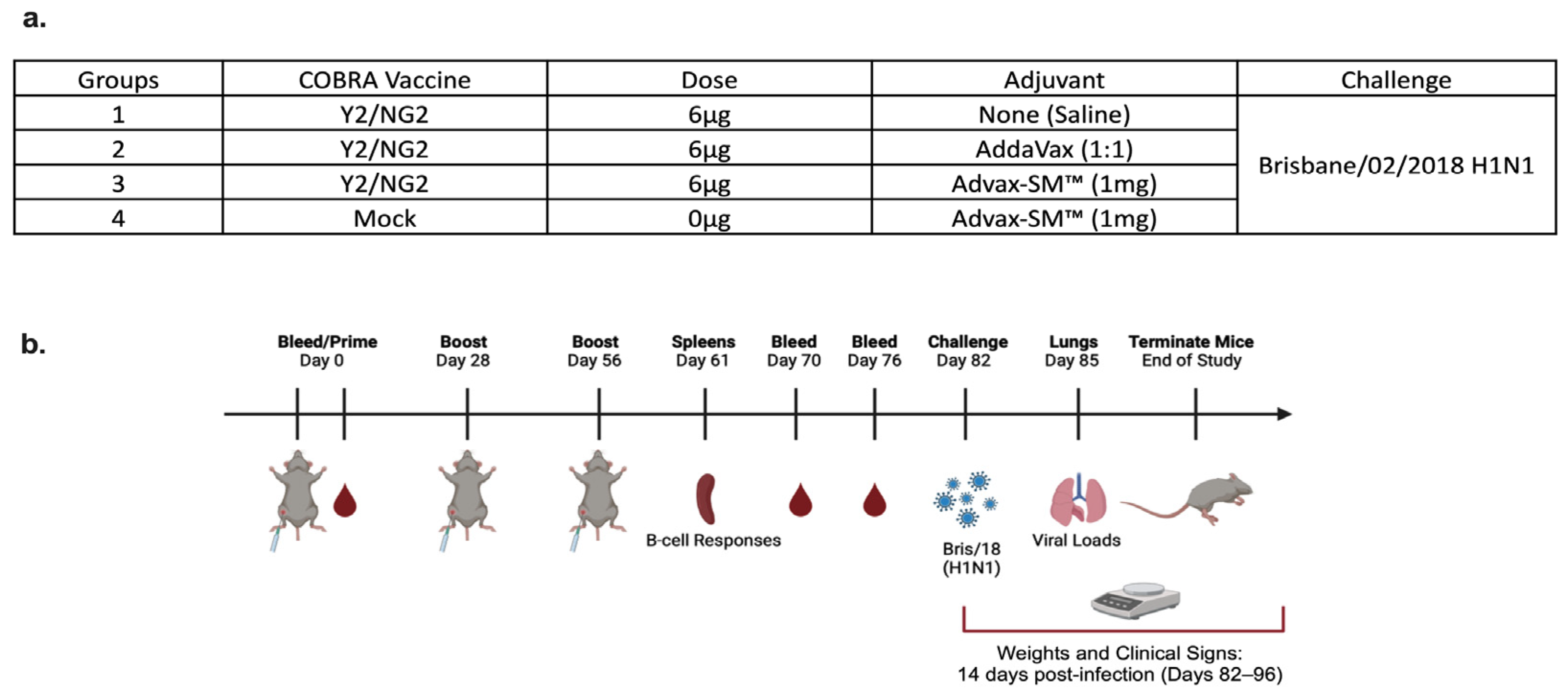
2.2. Vaccination
2.3. Viral Lung Titers
2.4. Histopathology
2.5. Enzyme-Linked Immunosorbent Assay (ELISA)
2.6. Hemagglutination Inhibition Assay (HAI)
2.7. FluoroSpot Assay
2.8. Statistical Analysis
3. Results
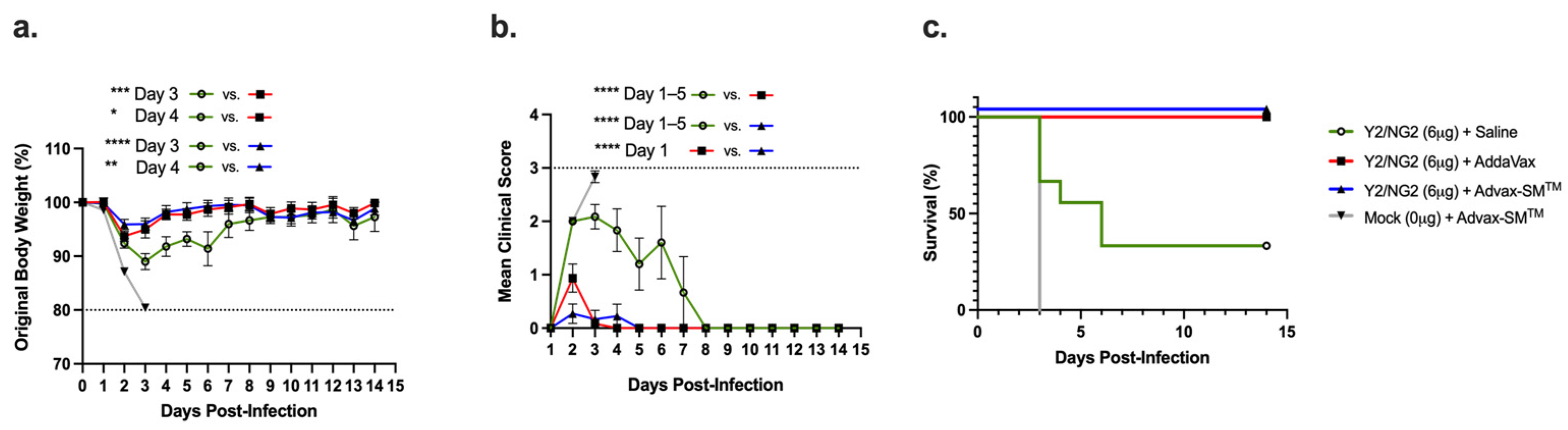
3.1. Advax-SM™-Adjuvanted COBRA HA Vaccines Protect Mice against Influenza Virus Challenge
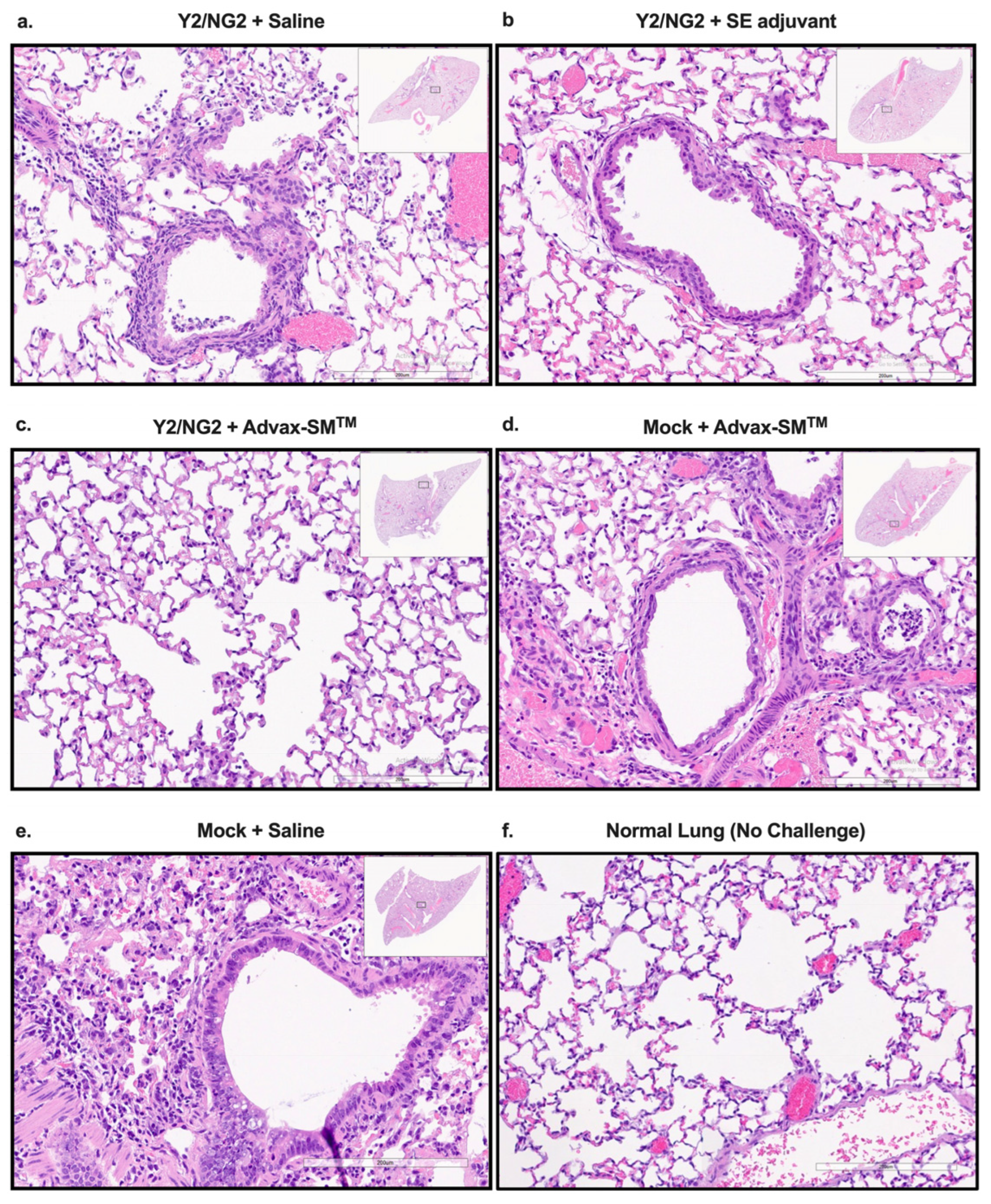
3.2. Advax-SM™ Protects Mice from Developing Lung Pathology following Influenza Infection
3.3. Advax-SM™ Adjuvant Enhances Serum Anti-Influenza IgG, IgA, and Subclass Switching following Vaccination
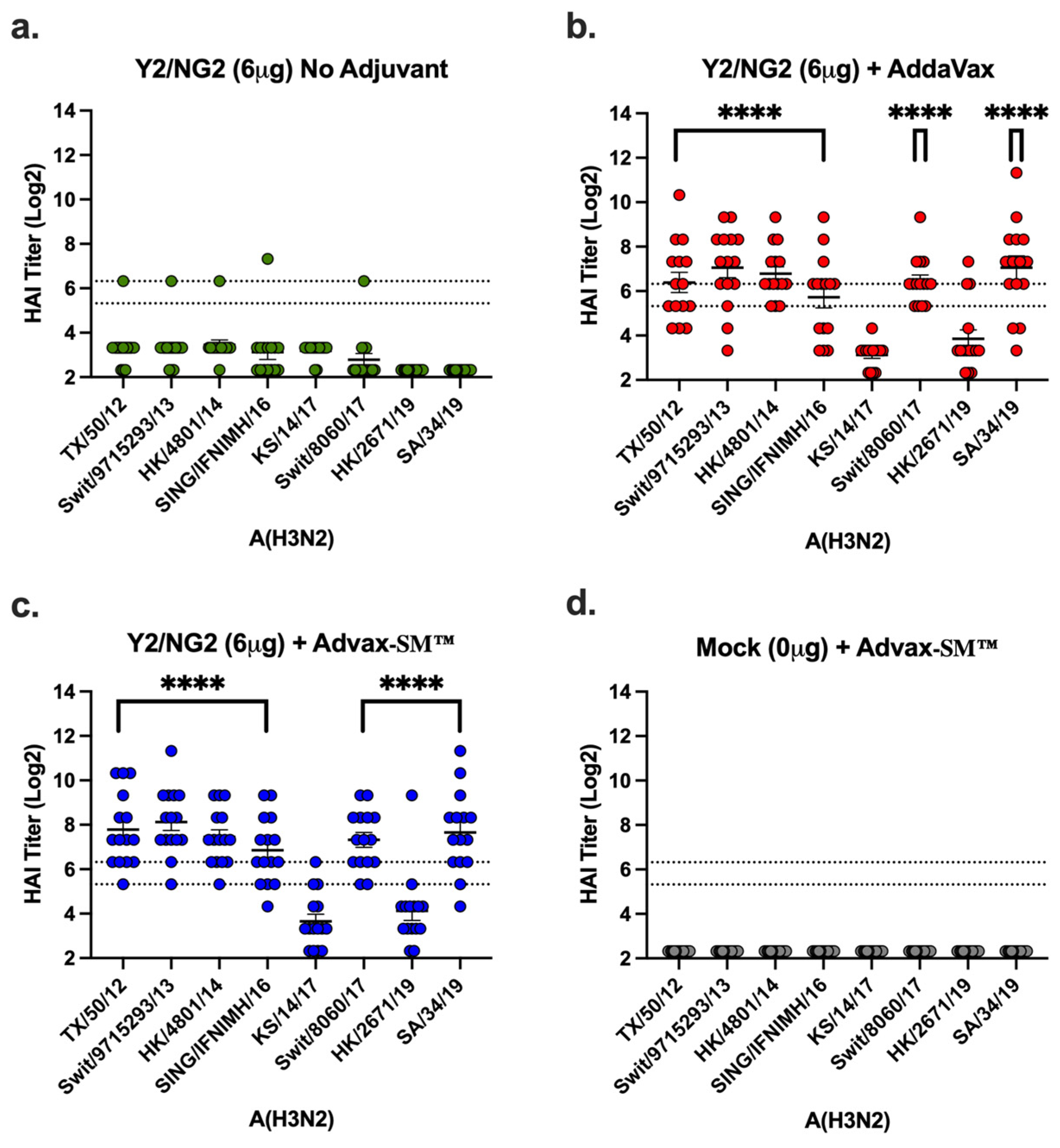
3.4. Advax-SM™ Adjuvant Enhances Serum Hemagglutination-Inhibition (HAI) Titers in Vaccinated Mice
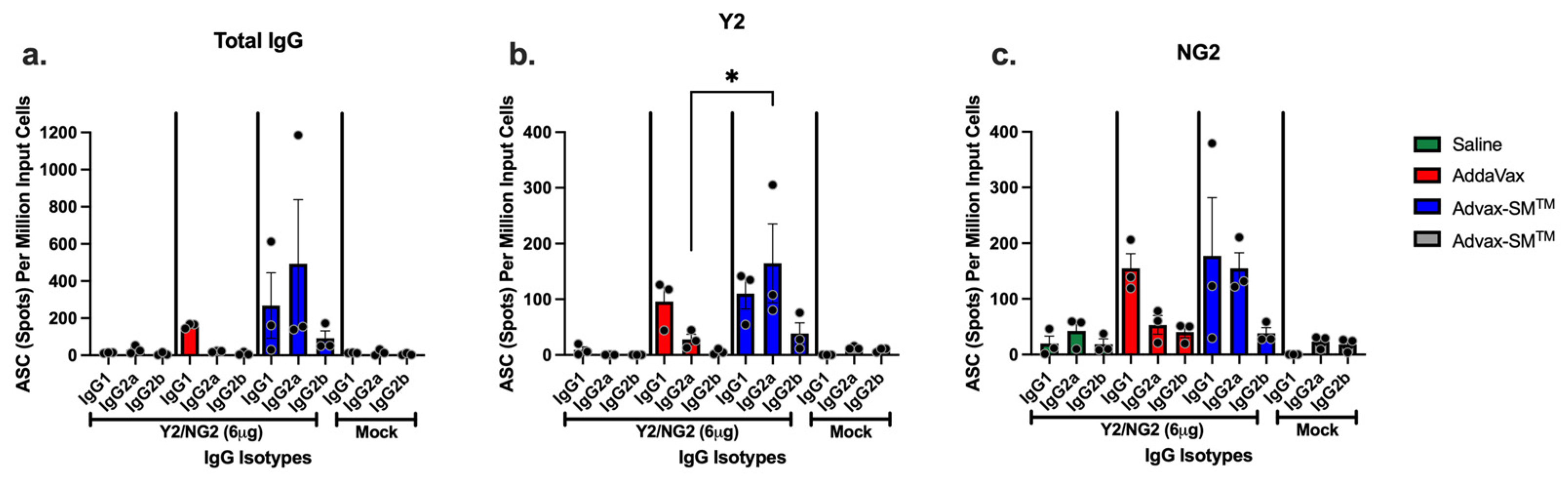
3.5. Advax-SM™ Adjuvant Drives Production of IgG1 and IgG2a Antibody-Secreting Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Paget, J.; Spreeuwenberg, P.; Charu, V.; Taylor, R.J.; Iuliano, A.D.; Bresee, J.; Simonsen, L.; Viboud, C.; Global Seasonal Influenza-Associated Mortality Collaborator Network and GLaMOR Collaborating Teams. Global mortality associated with seasonal influenza epidemics: New burden estimates and predictors from the GLaMOR Project. J. Glob. Health 2019, 9, 020421. [Google Scholar] [CrossRef]
- Simonsen, L.; Fukuda, K.; Schonberger, L.B.; Cox, N.J. The Impact of Influenza Epidemics on Hospitalizations. J. Infect. Dis. 2000, 181, 831–837. [Google Scholar] [CrossRef]
- Chen, J.; Deng, Y.-M. Influenza virus antigenic variation, host antibody production and new approach to control epidemics. Virol. J. 2009, 6, 30. [Google Scholar] [CrossRef] [PubMed]
- Blackburne, B.P.; Hay, A.J.; Goldstein, R.A. Changing Selective Pressure during Antigenic Changes in Human Influenza H3. PLoS Pathog. 2008, 4, e1000058. [Google Scholar] [CrossRef]
- Kim, H.; Webster, R.G.; Webby, R.J. Influenza Virus: Dealing with a Drifting and Shifting Pathogen. Viral Immunol. 2018, 31, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.P.; Gordon, M.L. An overview of influenza A virus genes, protein functions, and replication cycle highlighting important updates. Virus Genes 2022, 58, 255–269. [Google Scholar] [CrossRef]
