Differential Regulation of DC Function, Adaptive Immunity, and MyD88 Dependence by Two Squalene Emulsion-Based Vaccine Adjuvants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Adjuvants
2.3. Mice
2.4. Immunization
2.5. Serum Antibody Titer
2.6. Hemagglutination Inhibition (HAI) Titer
2.7. Lethal Viral Challenge
2.8. Single-Cell Suspension Preparation
2.9. Immunostaining and Flow Cytometry
2.10. Statistical Analysis
3. Results
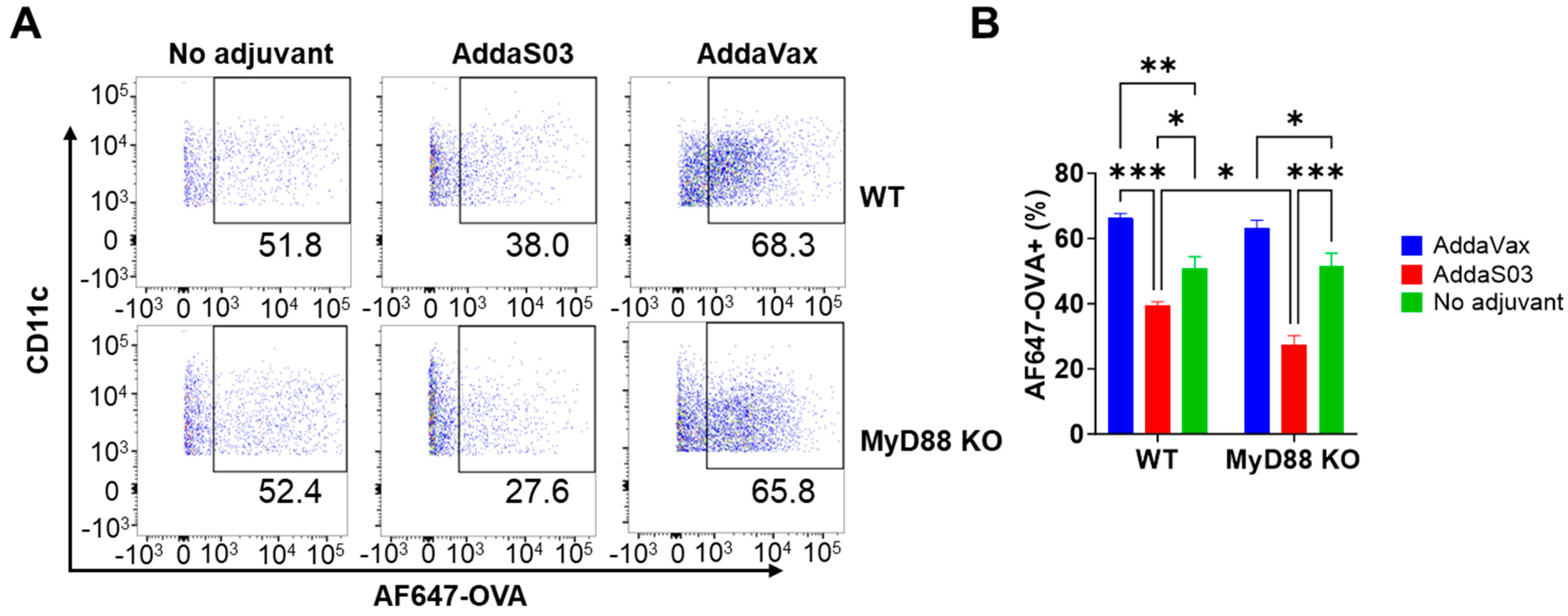
3.1. AddaVax and AddaS03 Differentially Increase Antigen Uptake and DC Maturation at Injection Site
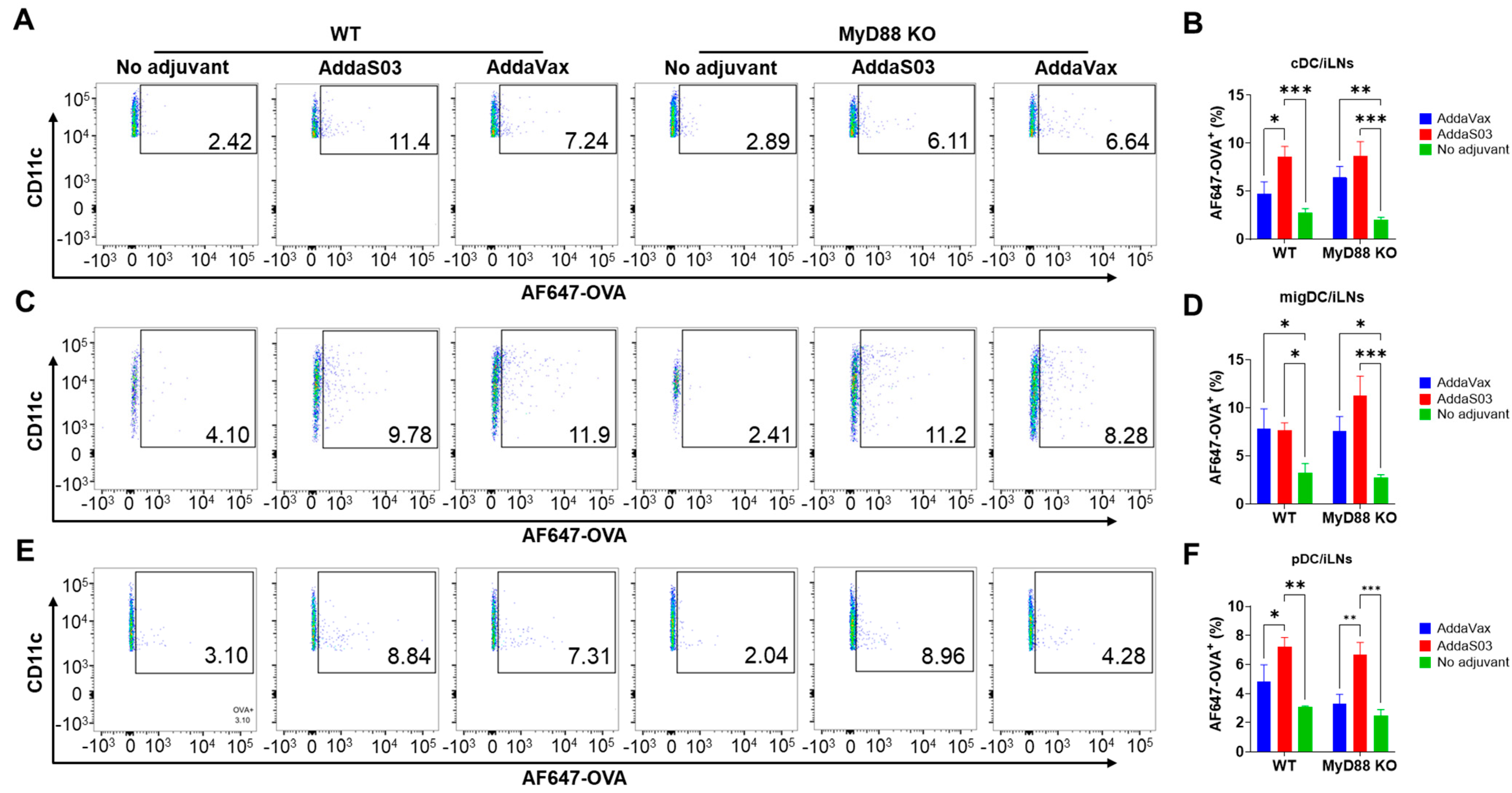
3.2. AddaS03 More Potently Increases Antigen Uptake in Draining LNs
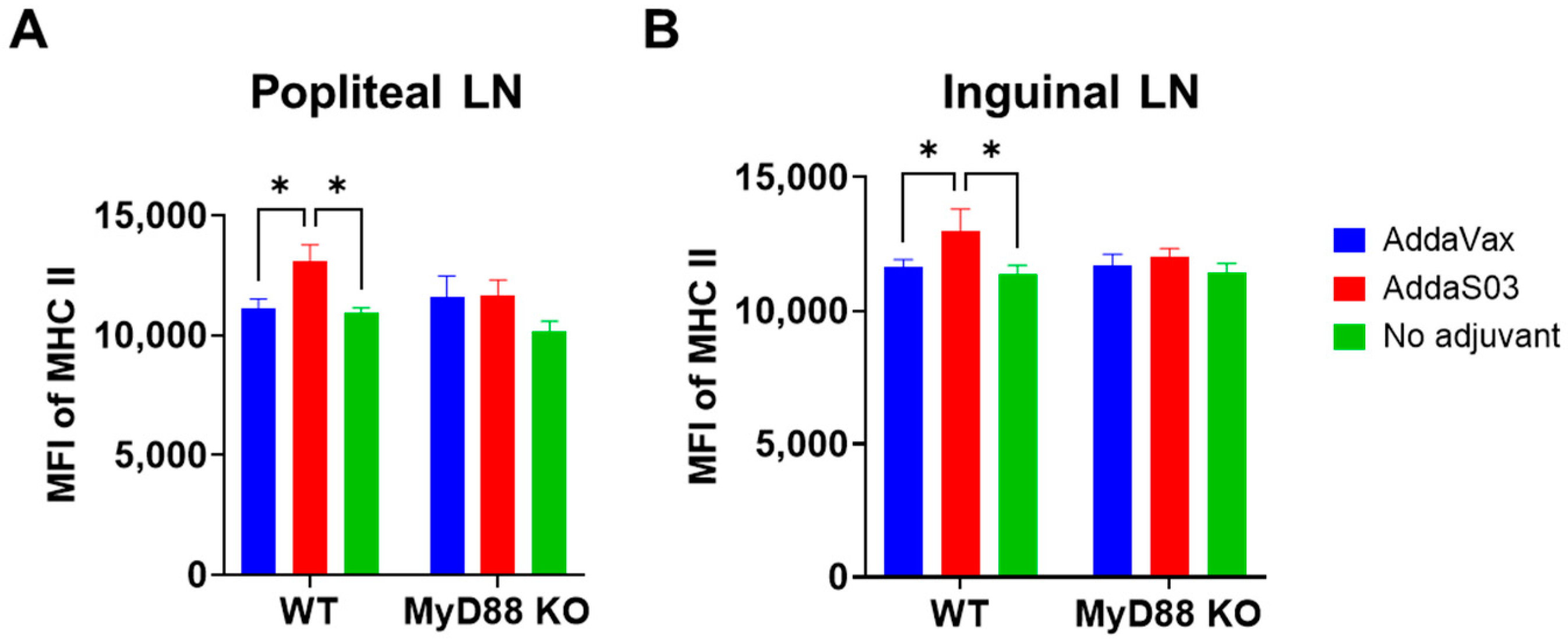
3.3. AddaS03 but Not AddaVax Stimulates DC Maturation in Draining LNs
3.4. AddaVax More Depends on MyD88 to Enhance OVA-Induced Antibody Responses
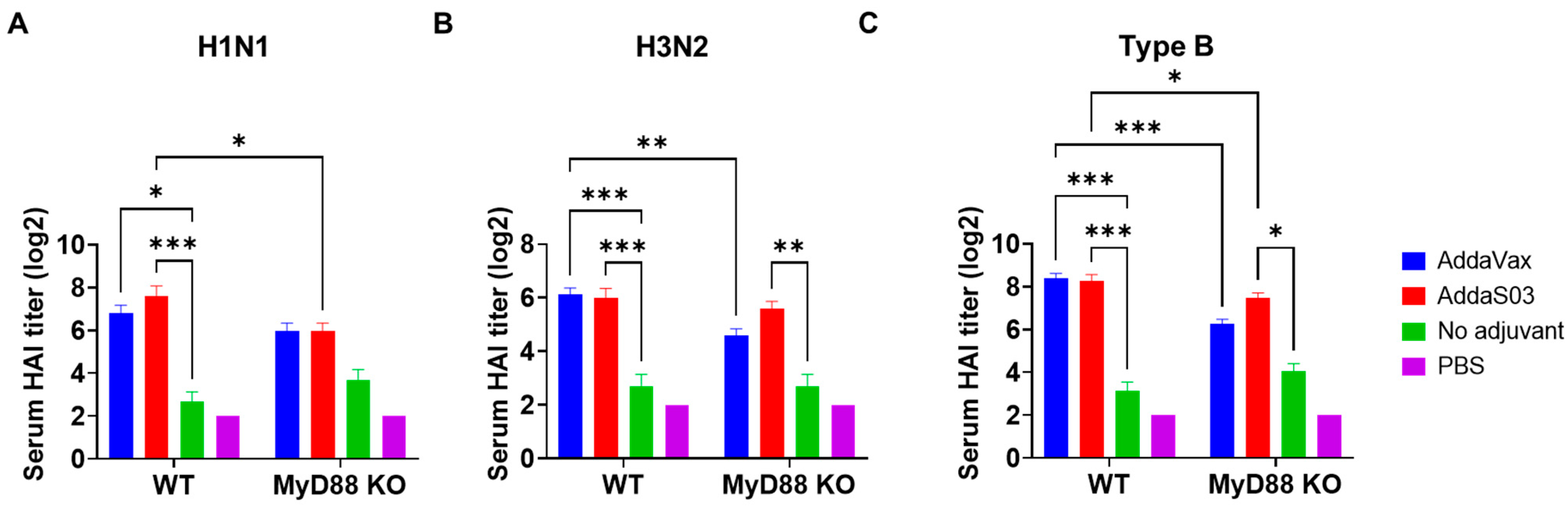
3.5. AddaVax Depends More on MyD88 to Enhance Influenza Vaccine-Induced Antibody Responses and Protection
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jensen, F.C.; Savary, J.R.; Diveley, J.P.; Chang, J.C. Adjuvant activity of incomplete Freund’s adjuvant. Adv. Drug Deliv. Rev. 1998, 32, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Billiau, A.; Matthys, P. Modes of action of Freund’s adjuvants in experimental models of autoimmune diseases. J. Leukoc. Biol. 2001, 70, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: Mechanisms and platforms. Signal Transduct. Target. Ther. 2023, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gong, H.; Sun, Q.; Yang, J.; Yan, X.; Xu, F. Research progress on emulsion vaccine adjuvants. Heliyon 2024, 10, e24662. [Google Scholar] [CrossRef]
- Stills, H.F., Jr. Adjuvants and antibody production: Dispelling the myths associated with Freund’s complete and other adjuvants. ILAR J. 2005, 46, 280–293. [Google Scholar] [CrossRef]
