Peptide Vaccines for Hypertension and Diabetes Mellitus
Abstract
:1. Introduction
2. Therapeutic Vaccine for Hypertension
2.1. History of Peptide Vaccine for Hypertension
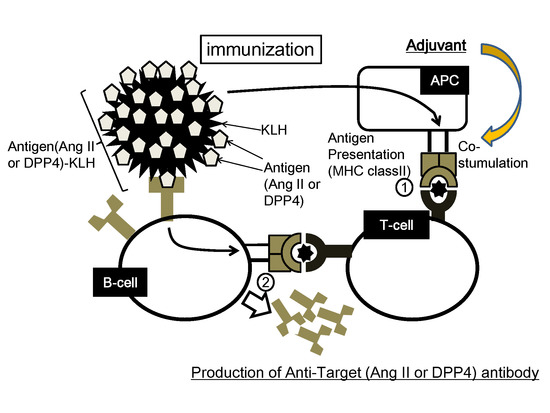
2.2. Concept of Peptide Vaccine for Hypertension
2.3. Experimental Results of Therapeutic Vaccine for Hypertension

3. Therapeutic Vaccine for Diabetes Mellitus
3.1. Design of DPP4 Vaccine
3.2. Experimental Results of Therapeutic Vaccine for Diabetes Mellitus
3.3. Evaluation of Safety Issues for DPP4 Vaccine
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References
- Delavallee, L.; Duvallet, E.; Semerano, L.; Assier, E.; Boissier, M.C. Anti-cytokine vaccination in autoimmune diseases. Swiss Med. Wkly. 2010. [Google Scholar] [CrossRef]
- Dillman, R.O. Cancer immunotherapy. Cancer Biother. Radiopharm. 2011, 26, 1–64. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.; Diamond, D.M.; Gottschall, P.E.; Ugen, K.E.; Dickey, C.; Hardy, J.; Duff, K.; Jantzen, P.; di Carlo, G.; Wilcock, D.; et al. A beta peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature 2000, 408, 982–985. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D. Amyloid-beta immunotherapy for Alzheimer’s disease: The end of the beginning. Nat. Rev. Neurosci. 2002, 3, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Schenk, D.; Barbour, R.; Dunn, W.; Gordon, G.; Grajeda, H.; Guido, T.; Hu, K.; Huang, J.; Johnson-Wood, K.; Khan, K.; et al. Immunization with amyloid-beta attenuates alzheimer-disease-like pathology in the pdapp mouse. Nature 1999, 400, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with ad after abeta42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, I.; Boada Rovira, M.; Sanchez Guerra, M.L.; Rey, M.J.; Costa-Jussa, F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004, 14, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, J.A.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of human alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat. Med. 2003, 9, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Tissot, A.C.; Maurer, P.; Nussberger, J.; Sabat, R.; Pfister, T.; Ignatenko, S.; Volk, H.D.; Stocker, H.; Müller, P.; Jennings, G.T.; et al. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: A double-blind, randomised, placebo-controlled phase IIa study. Lancet 2008, 371, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, S.M.; Auton, T.R.; Downham, M.R.; Sharp, H.L.; Kemp, P.A.; March, J.E.; Martin, H.; Morgan, P.J.; Rushton, A.; Bennett, T.; et al. Active immunization with angiotensin I peptide analogue vaccines selectively reduces the pressor effects of exogenous angiotensin I in conscious rats. Br. J. Pharmacol. 2000, 129, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.J.; Coltart, J.; Gunewardena, K.; Ritter, J.M.; Auton, T.R.; Glover, J.F. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin. Sci. (Lond.) 2004, 107, 167–173. [Google Scholar] [CrossRef]
- Ambuhl, P.M.; Tissot, A.C.; Fulurija, A.; Maurer, P.; Nussberger, J.; Sabat, R.; Nief, V.; Schellekens, C.; Sladko, K.; Roubicek, K.; et al. A vaccine for hypertension based on virus-like particles: Preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 2007, 25, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Wakerlin, G.E. Antibodies to renin as proof of the pathogenesis of sustained renal hypertension. Circulation 1958, 17, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.I.; Skom, J.H.; Wakerlin, G.E. Pathogenesis of spontaneous and pyelonephritic hypertension in the dog. Circ. Res. 1957, 5, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Helmer, O.M. Studies on renin antibodies. Circulation 1958, 17, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Frank, M.H. Renin in experimental renal hypertension in monkeys. Circ. Res. 1963, 12, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Deodhar, S.D.; Haas, E.; Goldblatt, H. Production of antirenin to homologous renin and its effect of experimental renal hypertension. J. Exp. Med. 1964, 119, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Skeggs, L.T.; Kahn, J.R.; Levine, M.; Dorer, F.E.; Lentz, K.E. Chronic one-kidney hypertension in rabbits. I. Treatment with kidney extracts. Circ. Res. 1975, 37, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Guettier, C.; Philippe, M.; Galen, F.X.; Corvol, P.; Menard, J. Active immunization against renin in normotensive marmoset. Proc. Natl. Acad. Sci. USA 1987, 84, 4346–4350. [Google Scholar] [CrossRef] [PubMed]