- Connaris, H.; Govorkova, E.A.; Ligertwood, Y.; Dutia, B.M.; Yang, L.; Tauber, S.; Taylor, M.A.; Alias, N.; Hagan, R.; Nash, A.A.; et al. Prevention of influenza by targeting host receptors using engineered proteins. Proc. Natl. Acad. Sci. USA 2014, 111, 6401–6406. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Bivalent H1 and H3 COBRA Recombinant Hemagglutinin Vaccines Elicit Seroprotective Antibodies against H1N1 and H3N2 Influenza Viruses from 2009 to 2019. J. Virol. 2022, 96, e0165221. [Google Scholar] [CrossRef]
- Alfelali, M.; Khandaker, G.; Booy, R.; Rashid, H. Mismatching between circulating strains and vaccine strains of influenza: Effect on Hajj pilgrims from both hemispheres. Hum. Vaccines Immunother. 2015, 12, 709–715. [Google Scholar] [CrossRef]
- Clem, A.S. Fundamentals of vaccine immunology. J. Glob. Infect. Dis. 2011, 3, 73–78. [Google Scholar] [CrossRef]
- Lu, X.; Edwards, L.E.; Desheva, J.A.; Nguyen, D.C.; Rekstin, A.; Stephenson, I.; Szretter, K.; Cox, N.J.; Rudenko, L.G.; Klimov, A.; et al. Cross-protective immunity in mice induced by live-attenuated or inactivated vaccines against highly pathogenic influenza A (H5N1) viruses. Vaccine 2006, 24, 6588–6593. [Google Scholar] [CrossRef]
- Vaccine Adjuvants: Mechanisms and Platforms|Signal Transduction and Targeted Therapy. Available online: https://www.nature.com/articles/s41392-023-01557-7#citeas (accessed on 11 December 2023).
- Perrie, Y.; Mohammed, A.R.; Kirby, D.J.; McNeil, S.E.; Bramwell, V.W. Vaccine adjuvant systems: Enhancing the efficacy of sub-unit protein antigens. Int. J. Pharm. 2008, 364, 272–280. [Google Scholar] [CrossRef] [PubMed]
- O’hagan, D.T.; Lodaya, R.N.; Lofano, G. The continued advance of vaccine adjuvants—‘We can work it out’. Semin. Immunol. 2020, 50, 101426. [Google Scholar] [CrossRef]
- O’Hagan, D.T. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Expert Rev. Vaccines 2007, 6, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, X.; Dang, Y.; Duan, P.; Xu, W.; Zhang, X.; Wang, S.; Luo, J.; Li, X. AddaVax-Adjuvanted H5N8 Inactivated Vaccine Induces Robust Humoral Immune Response against Different Clades of H5 Viruses. Vaccines 2022, 10, 1683. [Google Scholar] [CrossRef]