- Chen, X. Emerging adjuvants for intradermal vaccination. Int. J. Pharm. 2022, 632, 122559. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, Y.; Li, Y.; Chen, X. Adjuvantation of Influenza Vaccines to Induce Cross-Protective Immunity. Vaccines 2021, 9, 75. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; van der Most, R.; Lodaya, R.N.; Coccia, M.; Lofano, G. “World in motion”–emulsion adjuvants rising to meet the pandemic challenges. NPJ Vaccines 2021, 6, 158. [Google Scholar] [CrossRef]
- O’Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59((R)) adjuvant: A phoenix that arose from the ashes. Expert Rev. Vaccines 2013, 12, 13–30. [Google Scholar] [CrossRef]
- Shah, R.R.; Taccone, M.; Monaci, E.; Brito, L.A.; Bonci, A.; O’Hagan, D.T.; Amiji, M.M.; Seubert, A. The droplet size of emulsion adjuvants has significant impact on their potency, due to differences in immune cell-recruitment and -activation. Sci. Rep. 2019, 9, 11520. [Google Scholar] [CrossRef]
- Garcon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Expert Rev. Vaccines 2012, 11, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Hauser, M.I.; Muscatello, D.J.; Soh, A.C.Y.; Dwyer, D.E.; Turner, R.M. An indirect comparison meta-analysis of AS03 and MF59 adjuvants in pandemic influenza A(H1N1)pdm09 vaccines. Vaccine 2019, 37, 4246–4255. [Google Scholar] [CrossRef] [PubMed]
- Goll, J.B.; Jain, A.; Jensen, T.L.; Assis, R.; Nakajima, R.; Jasinskas, A.; Coughlan, L.; Cherikh, S.R.; Gelber, C.E.; Khan, S.; et al. The antibody landscapes following AS03 and MF59 adjuvanted H5N1 vaccination. NPJ Vaccines 2022, 7, 103. [Google Scholar] [CrossRef] [PubMed]
- Jackson, L.A.; Campbell, J.D.; Frey, S.E.; Edwards, K.M.; Keitel, W.A.; Kotloff, K.L.; Berry, A.A.; Graham, I.; Atmar, R.L.; Creech, C.B.; et al. Effect of Varying Doses of a Monovalent H7N9 Influenza Vaccine With and Without AS03 and MF59 Adjuvants on Immune Response: A Randomized Clinical Trial. JAMA 2015, 314, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Winokur, P.L.; Hegmann, T.E.; Keitel, W.A.; Bernstein, D.I.; Frey, S.E.; Bryant, C.; Group, D.S. Safety and Immunogenicity of a monovalent inactivated influenza A/H5N8 virus vaccine given with and without AS03 or MF59 adjuvants in healthy adults. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2023. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tegenge, M.A.; Von Tungeln, L.S.; Anderson, S.A.; Mitkus, R.J.; Vanlandingham, M.M.; Forshee, R.A.; Beland, F.A. Comparative pharmacokinetic and biodistribution study of two distinct squalene-containing oil-in-water emulsion adjuvants in H5N1 influenza vaccines. Regul. Toxicol. Pharmacol. 2019, 108, 104436. [Google Scholar] [CrossRef] [PubMed]
- Calabro, S.; Tortoli, M.; Baudner, B.C.; Pacitto, A.; Cortese, M.; O’Hagan, D.T.; De, G.E.; Seubert, A.; Wack, A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 2011, 29, 1812–1823. [Google Scholar] [CrossRef] [PubMed]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; Planty, C.; Elouahabi, A.; Harvengt, P.; Carlsen, H.; et al. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine 2011, 29, 2461–2473. [Google Scholar] [CrossRef] [PubMed]
- Seubert, A.; Calabro, S.; Santini, L.; Galli, B.; Genovese, A.; Valentini, S.; Aprea, S.; Colaprico, A.; D’Oro, U.; Giuliani, M.M.; et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc. Natl. Acad. Sci. USA 2011, 108, 11169–11174. [Google Scholar] [CrossRef]
- Vono, M.; Taccone, M.; Caccin, P.; Gallotta, M.; Donvito, G.; Falzoni, S.; Palmieri, E.; Pallaoro, M.; Rappuoli, R.; Di Virgilio, F.; et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Natl. Acad. Sci. USA 2013, 110, 21095–21100. [Google Scholar] [CrossRef]
- Kim, E.H.; Woodruff, M.C.; Grigoryan, L.; Maier, B.; Lee, S.H.; Mandal, P.; Cortese, M.; Natrajan, M.S.; Ravindran, R.; Ma, H.; et al. Squalene emulsion-based vaccine adjuvants stimulate CD8 T cell, but not antibody responses, through a RIPK3-dependent pathway. Elife 2020, 9, e52687. [Google Scholar] [CrossRef] [PubMed]
