Antibody Response after Homologous and Heterologous Prime–Boost COVID-19 Vaccination in a Bangladeshi Residential University Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Study Population and Sample Size Estimation
2.3. Antibody Titer Measurement Assays
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Vaccine Groups
3.3. Anti-Nucleocapsid Antibody Assessment
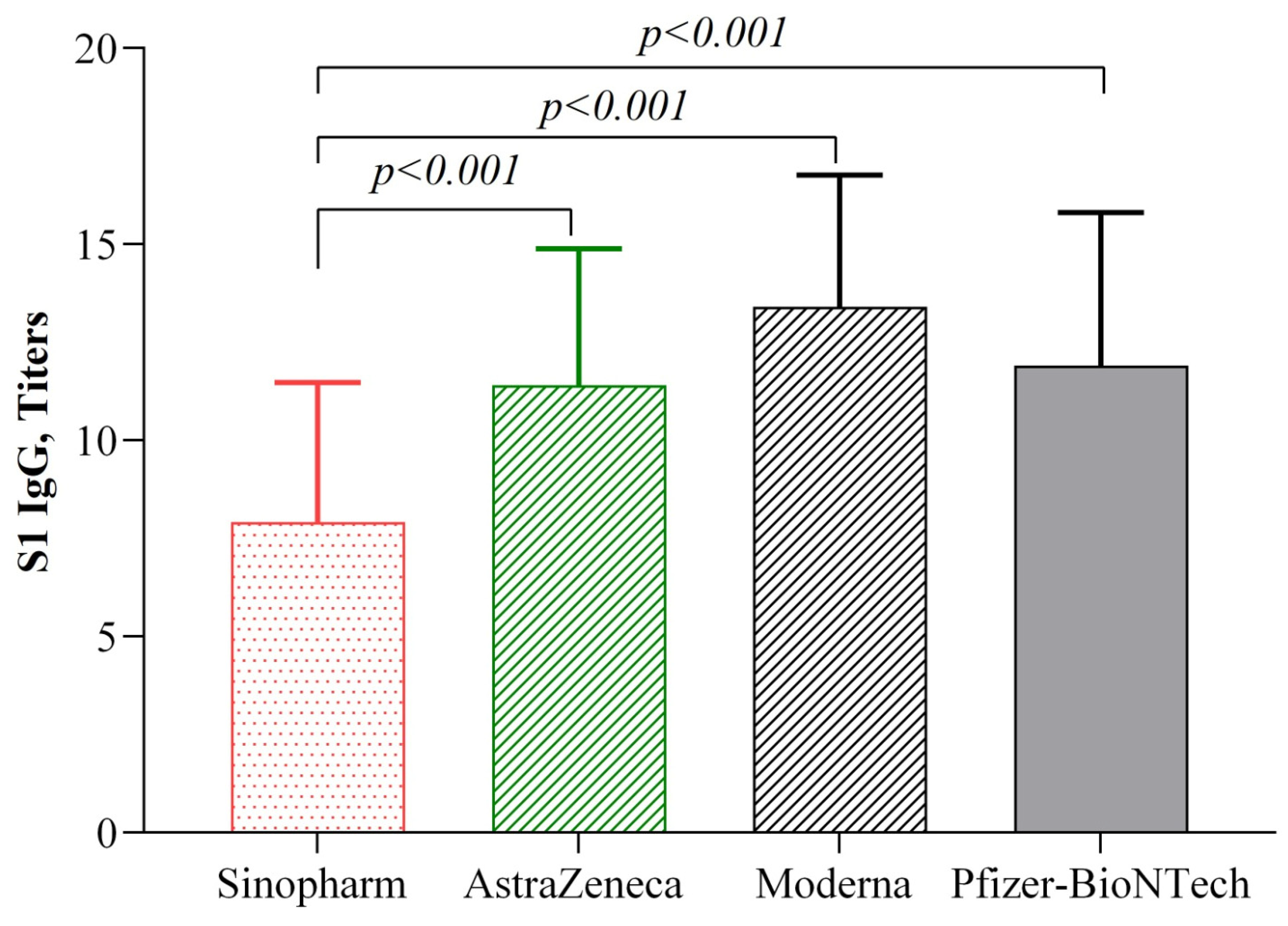
3.4. Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels Post-Second Dose
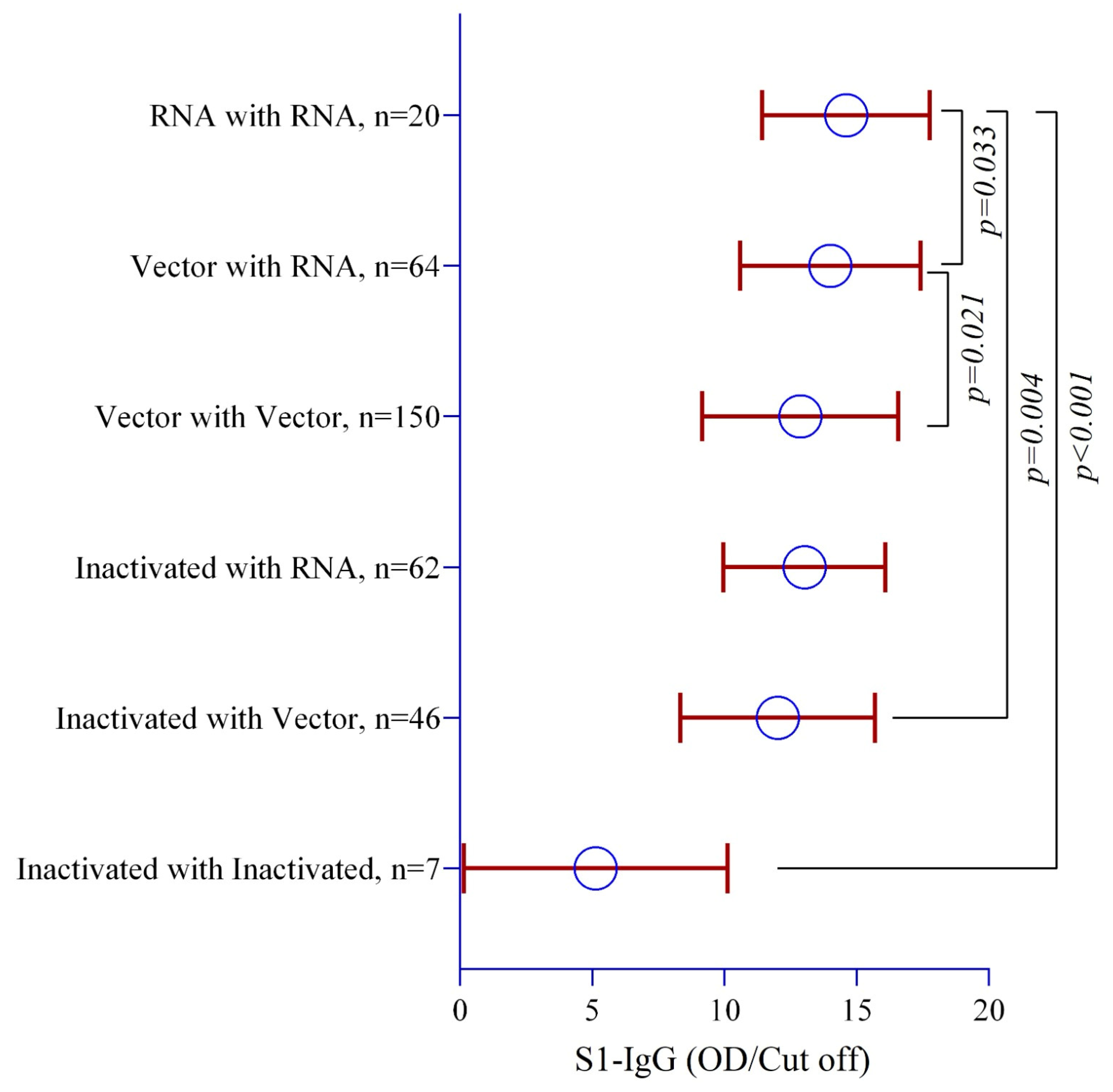