- Goff, P.H.; Eggink, D.; Seibert, C.W.; Hai, R.; Martínez-Gil, L.; Krammer, F.; Palese, P. Adjuvants and Immunization Strategies to Induce Influenza Virus Hemagglutinin Stalk Antibodies. PLoS ONE 2013, 8, e79194. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef]
- Huang, Y.; França, M.S.; Allen, J.D.; Shi, H.; Ross, T.M. Next generation of computationally optimized broadly reactive HA vaccines elicited cross-reactive immune responses and provided protection against H1N1 virus infection. Vaccines 2021, 9, 793. [Google Scholar] [CrossRef]
- Giles, B.M.; Crevar, C.J.; Carter, D.M.; Bissel, S.J.; Schultz-Cherry, S.; Wiley, C.A.; Ross, T.M. A Computationally Optimized Hemagglutinin Virus-like Particle Vaccine Elicits Broadly Reactive Antibodies that Protect Nonhuman Primates from H5N1 Infection. J. Infect. Dis. 2012, 205, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Carlock, M.A.; Ross, T.M. A computationally optimized broadly reactive hemagglutinin vaccine elicits neutralizing antibodies against influenza B viruses from both lineages. Sci. Rep. 2023, 13, 15911. [Google Scholar] [CrossRef]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Principi, N. Different influenza vaccine formulations and adjuvants for childhood influenza vaccination. Vaccine 2011, 29, 7535–7541. [Google Scholar] [CrossRef] [PubMed]
- Sakala, I.G.; Honda-Okubo, Y.; Petrovsky, N. Developmental and reproductive safety of Advax-CpG55. 2TM adjuvanted COVID-19 and influenza vaccines in mice. Vaccine 2023, 41, 6093–6104. [Google Scholar] [CrossRef] [PubMed]
- Honda-Okubo, Y.; Saade, F.; Petrovsky, N. Advax™, a polysaccharide adjuvant derived from delta inulin, provides improved influenza vaccine protection through broad-based enhancement of adaptive immune responses. Vaccine 2012, 30, 5373–5381. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Krieg, A.M. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv. Drug Deliv. Rev. 2009, 61, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Jegerlehner, A.; Maurer, P.; Bessa, J.; Hinton, H.J.; Kopf, M.; Bachmann, M.F. TLR9 Signaling in B Cells Determines Class Switch Recombination to IgG2a. J. Immunol. 2007, 178, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Yin, Y.; Yu, Q.; Huang, L.; Wang, X.; Lin, J.; Yang, Q. CpG Oligodeoxynucleotides Facilitate Delivery of Whole Inactivated H9N2 Influenza Virus via Transepithelial Dendrites of Dendritic Cells in Nasal Mucosa. J. Virol. 2015, 89, 5904–5918. [Google Scholar] [CrossRef] [PubMed]
- Saade, F.; Honda-Okubo, Y.; Trec, S.; Petrovsky, N. A novel hepatitis B vaccine containing Advax™, a polysaccharide adjuvant derived from delta inulin, induces robust humoral and cellular immunity with minimal reactogenicity in preclinical testing. Vaccine 2013, 31, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; A Prow, N.; Wang, W.; Tan, C.S.; Coyle, M.; Douma, A.; Hobson-Peters, J.; Kidd, L.; A Hall, R.; Petrovsky, N. Safety and immunogenicity of a delta inulin-adjuvanted inactivated Japanese encephalitis virus vaccine in pregnant mares and foals. Veter. Res. 2014, 45, 130. [Google Scholar] [CrossRef]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Ecker, J.W.; Kirchenbaum, G.A.; Pierce, S.R.; Skarlupka, A.L.; Abreu, R.B.; Cooper, R.E.; Taylor-Mulneix, D.; Ross, T.M.; Sautto, G.A. High-yield expression and purification of recombinant influenza virus proteins from stably-transfected mammalian cell Lines. Vaccines 2020, 8, 462. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. WHO Global Influenza Surveillance Network: Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza. 2011. Available online: https://apps.who.int/iris/handle/10665/44518 (accessed on 10 August 2023).
- Carter, D.M.; Darby, C.A.; Lefoley, B.C.; Crevar, C.J.; Alefantis, T.; Oomen, R.; Anderson, S.F.; Strugnell, T.; Cortés-Garcia, G.; Vogel, T.U.; et al. Design and characterization of a computationally optimized broadly reactive hemagglutinin vaccine for H1N1 influenza viruses. J. Virol. 2016, 90, 4720–4734. [Google Scholar] [CrossRef] [PubMed]