- Givord, C.; Welsby, I.; Detienne, S.; Thomas, S.; Assabban, A.; Lima Silva, V.; Molle, C.; Gineste, R.; Vermeersch, M.; Perez-Morga, D.; et al. Activation of the endoplasmic reticulum stress sensor IRE1alpha by the vaccine adjuvant AS03 contributes to its immunostimulatory properties. NPJ Vaccines 2018, 3, 20. [Google Scholar] [CrossRef]
- Steinman, R.M.; Hemmi, H. Dendritic cells: Translating innate to adaptive immunity. Curr. Top. Microbiol. Immunol. 2006, 311, 17–58. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Li, Y.; Zhao, Y.; Chen, X. Overcoming Aging-Associated Poor Influenza Vaccine Responses with CpG 1018 Adjuvant. Vaccines 2022, 10, 1894. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Kang, X.; Kim, K.H.; Zhao, Y.; Li, Y.; Kang, S.M.; Chen, X. Effective adjuvantation of nanograms of influenza vaccine and induction of cross-protective immunity by physical radiofrequency adjuvant. Sci. Rep. 2022, 12, 21249. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, X.; Hossen, M.N.; Kakar, P.; Zhao, Y.; Chen, X. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat. Commun. 2018, 9, 3695. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Y.; Li, Y.; Zhao, Y.; Chen, X. Vaccine delivery alerts innate immune systems for more immunogenic vaccination. JCI Insight 2021, 6, e144627. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.; Chen, X. Comparative tissue proteomics reveals unique action mechanisms of vaccine adjuvants. iScience 2023, 26, 105800. [Google Scholar] [CrossRef]
- Oprescu, S.N.; Yue, F.; Kuang, S. Single-Cell Isolation from Regenerating Murine Muscles for RNA-Sequencing Analysis. STAR Protoc. 2020, 1, 100051. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Voyer, J.; Li, Y.; Chen, X. Flagellin/Virus-like Particle Hybrid Platform with High Immunogenicity, Safety, and Versatility for Vaccine Development. ACS Appl. Mater. Interfaces 2022, 14, 21872–21885. [Google Scholar] [CrossRef]
