A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant
Abstract
:1. Introduction
2. Material and Methods
2.1. Mice
2.2. Expression and Purification of Recombinant Vaults
2.3. Chlamydia Preparation, Immunization and Challenge of Mice
2.4. Histology, Hematoxylin, and Eosin and Trichrome Stain
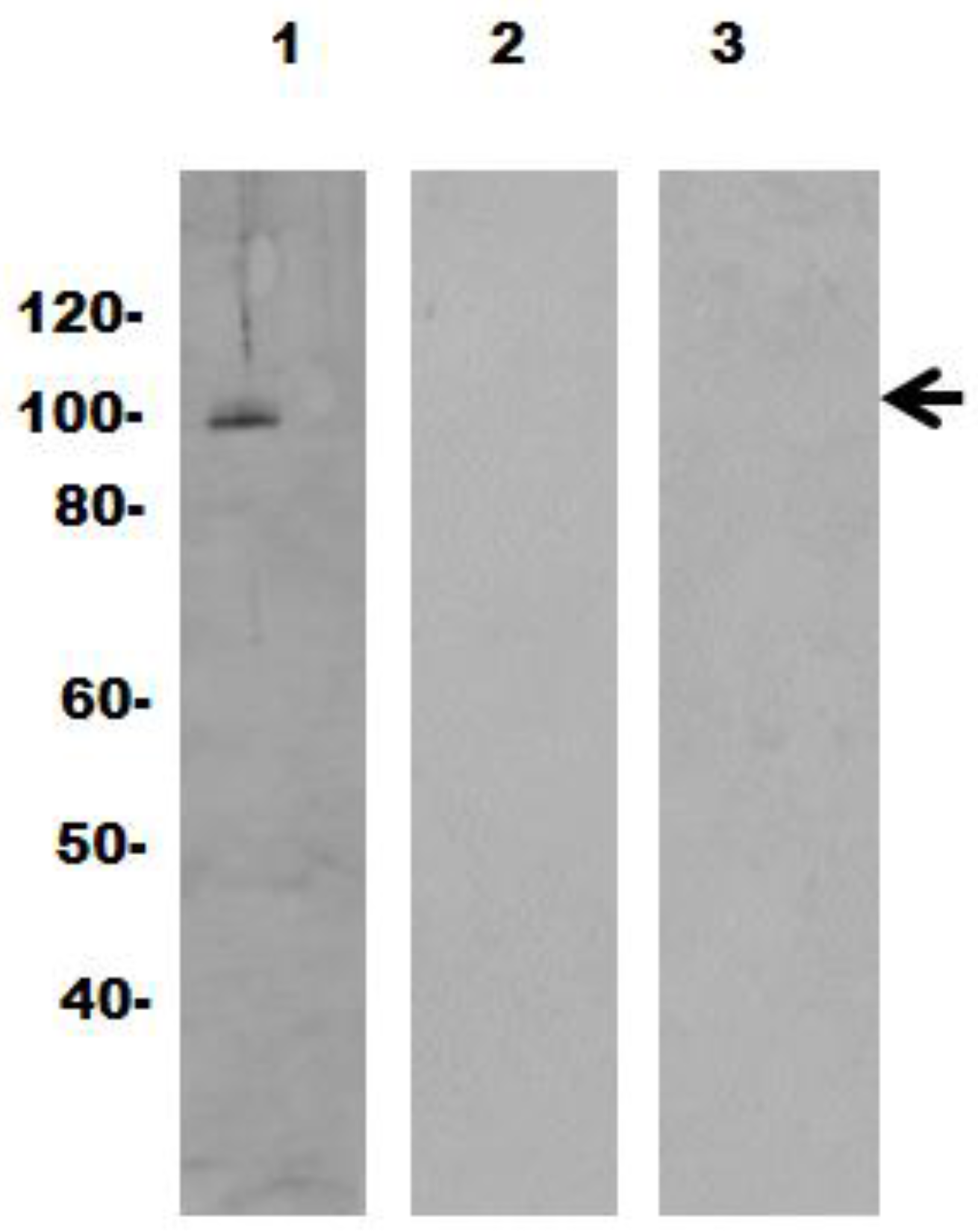
2.5. Gel Electrophoresis and Immunoblotting
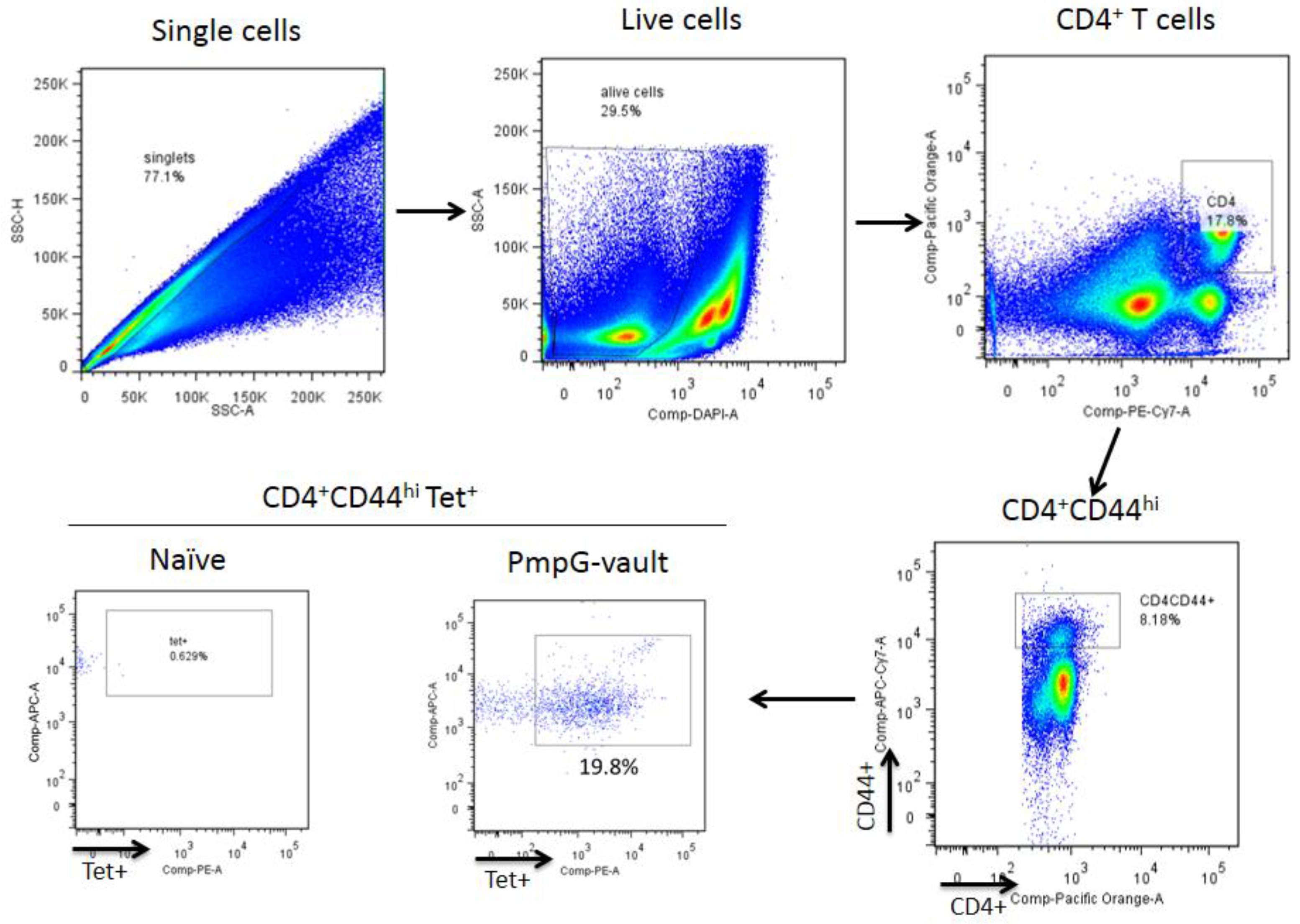
2.6. Isolation of Lymphocytes and Tetramer Enrichment