- Committee for Medicinal Products for Human Use. Guideline on Influenza Vaccines—Non-Clinical and Clinical Module; EMA/CHMP/VWP/457259/2014; European Medicines Agency: Amsterdam, The Netherlands, 2016; Volume 44, pp. 1–31. [Google Scholar]
- Hannoun, C. The evolving history of influenza viruses and influenza vaccines. Expert Rev. Vaccines 2013, 12, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-S.; Webby, R.J. Traditional and New Influenza Vaccines. Clin. Microbiol. Rev. 2013, 26, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.C.; Eick, A.A.; Blazes, D.L.; Lee, S.-E.; Perdue, C.L.; Lipnick, R.; Vest, K.G.; Russell, K.L.; DeFraites, R.F.; Sanchez, J.L. Seasonal Influenza Vaccine and Protection against Pandemic (H1N1) 2009-Associated Illness among US Military Personnel. PLoS ONE 2010, 5, e10722. [Google Scholar] [CrossRef] [PubMed]
- Tsilibary, E.-P.; Charonis, S.A.; Georgopoulos, A.P. Vaccines for Influenza. Vaccines 2021, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Buckland, B.C. The development and manufacture of influenza vaccines. Hum. Vaccine. Immunother. 2015, 11, 1357–1360. [Google Scholar] [CrossRef]
- Arunachalam, A.B.; Post, P.; Rudin, D. Unique features of a recombinant haemagglutinin influenza vaccine that influence vaccine performance. NPJ Vaccines 2021, 6, 144. [Google Scholar] [CrossRef]
- Rudenko, L.; Yeolekar, L.; Kiseleva, I.; Isakova-Sivak, I. Development and approval of live attenuated influenza vaccines based on Russian master donor viruses: Process challenges and success stories. Vaccine 2016, 34, 5436–5441. [Google Scholar] [CrossRef]
- Dunkle, L.M.; Izikson, R. Recombinant hemagglutinin influenza vaccine provides broader spectrum protection. Expert Rev. Vaccines 2016, 15, 957–966. [Google Scholar] [CrossRef]
- Wilkins, A.L.; Kazmin, D.; Napolitani, G.; Clutterbuck, E.A.; Pulendran, B.; Siegrist, C.-A.; Pollard, A.J. AS03- and MF59-Adjuvanted Influenza Vaccines in Children. Front. Immunol. 2017, 8, 1760. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Russell, R.F.; Kinnear, E. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2017, 14, 550–564. [Google Scholar] [CrossRef]