- Pulendran, B.; SArunachalam, P.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Ho, N.I.; Huis In‘t Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 2018, 9, 2874. [Google Scholar] [CrossRef] [PubMed]
- Lian, Z.X.; Okada, T.; He, X.S.; Kita, H.; Liu, Y.J.; Ansari, A.A.; Kikuchi, K.; Ikehara, S.; Gershwin, M.E. Heterogeneity of dendritic cells in the mouse liver: Identification and characterization of four distinct populations. J. Immunol. 2003, 170, 2323–2330. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.A., 3rd; Murphy, K.M.; Briseno, C.G. Development, Diversity, and Function of Dendritic Cells in Mouse and Human. Cold Spring Harb. Perspect. Biol. 2018, 10, a028613. [Google Scholar] [CrossRef] [PubMed]
- Hongo, D.; Zheng, P.; Dutt, S.; Pawar, R.D.; Meyer, E.; Engleman, E.G.; Strober, S. Identification of Two Subsets of Murine DC1 Dendritic Cells That Differ by Surface Phenotype, Gene Expression, and Function. Front. Immunol. 2021, 12, 746469. [Google Scholar] [CrossRef] [PubMed]
- Harrell, M.I.; Iritani, B.M.; Ruddell, A. Lymph node mapping in the mouse. J. Immunol. Methods 2008, 332, 170–174. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.Y.; Han, S.N. The Role of Vitamin E in Immunity. Nutrients 2018, 10, 1614. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Sagoo, P.; Chan, C.; Yates, J.B.; Campbell, J.; Beutelspacher, S.C.; Foxwell, B.M.; Lombardi, G.; George, A.J. Inhibition of NF-kappa B and oxidative pathways in human dendritic cells by antioxidative vitamins generates regulatory T cells. J. Immunol. 2005, 174, 7633–7644. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Kobiyama, K.; Hayashi, T.; Onishi, M.; Yanagida, Y.; Nakagawa, T.; Hashimoto, M.; Nishinaka, A.; Hirose, J.; Asaoka, Y.; et al. A-910823, a squalene-based emulsion adjuvant, induces T follicular helper cells and humoral immune responses via alpha-tocopherol component. Front. Immunol. 2023, 14, 1116238. [Google Scholar] [CrossRef]