2.7. Tetramer Enrichment and Flow Cytometry Staining
2.8. Statistical Analysis
3. Results
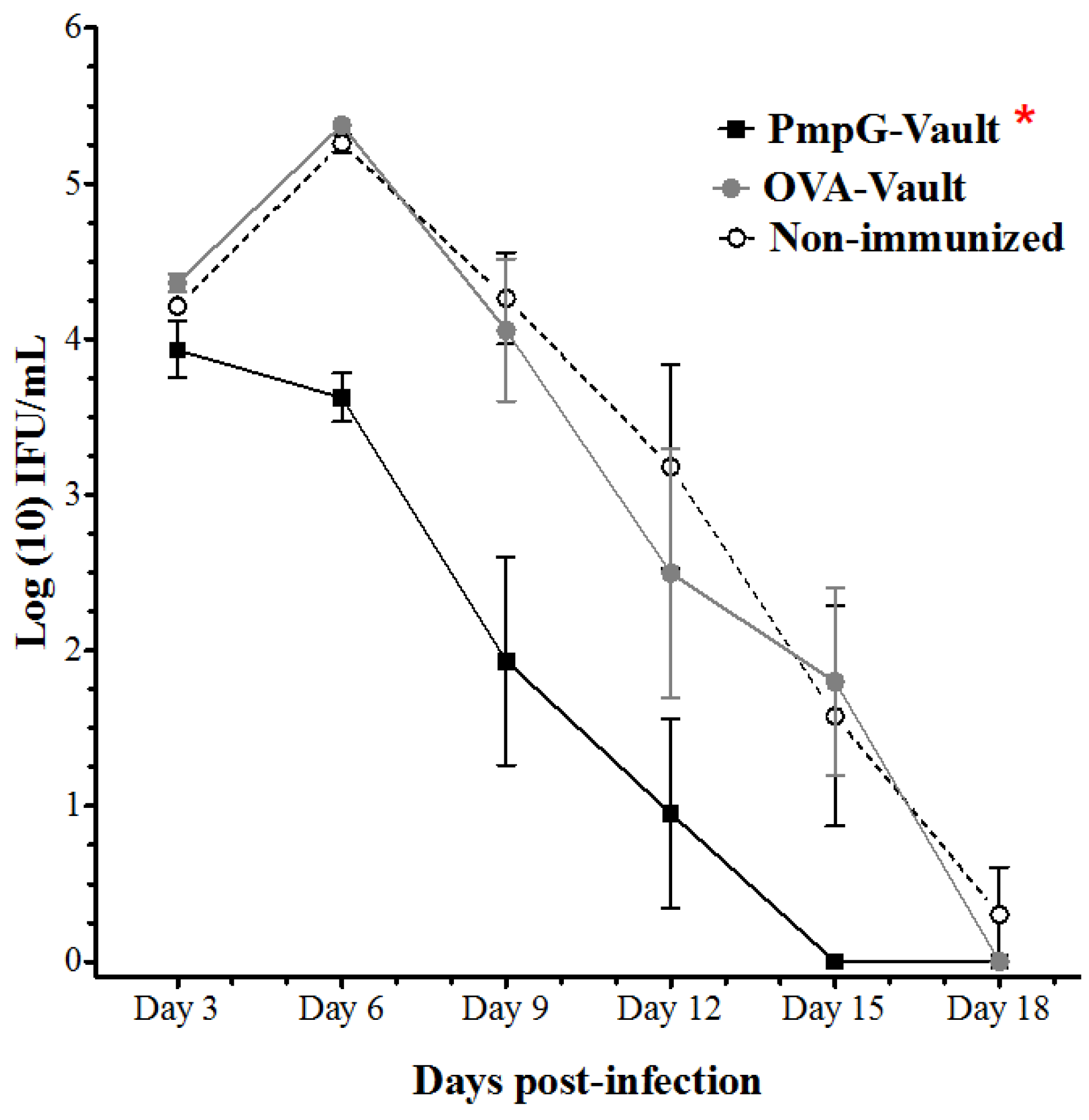
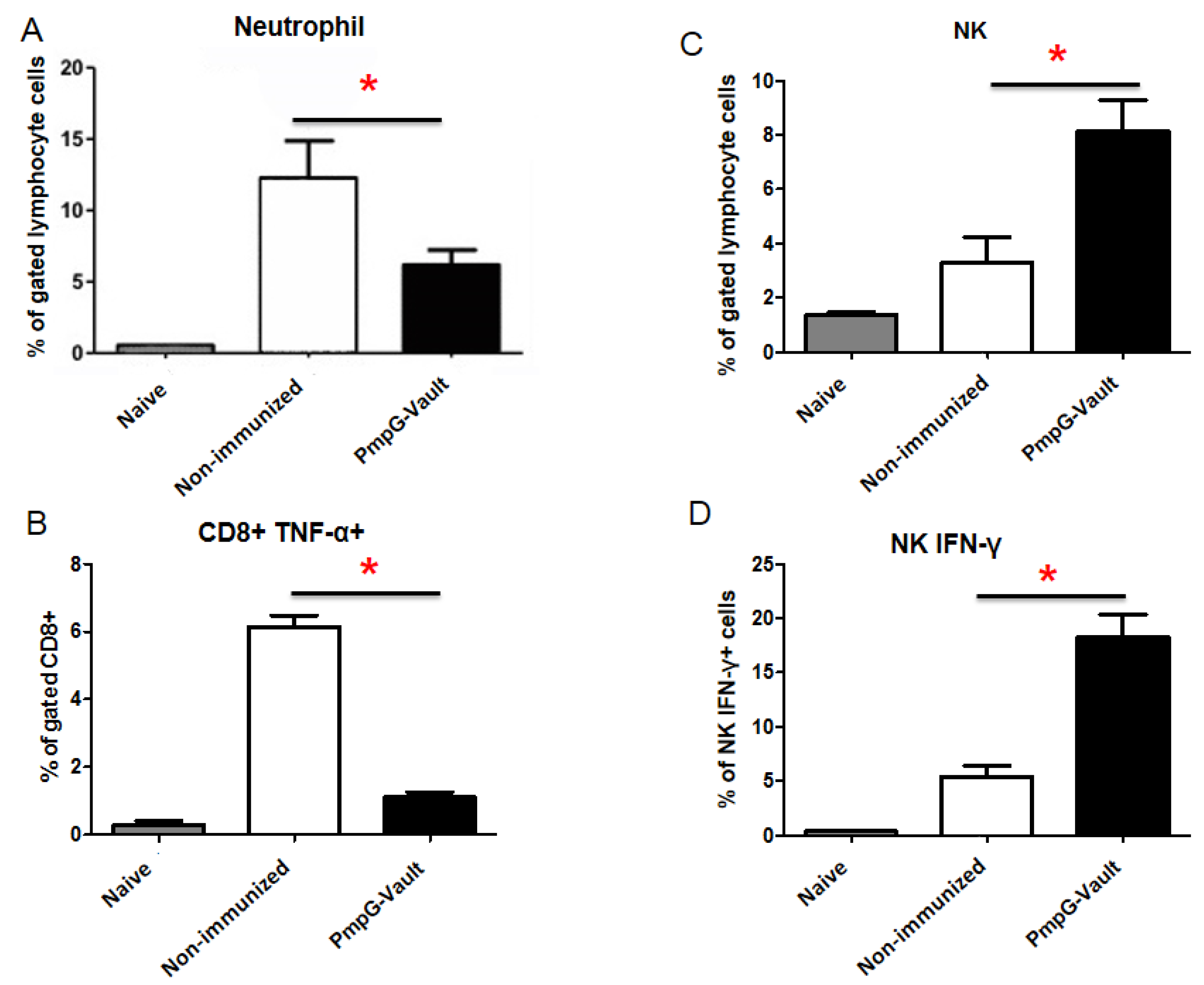
3.1. PmpG-Vault Immunization Reduces