- Lindert, K.; Leav, B.; Heijnen, E.; Barrett, J.; Nicolay, U. Cumulative clinical experience with MF59-adjuvanted trivalent seasonal influenza vaccine in young children and adults 65 years of age and older. Int. J. Infect. Dis. 2019, 85, S10–S17. [Google Scholar] [CrossRef]
- Podda, A. The adjuvanted influenza vaccines with novel adjuvants: Experience with the MF59-adjuvanted vaccine. Vaccine 2001, 19, 2673–2680. [Google Scholar] [CrossRef]
- Orsi, A.; Ansaldi, F.; de Florentiis, D.; Ceravolo, A.; Parodi, V.; Canepa, P.; Coppelli, M.; Icardi, G.; Durando, P. Cross-protection against drifted influenza viruses. Hum. Vaccines Immunother. 2013, 9, 582–590. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, D.T.; Rappuoli, R.; De Gregorio, E.; Tsai, T.; Del Giudice, G. MF59 adjuvant: The best insurance against influenza strain diversity. Expert Rev. Vaccines 2011, 10, 447. [Google Scholar] [CrossRef]
- Principi, N.; Esposito, S. Adjuvanted influenza vaccines. Hum. Vaccines Immunother. 2012, 8, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Even-Or, O.; Samira, S.; Ellis, R.; Kedar, E.; Barenholz, Y. Adjuvanted influenza vaccines. Expert Rev. Vaccines 2013, 12, 1095–1108. [Google Scholar] [CrossRef]
- Soema, P.C.; Kompier, R.; Amorij, J.-P.; Kersten, G.F. Current and next generation influenza vaccines: Formulation and production strategies. Eur. J. Pharm. Biopharm. 2015, 94, 251–263. [Google Scholar] [CrossRef]
- Tate, M.D.; Mansell, A. An update on the NLRP3 inflammasome and influenza: The road to redemption or perdition? Curr. Opin. Immunol. 2018, 54, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Patron, M.; Moreno, M.; Baz, M.; Roehe, P.M.; Cibulski, S.P.; Silveira, F. ISCOM-like Nanoparticles Formulated with Quillaja brasiliensis Saponins Are Promising Adjuvants for Seasonal Influenza Vaccines. Vaccines 2021, 9, 1350. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, E.; Caproni, E.; Ulmer, J.B. Vaccine Adjuvants: Mode of Action. Front. Immunol. 2013, 4, 214. [Google Scholar] [CrossRef]
- Allison, A.C. Squalene and Squalane Emulsions as Adjuvants. Methods 1999, 19, 87–93. [Google Scholar] [CrossRef]