3.5. Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels Post-COVID-19 Vaccine Homologous and Heterologous Prime and Booster Doses
3.6. COVID-19 Vaccine-Wise Waning of Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels
3.7. Association of Gender with Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels
3.8. Effect of COVID-19 Vaccination on the Persistence of Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels
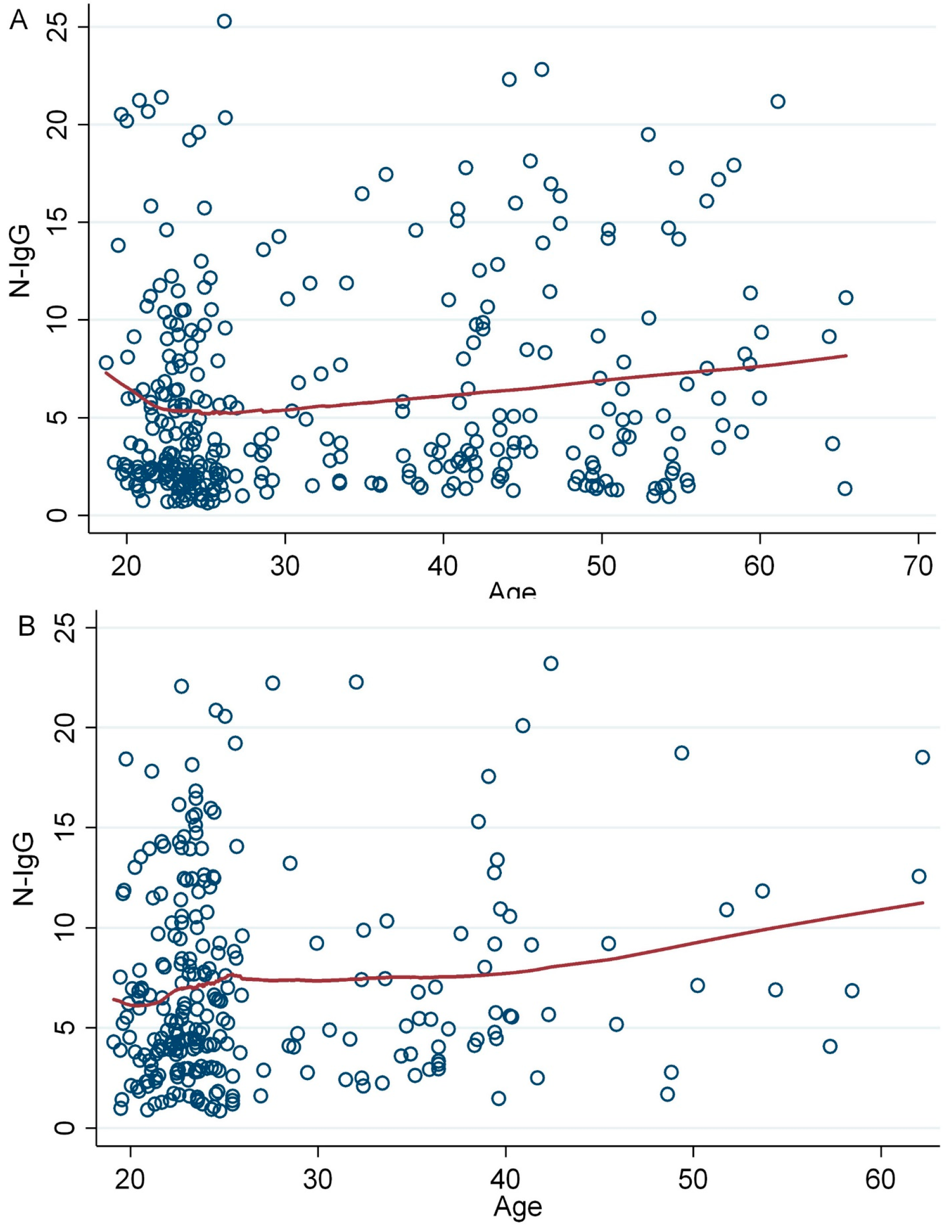
3.9. Association of Age with Anti-Spike IgG (Anti-S1 + RBD IgG) Antibody Levels
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- COVID-19 Epidemiological Update, World Health Organization, Edition 163 Published January 19 2024. Available online: https://www.who.int/publications/m/item/covid-19-epidemiological-update---19-january-2024 (accessed on 10 March 2024).
- A Brief History of Vaccination, World Health Organization. Available online: https://www.who.int/news-room/spotlight/history-of-vaccination/a-brief-history-of-vaccination (accessed on 10 March 2024).