Early Bacterial Burden Following Chlamydial Genital Infection and Does Not Induce Systemic Inflammation
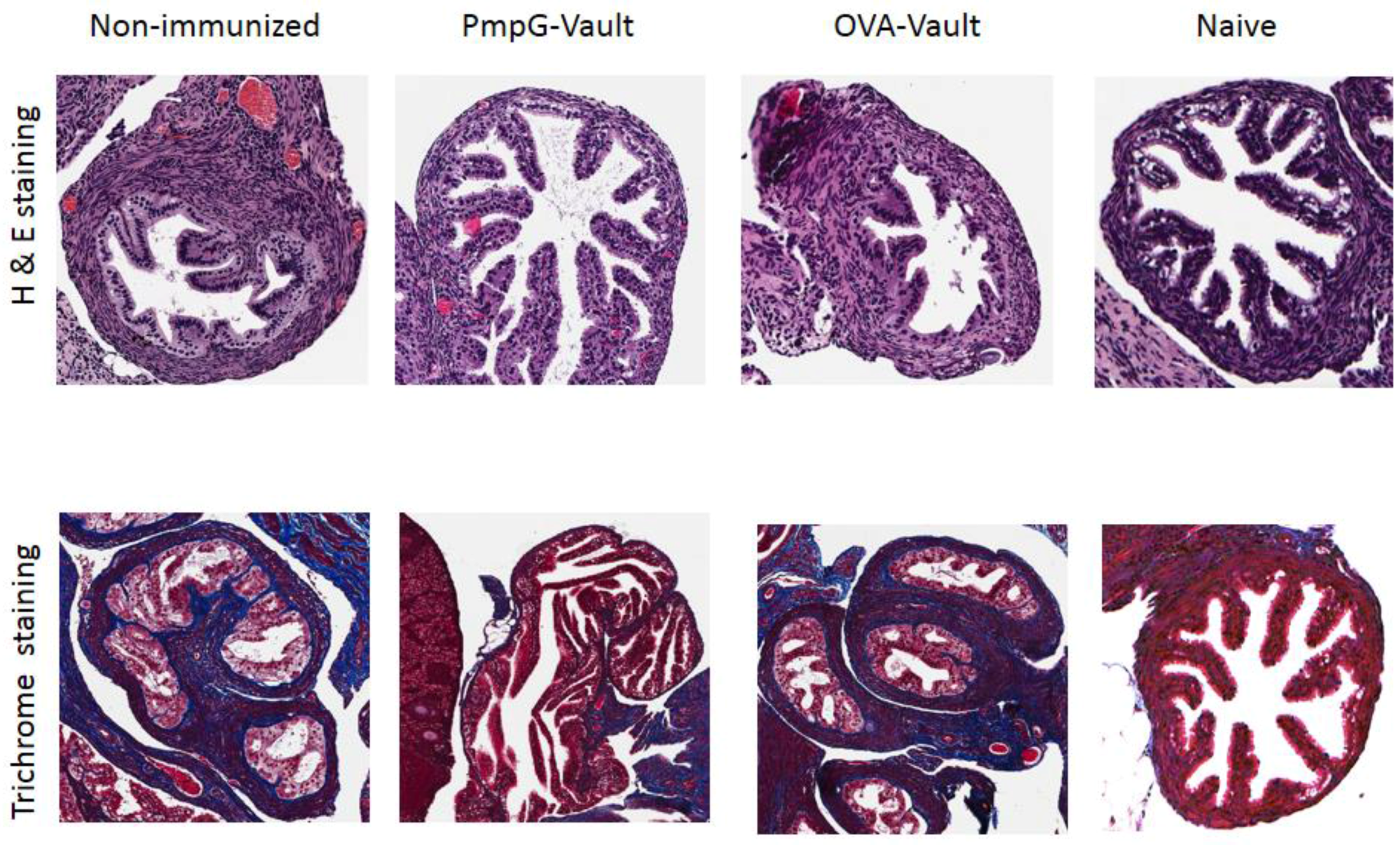
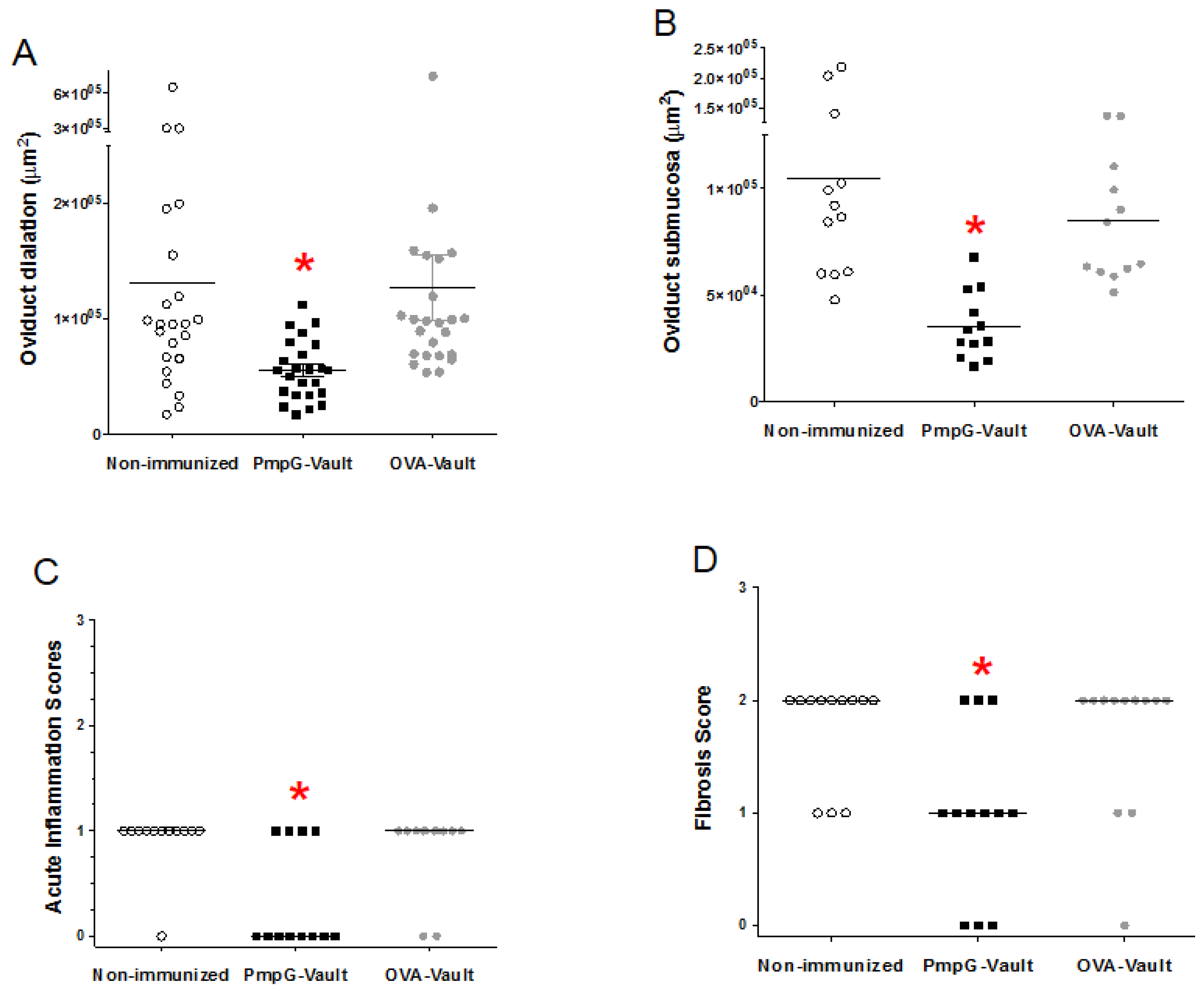
3.2. Genital Tract Histopathology