- Nguyen-Contant, P.; Sangster, M.Y.; Topham, D.J. Squalene-Based Influenza Vaccine Adjuvants and Their Impact on the Hemagglutinin-Specific B Cell Response. Pathogens 2021, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Schetters, S.T.T.; Kruijssen, L.J.W.; Crommentuijn, M.H.W.; Kalay, H.; Haan, J.M.M.D.; van Kooyk, Y. Immunological dynamics after subcutaneous immunization with a squalene-based oil-in-water adjuvant. FASEB J. 2020, 34, 12406–12418. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 855. [Google Scholar] [CrossRef]
- Zhu, J.; Jankovic, D.; Oler, A.J.; Wei, G.; Sharma, S.; Hu, G.; Guo, L.; Yagi, R.; Yamane, H.; Punkosdy, G.; et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 2012, 37, 660–673. [Google Scholar] [CrossRef]
- Maier, E.; Duschl, A.; Horejs-Hoeck, J. STAT6-dependent and -independent mechanisms in Th2 polarization. Eur. J. Immunol. 2012, 42, 2827–2833. [Google Scholar] [CrossRef]
- Monteleone, I.; Monteleone, G.; Blanco, G.D.V.; Vavassori, P.; Cucchiara, S.; MacDonald, T.T.; Pallone, F. Regulation of the T helper cell type 1 transcription factor T-bet in coeliac disease mucosa. Gut 2004, 53, 1090–1095. [Google Scholar] [CrossRef]
- Thieu, V.T.; Yu, Q.; Chang, H.C.; Yeh, N.; Nguyen, E.T.; Sehra, S.; Kaplan, M.H. Stat4 is required for T-bet to promote IL-12-dependent Th1 fate determination. Immunity 2008, 29, 679. [Google Scholar] [CrossRef] [PubMed]
- Severinson, E. Identification of the IgG1 Induction Factor (Interleukin 4). Front. Immunol. 2014, 5, 628. [Google Scholar] [CrossRef]
- Yalcindag, A.; He, R.; Laouini, D.; Alenius, H.; Carroll, M.; Oettgen, H.C.; Geha, R.S. The complement component C3 plays a critical role in both Th1 and Th2 responses to antigen. J. Allergy Clin. Immunol. 2006, 117, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.; Reiser, H. IL-4: Role in disease and regulation of production. Clin. Exp. Immunol. 1998, 113, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Petrovsky, N.; Cooper, P.D. Advax™, a novel microcrystalline polysaccharide particle engineered from delta inulin, provides robust adjuvant potency together with tolerability and safety. Vaccine 2015, 33, 5920–5926. [Google Scholar] [CrossRef] [PubMed]