- COVID-19 Vaccination, World Data, World Health Organization. Available online: https://data.who.int/dashboards/covid19/vaccines?n=c (accessed on 10 March 2024).
- Han, X.; Xu, P.; Ye, Q. Analysis of COVID-19 vaccines: Types, thoughts, and application. J. Clin. Lab. Anal. 2021, 35, e23937. [Google Scholar] [CrossRef] [PubMed]
- Khandker, S.S.; Godman, B.; Jawad, M.I.; Meghla, B.A.; Tisha, T.A.; Khondoker, M.U.; Haq, M.A.; Charan, J.; Talukder, A.A.; Azmuda, N.; et al. A systematic review on COVID-19 vaccine strategies, their effectiveness, and issues. Vaccines 2021, 9, 1387. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, M.; Mahmud, S.; Mian, A.U.; Hasan, P.; Muyeed, A.; Ali, M.T.; Ahmed, F.F.; Islam, A.; Rahman, M.M.; Islam, M.; et al. Side effects of COVID-19 vaccines and perceptions about COVID-19 and its vaccines in Bangladesh: A Cross-sectional study. Vaccine X 2022, 12, 100207. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA vaccine against SARS-CoV-2—Preliminary report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Saeed, B.Q.; Al-Shahrabi, R.; Alhaj, S.S.; Alkokhardi, Z.M.; Adrees, A.O. Side effects and perceptions following Sinopharm COVID-19 vaccination. Int. J. Infect. Dis. 2021, 111, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Siegrist, C.A. Vaccine immunology. Vaccines 2008, 5, 17–36. [Google Scholar]
- Iwasaki, A.; Omer, S.B. Why and how vaccines work. Cell 2020, 183, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Deming, M.E.; Lyke, K.E. A ‘mix and match’ approach to SARS-CoV-2 vaccination. Nat. Med. 2021, 27, 1510–1511. [Google Scholar] [CrossRef] [PubMed]
- Kunal, S.; Sakthivel, P.; Gupta, N.; Ish, P. Mix and match COVID-19 vaccines: Potential benefit and perspective from India. Postgrad. Med. J. 2022, 98, e99–e101. [Google Scholar] [CrossRef]
- Jamiruddin, R.; Haq, A.; Khondoker, M.U.; Ali, T.; Ahmed, F.; Khandker, S.S.; Jawad, I.; Hossain, R.; Ahmed, S.; Rahman, S.R.; et al. Antibody response to the first dose of AZD1222 vaccine in COVID-19 convalescent and uninfected individuals in Bangladesh. Expert Rev. Vaccines 2021, 20, 1651–1660. [Google Scholar] [CrossRef]
- Roy, D.; Rahman, M.M.; Chaity, A.S.; Reza, M.A.; Haque, A. Differential persistence of neutralizing antibody against SARS-CoV-2 in post-immunized Bangladeshi population. Sci. Rep. 2022, 12, 14681. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.; Haq, M.A.; Akhtar, E.; Roy, A.K.; Hosen, M.B.; Huda, T.M.N.; Akter, S.; Ahmed, R.; Chowdhury, M.R.; Ferdous, J.; et al. Serosurveillance among urban slum and non-slum populations immunized with COVID-19 vaccines in Bangladesh. Epidemiol. Infect. 2024, 152, e14. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Laurie, K.L.; Cowling, B.J.; Engelhardt, O.G.; Sturm-Ramirez, K.; Sanchez, J.L.; Katz, J.M.; Uyeki, T.M.; Wood, J.; Van Kerkhove, M.D.; et al. CONSISE statement on the reporting of Seroepidemiologic Studies for influenza (ROSES-I statement): An extension of the STROBE statement. Influenza Other Respir Viruses 2017, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Jamiruddin, M.R.; Haq, M.A.; Tomizawa, K.; Kobatake, E.; Mie, M.; Ahmed, S.; Khandker, S.S.; Ali, T.; Jahan, N.; Oishee, M.J.; et al. Longitudinal antibody dynamics against structural proteins of SARS-CoV-2 in three COVID-19 patients shows concurrent development of IgA, IgM, and IgG. J. Inflamm. Res. 2021, 14, 2497. [Google Scholar] [CrossRef]