Following C. muridarum Challenge is Reduced by Vaccination with Vaults Containing PmpG
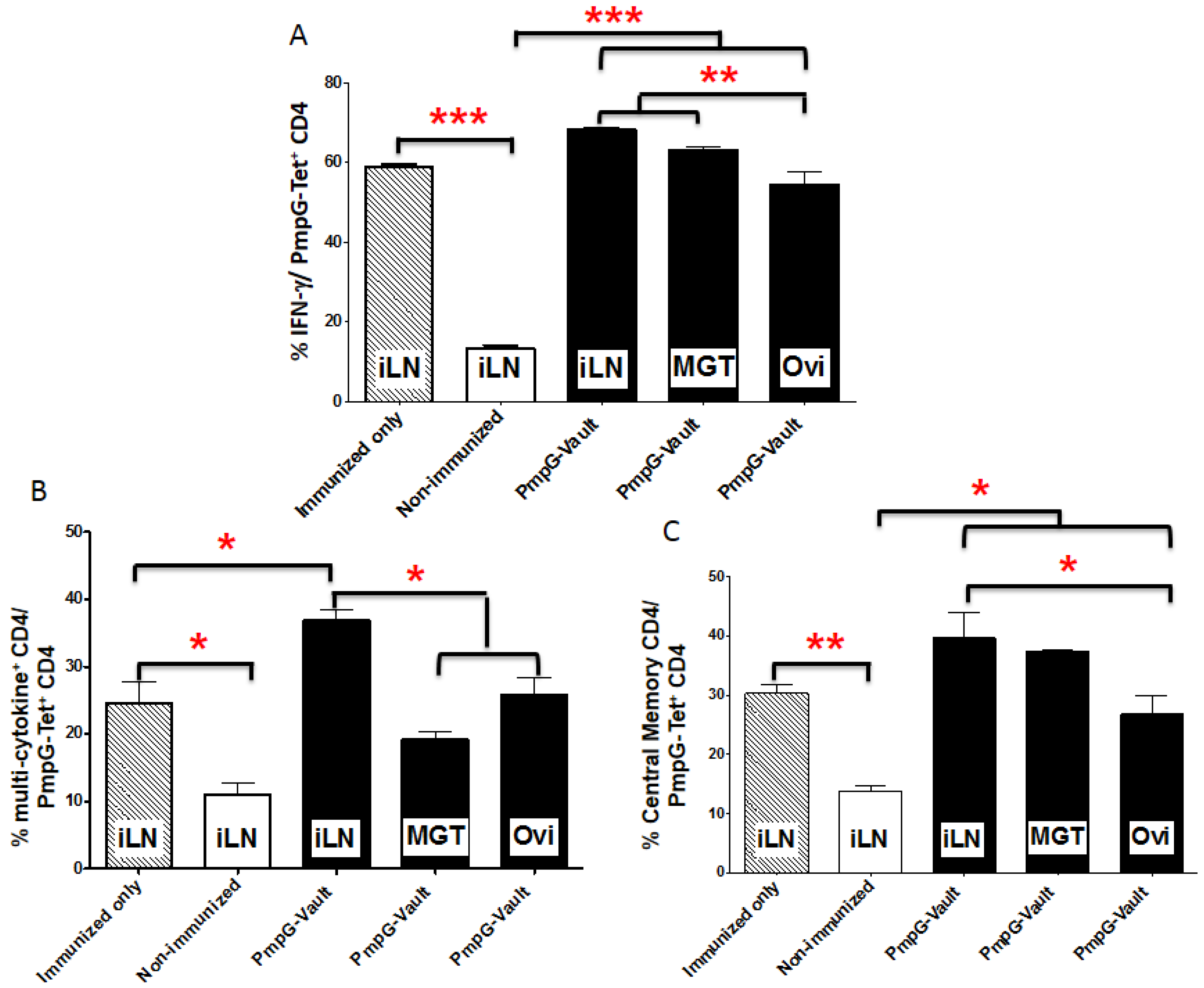
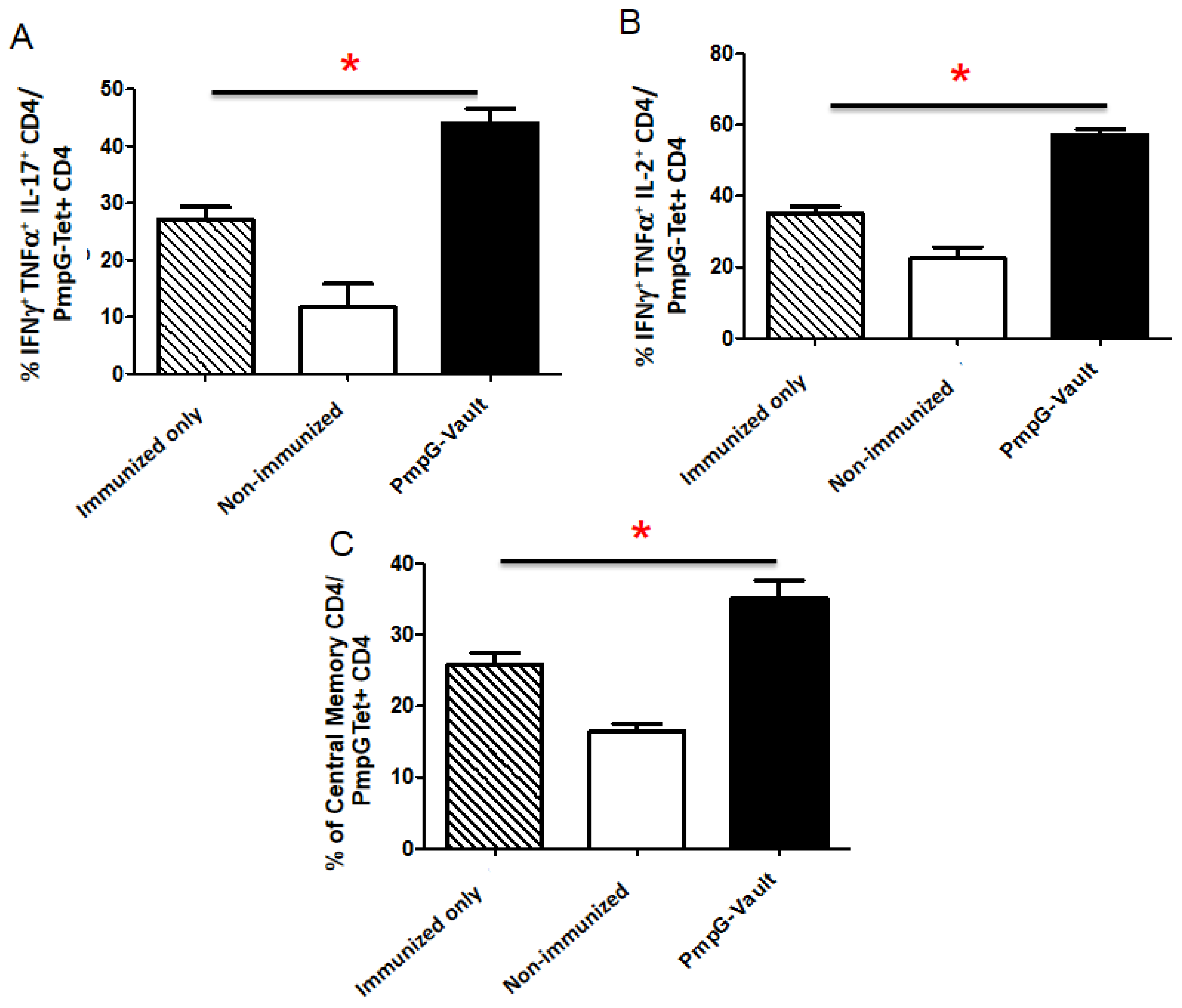
3.3. Vault-Vaccines Induce Antigen-Specific IFN-γ Secreting CD4 