- Salehen, N.; Stover, C. The role of complement in the success of vaccination with conjugated vs. unconjugated polysaccharide antigen. Vaccine 2008, 26, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Toapanta, F.R.; Ross, T.M. Complement-Mediated Activation of the Adaptive Immune Responses: Role of C3d in Linking the Innate and Adaptive Immunity. Immunol. Res. 2006, 36, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Aleebrahim-Dehkordi, E.; Molavi, B.; Mokhtari, M.; Deravi, N.; Fathi, M.; Fazel, T.; Mohebalizadeh, M.; Koochaki, P.; Shobeiri, P.; Hasanpour-Dehkordi, A. T helper type (Th1/Th2) responses to SARS-CoV-2 and influenza A (H1N1) virus: From cytokines produced to immune responses. Transpl. Immunol. 2022, 70, 101495. [Google Scholar] [CrossRef]
- Cao, P.; Wang, Z.; Yan, A.W.C.; McVernon, J.; Xu, J.; Heffernan, J.M.; Kedzierska, K.; McCaw, J.M. On the Role of CD8+ T Cells in Determining Recovery Time from Influenza Virus Infection. Front. Immunol. 2016, 7, 611. [Google Scholar] [CrossRef]
- Jia, R.; Liu, S.; Xu, J.; Liang, X. IL16 deficiency enhances Th1 and cytotoxic T lymphocyte response against influenza A virus infection. Biosci. Trends 2019, 13, 516–522. [Google Scholar] [CrossRef]
- Kidd, P. Th1/Th2 balance: The hypothesis, its limitations, and implications for health and disease. Altern. Med. Rev. 2003, 8, 223–246. [Google Scholar]



 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez, P.L.; Andre, G.; Antipov, A.; Petrovsky, N.; Ross, T.M. Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines. Vaccines 2024, 12, 455. https://doi.org/10.3390/vaccines12050455
Sanchez PL, Andre G, Antipov A, Petrovsky N, Ross TM. Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines. Vaccines. 2024; 12(5):455. https://doi.org/10.3390/vaccines12050455
Chicago/Turabian StyleSanchez, Pedro L., Greiciely Andre, Anna Antipov, Nikolai Petrovsky, and Ted M. Ross. 2024. "Advax-SM™-Adjuvanted COBRA (H1/H3) Hemagglutinin Influenza Vaccines" Vaccines 12, no. 5: 455. https://doi.org/10.3390/vaccines12050455