- Adnan, N.; Haq, M.A.; Tisha, T.A.; Khandker, S.S.; Jamiruddin, M.R.; Sajal, S.S.A.; Akter, S.; Ahmed, M.F.; Raqib, R.; Khondoker, M.U.; et al. Optimizing SARS-CoV-2 Immunoassays for Specificity in Dengue-Co-Endemic Areas. Cureus 2023, 15, e47683. [Google Scholar] [CrossRef]
- Sil, B.K.; Jahan, N.; Haq, M.A.; Oishee, M.J.; Ali, T.; Khandker, S.S.; Kobatake, E.; Mie, M.; Khondoker, M.U.; Jamiruddin, M.R.; et al. Development and performance evaluation of a rapid in-house ELISA for retrospective serosurveillance of SARS-CoV-2. PLoS ONE 2021, 16, e0246346. [Google Scholar] [CrossRef]
- Ciarambino, T.; Para, O.; Giordano, M. Immune system and COVID-19 by sex differences and age. Women’s Health 2021, 17, 17455065211022262. [Google Scholar] [CrossRef]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008, 16, 1833–1840. [Google Scholar] [CrossRef]
- Karikó, K.; Buckstein, M.; Ni, H.; Weissman, D. Suppression of RNA recognition by Toll-like receptors: The impact of nucleoside modification and the evolutionary origin of RNA. Immunity 2005, 23, 165–175. [Google Scholar] [CrossRef]
- Hou, X.; Zaks, T.; Langer, R.; Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 2021, 6, 1078–1094. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Slomka, S.; Zieba, P.; Rosiak, O.; Piekarska, A. Comparison of Post-Vaccination Response between mRNA and Vector Vaccines against SARS-CoV-2 in Terms of Humoral Response after Six Months of Observation. Vaccines 2023, 11, 1625. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Yun, S.G.; Kim, S.W.; Kim, C.G.; Cha, J.H.; Lee, C.; Kang, S.; Park, S.G.; Kim, S.B.; Lee, K.B.; et al. Humoral and Cellular Immune Responses to Vector, Mix-and-Match, or mRNA Vaccines against SARS-CoV-2 and the Relationship between the Two Immune Responses. Microbiol. Spectr. 2022, 10, e02495-21. [Google Scholar] [CrossRef] [PubMed]
- Noor, R. Developmental Status of the Potential Vaccines for the Mitigation of the COVID-19 Pandemic and a Focus on the Effectiveness of the Pfizer-BioNTech and Moderna mRNA Vaccines. Curr. Clin. Microbiol. Rep. 2021, 8, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Kaki, M.; Potluri, V.S.; Kahar, P.; Khanna, D. A comprehensive review of SARS-CoV-2 vaccines: Pfizer, moderna & Johnson & Johnson. Hum. Vaccines Immunother. 2022, 18, 2002083. [Google Scholar]
- Ghiasi, N.; Valizadeh, R.; Arabsorkhi, M.; Hoseyni, T.S.; Esfandiari, K.; Sadighpour, T.; Jahantigh, H.R. Efficacy and side effects of Sputnik V, Sinopharm and AstraZeneca vaccines to stop COVID-19; a review and discussion. Immunopathol. Persa 2021, 7, e31. [Google Scholar] [CrossRef]
- Jahromi, M.; Al Sheikh, M.H. Partial protection of Sinopharm vaccine against SARS-COV-2 during recent outbreak in Bahrain. Microb. Pathog. 2021, 158, 105086. [Google Scholar] [CrossRef] [PubMed]
- Garg, I.; Sheikh, A.B.; Pal, S.; Shekhar, R. Mix-and-match COVID-19 vaccinations (heterologous boost): A review. Infect. Dis. Rep. 2022, 14, 537–546. [Google Scholar] [CrossRef]
- Callaway, E. Mix-and-match COVID vaccines ace the effectiveness test. Nature 2021, 21. [Google Scholar] [CrossRef]
- Rzymski, P.; Szuster-Ciesielska, A.; Dzieciątkowski, T.; Gwenzi, W.; Fal, A. mRNA vaccines: The future of prevention of viral infections? J. Med. Virol. 2023, 95, e28572. [Google Scholar] [CrossRef]