Cells That Also Produce Multiple Other Cytokines and Contain Central Memory Cells
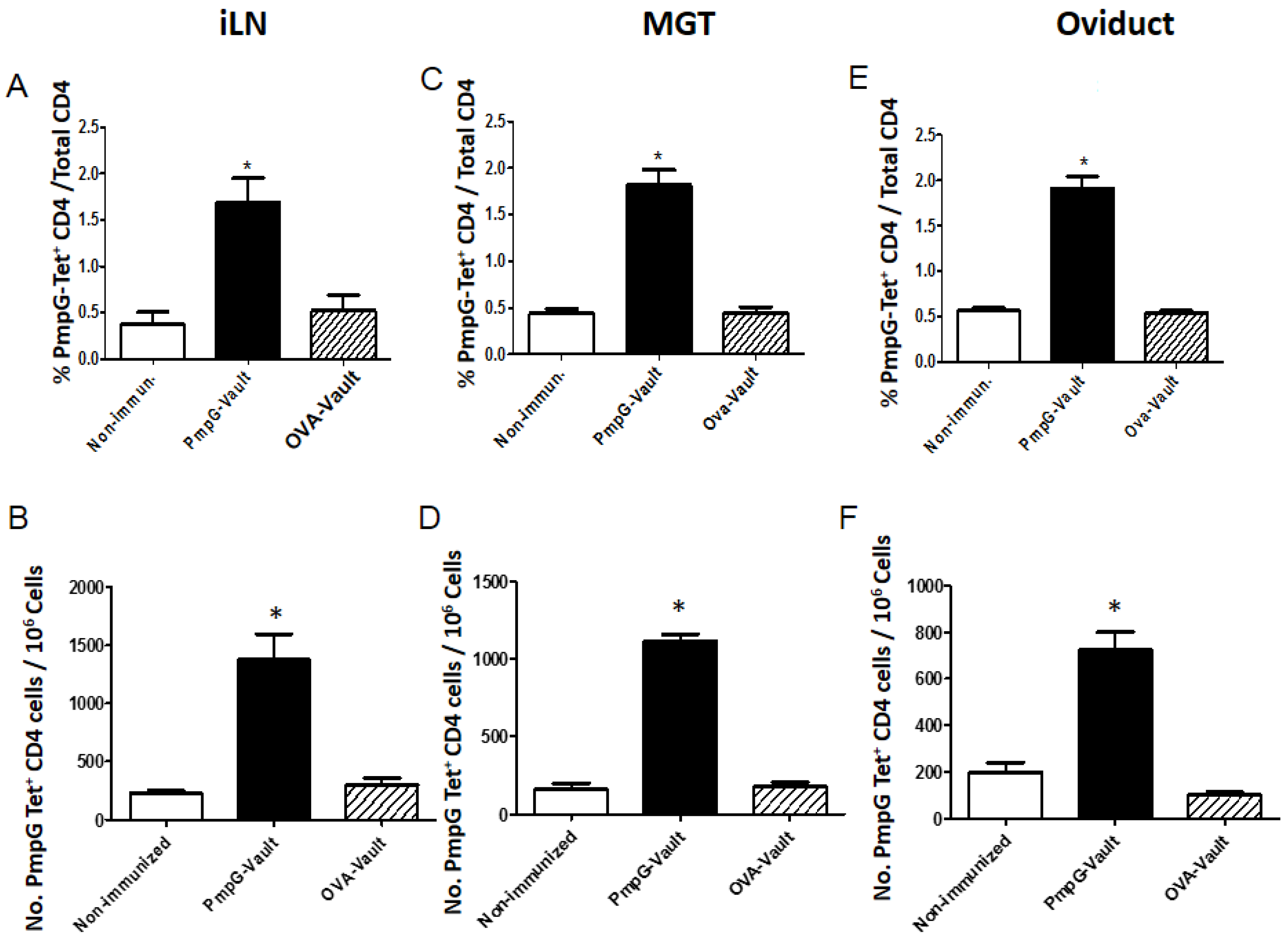
3.4. Induction of Systemic Antigen-Specific Cellular Immune Responses
3.5. Inflammatory Factors Related to Tissue Pathology
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- US Department of Health and Human Services. Sexually Transmitted Disease Surveillance, 2007; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2007.