- Khurana, S.; Coyle, E.M.; Manischewitz, J.; King, L.R.; Gao, J.; Germain, R.N.; Schwartzberg, P.L.; Tsang, J.S.; Golding, H.; the CHI Consortium. AS03-adjuvanted H5N1 vaccine promotes antibody diversity and affinity maturation, NAI titers, cross-clade H5N1 neutralization, but not H1N1 cross-subtype neutralization. NPJ Vaccines 2018, 3, 40. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Chearwae, W.; Castellino, F.; Manischewitz, J.; King, L.R.; Honorkiewicz, A.; Rock, M.T.; Edwards, K.M.; Del Giudice, G.; Rappuoli, R.; et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2010, 2, 15ra15. [Google Scholar] [CrossRef] [PubMed]




| AddaVax | MF59® | AddaS03 | AS03® | |
|---|---|---|---|---|
| Squalene | 5% | 4.3% | 5% | 4.3% |
| Span 85 | 0.5% | 0.5% | - | - |
| Tween 80 | 0.5% | 0.5% | 1.8% | 1.9% |
| α-tocopherol | - | - | 5% | 4.7% |
| Buffer | Citrate | Citrate | Phosphate | Phosphate |
| Size | ~160 nm | ~160 nm | ~160 nm | ~160 nm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakkala, J.R.; Li, Y.; Akter, L.; Kang, X.; Chen, X. Differential Regulation of DC Function, Adaptive Immunity, and MyD88 Dependence by Two Squalene Emulsion-Based Vaccine Adjuvants. Vaccines 2024, 12, 531. https://doi.org/10.3390/vaccines12050531
Nakkala JR, Li Y, Akter L, Kang X, Chen X. Differential Regulation of DC Function, Adaptive Immunity, and MyD88 Dependence by Two Squalene Emulsion-Based Vaccine Adjuvants. Vaccines. 2024; 12(5):531. https://doi.org/10.3390/vaccines12050531
Chicago/Turabian StyleNakkala, Jayachandra Reddy, Yibo Li, Labone Akter, Xinliang Kang, and Xinyuan Chen. 2024. "Differential Regulation of DC Function, Adaptive Immunity, and MyD88 Dependence by Two Squalene Emulsion-Based Vaccine Adjuvants" Vaccines 12, no. 5: 531. https://doi.org/10.3390/vaccines12050531
APA StyleNakkala, J. R., Li, Y., Akter, L., Kang, X., & Chen, X. (2024). Differential Regulation of DC Function, Adaptive Immunity, and MyD88 Dependence by Two Squalene Emulsion-Based Vaccine Adjuvants. Vaccines, 12(5), 531. https://doi.org/10.3390/vaccines12050531