- Bayram, A.; Demirbakan, H.; Günel Karadeniz, P.; Erdoğan, M.; Koçer, I. Quantitation of antibodies against SARS-CoV-2 spike protein after two doses of CoronaVac in healthcare workers. J. Med. Virol. 2021, 93, 5560–5567. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xiang, T.; Liang, B.; Deng, H.; Wang, H.; Feng, X.; Quan, X.; Wang, X.; Li, S.; Lu, S.; et al. Characterization of SARS-CoV-2-Specific humoral and cellular immune responses induced by inactivated COVID-19 vaccines in a real-world setting. Front. Immunol. 2021, 12, 802858. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.H.G.; de Souza, T.D.F.G.; de Carvalho Araújo, F.M.; de Andrade, L.O.M. Dynamics of antibody response to CoronaVac vaccine. J. Med. Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Antoni, D.; Manoussopoulos, Y.; Stefanou, P.; Argyropoulou, S.; Vrioni, G.; Tsakris, A. Age and sex associations of SARS-CoV-2 antibody responses post BNT162b2 vaccination in healthcare workers: A mixed effects model across two vaccination periods. PLoS ONE 2022, 17, e0266958. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.J.; Huang, H.; Zheng, P.; Xue, M.; Ma, J.; Zhan, Z.; Gan, H.; Zeng, Y.; Lin, R.; Li, S.; et al. Humoral immune response of BBIBP COVID-19 vaccination before and after the booster immunization. Allergy 2022, 77, 2404–2414. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.K.; Pradhan, S.K.; Pati, S.; Sahu, S.; Nanda, R.K. Waning of anti-spike antibodies in AZD1222 (ChAdOx1) vaccinated healthcare providers: A prospective longitudinal study. Cureus 2021, 13, e19879. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Guo, Y.; Mao, R.; Zhang, J. Proportion of asymptomatic coronavirus disease 2019: A systematic review and meta-analysis. J. Med. Virol. 2021, 93, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Farid, E.; Herrera-Uribe, J.; Stevenson, N.J. The effect of age, gender and comorbidities upon SARS-CoV-2 spike antibody induction after two doses of sinopharm vaccine and the effect of a Pfizer/BioNtech booster vaccine. Front. Immunol. 2022, 13, 817597. [Google Scholar] [CrossRef]
- Nomura, Y.; Sawahata, M.; Nakamura, Y.; Koike, R.; Katsube, O.; Hagiwara, K.; Niho, S.; Masuda, N.; Tanaka, T.; Sugiyama, K. Attenuation of antibody titers from 3 to 6 months after the second dose of the BNT162b2 vaccine depends on sex, with age and smoking risk factors for lower antibody titers at 6 months. Vaccines 2021, 9, 1500. [Google Scholar] [CrossRef]
- Kusunoki, H.; Ekawa, K.; Ekawa, M.; Kato, N.; Yamasaki, K.; Motone, M.; Shimizu, H. Trends in Antibody Titers after SARS-CoV-2 Vaccination—Insights from Self-Paid Tests at a General Internal Medicine Clinic. Medicines 2023, 10, 27. [Google Scholar] [CrossRef]



| Variables | Outcomes |
|---|---|
| Age | 30.6 ± 11.3 |
| Age category | |
| 18–25 years | 329 (54.1%) |
| 25–45 years | 191 (31.4%) |
| 45–65 years | 88 (15.5%) |
| Gender | |
| Male | 387 (63.7%) |
| Female | 221 (36.3%) |
| Education | |
| Below HSC | 37 (6.1%) |
| HSC | 210 (34.5%) |
| Bachelor | 180 (29.6%) |
| Masters | 106 (17.4%) |
| PhD | 75 (12.3%) |
| Occupation | |
| Student | 375 (61.7%) |
| Teacher | 122 (20.1%) |
| University staff | 111 (18.3%) |
| BMI, Kg/m2 | |