- Michel, J.B.; Sayah, S.; Guettier, C.; Nussberger, J.; Philippe, M.; Gonzalez, M.F.; Carelli, C.; Galen, F.X.; Menard, J.; Corvol, P. Physiological and immunopathological consequences of active immunization of spontaneously hypertensive and normotensive rats against murine renin. Circulation 1990, 81, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qiu, Z.; Yang, S.; Ding, D.; Chen, F.; Zhou, Y.; Wang, M.; Lin, J.; Yu, X.; Zhou, Z.; et al. Effectiveness and safety of a therapeutic vaccine against angiotensin II receptor type 1 in hypertensive animals. Hypertension 2013, 61, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Chen, X.; Zhou, Y.; Lin, J.; Ding, D.; Yang, S.; Chen, F.; Wang, M.; Zhu, F.; Yu, X.; et al. Therapeutic vaccines against human and rat renin in spontaneously hypertensive rats. PLoS One 2013, 8, e66420. [Google Scholar] [CrossRef] [PubMed]
- Nakagami, F.; Koriyama, H.; Nakagami, H.; Osako, M.K.; Shimamura, M.; Kyutoku, M.; Miyake, T.; Katsuya, T.; Rakugi, H.; Morishita, R. Decrease in blood pressure and regression of cardiovascular complications by angiotensin II vaccine in mice. PLoS One 2013, 8, e60493. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J.; Nauck, M.A. The incretin system: Glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet 2006, 368, 1696–1705. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.; Wiesenthal, S.R.; MacDonald, P.E.; McCall, R.H.; Tchipashvili, V.; Rashid, S.; Satkunarajah, M.; Irwin, D.M.; Shi, Z.Q.; Brubaker, P.L.; et al. Glucagon-like peptide 1 increases insulin sensitivity in depancreatized dogs. Diabetes 1999, 48, 1045–1053. [Google Scholar] [CrossRef] [PubMed]
- Zander, M.; Madsbad, S.; Madsen, J.L.; Holst, J.J. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and β-cell function in type 2 diabetes: A parallel-group study. Lancet 2002, 359, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; McIntosh, C.H.; Pederson, R.A. Degradation of glucose-dependent insulinotropic polypeptide and truncated glucagon-like peptide 1 in vitro and in vivo by dipeptidyl peptidase IV. Endocrinology 1995, 136, 3585–3596. [Google Scholar] [PubMed]
- Baggio, L.L.; Drucker, D.J. Biology of Incretins: GLP-1 and GIP. Gastroenterology 2007, 132, 2131–2157. [Google Scholar] [CrossRef] [PubMed]
- Aschner, P.; Kipnes, M.S.; Lunceford, J.K.; Sanchez, M.; Mickel, C.; Williams-Herman, D.E.; Sitagliptin Study 021 Group. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care 2006, 29, 2632–2637. [Google Scholar] [CrossRef] [PubMed]
- Deacon, C.F.; Holst, J.J. Saxagliptin: A new dipeptidyl peptidase-4 inhibitor for the treatment of type 2 diabetes. Adv. Ther. 2009, 26, 488–499. [Google Scholar]
- Pang, Z.; Nakagami, H.; Osako, M.K.; Koriyama, H.; Nakagami, F.; Tomioka, H.; Shimamura, M.; Kurinami, H.; Takami, Y.; Morishita, Y.; et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E1256–E1263. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagami, H.; Koriyama, H.; Morishita, R. Peptide Vaccines for Hypertension and Diabetes Mellitus. Vaccines 2014, 2, 832-840. https://doi.org/10.3390/vaccines2040832
Nakagami H, Koriyama H, Morishita R. Peptide Vaccines for Hypertension and Diabetes Mellitus. Vaccines. 2014; 2(4):832-840. https://doi.org/10.3390/vaccines2040832
Chicago/Turabian StyleNakagami, Hironori, Hiroshi Koriyama, and Ryuichi Morishita. 2014. "Peptide Vaccines for Hypertension and Diabetes Mellitus" Vaccines 2, no. 4: 832-840. https://doi.org/10.3390/vaccines2040832