| Underweight (<18.5) | (0.70%) |
| Normal (18.5–24.9) | 439 (72.2%) |
| Overweight (25–29.9) | 161 (26.5%) |
| Comorbidities * | |
| Overall | 178 (29.3%) |
| Male | 124 (32.0%) |
| Female | 54 (24.4%) |
| Fever and pain distribution after the first dose of vaccination | |
| AstraZeneca | 114 (40.4%) |
| Moderna | 22 (61.1%) |
| Pfizer-BioNTech | 2 (10.5%) |
| Sinopharm | 36 (13.4%) |
| Previously infected with COVID-19 | 76 (12.5%) |
| Doses of Vaccine | Overall (n = 606) | Male (n = 385) | Female (n = 221) |
|---|---|---|---|
| Only received first dose | 17 (2.81%) | 16 (4.16%) | 1 (0.45%) |
| Only received both first and second dose | 227 (37.3%) | 118 (30.7%) | 109 (49.3%) |
| Received booster dose | 362 (59.5%) | 251 (65.2%) | 211 (50.2%) |
| Days between Vaccines | |||
| Days between first and second doses | 57.3 ± 35.1 | 58.2 ± 34.5 | 56.2 ± 35.8 |
| Days between second and booster doses | 102.2 ± 42.9 | 101.8 ± 45.3 | 102.7 ± 36.6 |
| Vaccine Types | |||
| Vaccine name (first dose) | |||
| AstreZeneca | 282 (46.5%) | 191 (49.6%) | 91 (41.2%) |
| Moderna | 36 (5.94%) | 19 (4.94%) | 17 (7.69%) |
| Pfizer-BioNTech | 19 (3.10%) | 11 (2.86%) | 8 (3.62%) |
| Sinopharm | 269 (44.2%) | 164 (42.6%) | 105 (47.5%) |
| Vaccine name (second dose) | |||
| AstreZeneca | 280 (46.1%) | 189 (51.2%) | 91 (41.4%) |
| Moderna | 36 (5.90%) | 19 (5.15%) | 17 (7.73%) |
| Pfizer-BioNTech | 18 (3.00%) | 11 (2.98%) | 7 (3.18%) |
| Sinopharm | 255 (41.9%) | 150 (40.7%) | 105 (47.7%) |
| Vaccine name (booster dose) | |||
| AstreZeneca | 208 (57.5%) | 141 (56.2%) | 67 (60.4%) |
| Moderna | 86 (14.1%) | 59 (23.5%) | 27 (24.3%) |
| Pfizer-BioNTech | 60 (9.90%) | 45 (17.9%) | 15 (13.5%) |
| Sinoppharm | 8 (1.30%) | 6 (2.39%) | 2 (1.80%) |
| Variables | Overall (n = 590) | Male (n = 375) | Female (n = 215) | |||
|---|---|---|---|---|---|---|
| β-Coefficient (95% CI) | p-Value | β-Coefficient (95% CI) | p-Value | β-Coefficient (95% CI) | p-Value | |
| First dose | Ref. | Ref. | Ref. | |||
| Second dose | 2.47 (0.59, 4.34) | 0.018 | 2.35 (0.28, 4.43) | 0.035 | 1.24 (−5.37, 7.84) | 0.855 |
| Booster dose | 6.83 (4.97, 8.68) | <0.001 | 6.96 (4.92, 9.00) | <0.001 | 5.19 (−1.49, 11.9) | 0.127 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adnan, N.; Haq, M.A.; Akter, S.; Sajal, S.M.S.A.; Islam, M.F.; Mou, T.J.; Jamiruddin, M.R.; Jubyda, F.T.; Islam, M.S.; Tuli, J.F.; et al. Antibody Response after Homologous and Heterologous Prime–Boost COVID-19 Vaccination in a Bangladeshi Residential University Cohort. Vaccines 2024, 12, 482. https://doi.org/10.3390/vaccines12050482
Adnan N, Haq MA, Akter S, Sajal SMSA, Islam MF, Mou TJ, Jamiruddin MR, Jubyda FT, Islam MS, Tuli JF, et al. Antibody Response after Homologous and Heterologous Prime–Boost COVID-19 Vaccination in a Bangladeshi Residential University Cohort. Vaccines. 2024; 12(5):482. https://doi.org/10.3390/vaccines12050482
Chicago/Turabian StyleAdnan, Nihad, Md. Ahsanul Haq, Salma Akter, S. M. Shafiul Alam Sajal, Md. Fokhrul Islam, Taslin Jahan Mou, Mohd. Raeed Jamiruddin, Fatema Tuz Jubyda, Md. Salequl Islam, Jamsheda Ferdous Tuli, and et al. 2024. "Antibody Response after Homologous and Heterologous Prime–Boost COVID-19 Vaccination in a Bangladeshi Residential University Cohort" Vaccines 12, no. 5: 482. https://doi.org/10.3390/vaccines12050482