- World Health Organization. Global Prevalence and Incidence of Selected Curable Sexually Transmitted Infections; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Rey-Ladino, J.; Ross, A.G.; Cripps, A.W. Immunity, immunopathology, and human vaccine development against sexually transmitted Chlamydia trachomatis. Hum. Vaccines Immunother. 2014, 10, 2664–2673. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Karunakaran, K.P.; Jiang, X.; Brunham, R.C. Evaluation of a multisubunit recombinant polymorphic membrane protein and major outer membrane protein T cell vaccine against Chlamydia muridarum genital infection in three strains of mice. Vaccine 2014, 32, 4672–4680. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Caldwell, H.D. CD4+ T cells play a significant role in adoptive immunity to Chlamydia trachomatis infection of the mouse genital tract. Infect. Immun. 1995, 63, 3302–3308. [Google Scholar] [PubMed]
- Morrison, S.G.; Su, H.; Caldwell, H.D.; Morrison, R.P. Immunity to murine Chlamydia trachomatis genital tract reinfection involves B cells and CD4+ T cells but not CD8+ T cells. Infect. Immun. 2000, 68, 6979–6987. [Google Scholar] [CrossRef] [PubMed]
- Morrison, R.P.; Feilzer, K.; Tumas, D.B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 1995, 63, 4661–4668. [Google Scholar] [PubMed]
- Karunakaran, K.P.; Rey-Ladino, J.; Stoynov, N.; Berg, K.; Shen, C.; Jiang, X.; Gabel, B.R.; Yu, H.; Foster, L.J.; Brunham, R.C. Immunoproteomic discovery of novel T cell antigens from the obligate intracellular pathogen Chlamydia. J. Immunol. 2008, 180, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Kickhoefer, V.A.; Stephen, A.G.; Harrington, L.; Robinson, M.O.; Rome, L.H. Vaults and telomerase share a common subunit, TEP1. J. Biol. Chem. 1999, 274, 32712–32717. [Google Scholar] [CrossRef] [PubMed]
- Rome, L.H.; Kickhoefer, V.A. Development of the vault particle as a platform technology. ACS Nano 2013, 7, 889–902. [Google Scholar] [CrossRef] [PubMed]
- Stephen, A.G.; Raval-Fernandes, S.; Huynh, T.; Torres, M.; Kickhoefer, V.A.; Rome, L.H. Assembly of vault-like particles in insect cells expressing only the major vault protein. J. Biol. Chem. 2001, 276, 23217–23220. [Google Scholar] [CrossRef] [PubMed]
- Champion, C.I.; Kickhoefer, V.A.; Liu, G.; Moniz, R.J.; Freed, A.S.; Bergmann, L.L.; Vaccari, D.; Raval-Fernandes, S.; Chan, A.M.; Rome, L.H.; et al. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS ONE 2009, 4, e5409. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Srivastava, M.K.; Andersson, Å.; Baratelli, F.; Huang, M.; Kickhoefer, V.A.; Dubinett, S.M.; Rome, L.H.; Sharma, S. Novel CCL21-Vault Nanocapsule Intratumoral Delivery Inhibits Lung Cancer Growth. PLoS ONE 2011, 6, e18758. [Google Scholar] [CrossRef] [PubMed]
- Buehler, D.C.; Marsden, M.D.; Shen, S.; Toso, D.B.; Wu, X.; Loo, J.A.; Zhou, Z.H.; Kickhoefer, V.A.; Wender, P.A.; Zack, J.A.; et al. Bioengineered vaults: Self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano 2014, 8, 7723–7732. [Google Scholar] [CrossRef] [PubMed]
- Eko, F.O.; He, Q.; Brown, T.; McMillan, L.; Ifere, G.O.; Ananaba, G.A.; Lyn, D.; Lubitz, W.; Kellar, K.L.; Black, C.M.; et al. A novel recombinant multisubunit vaccine against Chlamydia. J. Immunol. 2004, 173, 3375–3382. [Google Scholar] [CrossRef] [PubMed]
- Kar, U.K.; Jiang, J.; Champion, C.I.; Salehi, S.; Srivastava, M.; Sharma, S.; Rabizadeh, S.; Niazi, K.; Kickhoefer, V.; Rome, L.H.; et al. Vault nanocapsules as adjuvants favor cell-mediated over antibody-mediated immune responses following immunization of mice. PLoS ONE 2012, 7, e38553. [Google Scholar] [CrossRef] [PubMed]
- Maxion, H.K.M.; Liu, W.; Chang, M.H.; Kelly, K.A. The infecting dose of Chlamydia muridarum modulates the innate immune response and ascending infection. Infect. Immun. 2004, 72, 6330–6340. [Google Scholar] [CrossRef] [PubMed]
- Darville, T.; O’Neill, J.M.; Andrews, C.W., Jr.; Nagarajan, U.M.; Stahl, L.; Ojcius, D.M. Toll-like receptor-2, but not toll-like receptor-4, is essential for development of oviduct pathology in chlamydial genital tract infection. J. Immunol. 2003, 171, 6187–6197. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; He, X.S.; Feng, N.; Greenberg, H.B. Qualitative and quantitative characteristics of rotavirus-specific CD8 T cells vary depending on the route of infection. J. Virol. 2008, 82, 6812–6819. [Google Scholar] [CrossRef] [PubMed]
- Darville, T.; Andrews, C.W.; Laffoon, K.K.; Shymasani, W.; Kishen, L.R.; Rank, R.G. Mouse strain-dependent variation in the course and outcome of chlamydial genital tract infection is associated with differences in host response. Infect. Immun. 1997, 65, 3065–3073. [Google Scholar] [PubMed]
- Jiang, J.; Kelly, K.A. Isolation of lymphocytes from mouse genital tract mucosa. J. Vis. Exp. 2012, e4391. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.Q.; Patrick, A.; Moss, R.B.; Rosenthal, K.L. CD8+ T-cell-mediated cross-clade protection in the genital tract following intranasal immunization with inactivated human immunodeficiency virus antigen plus CpG oligodeoxynucleotides. J. Virol. 2005, 79, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Li, L.X.; McSorley, S.J. B cells enhance antigen-specific CD4 T cell priming and prevent bacteria dissemination following Chlamydia muridarum genital tract infection. PLoS Pathog. 2013, 9, e1003707. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Karunakaran, K.P.; Jiang, X.; Shen, C.; Andersen, P.; Brunham, R.C. Chlamydia muridarum T cell antigens and adjuvants that induce protective immunity in mice. Infect. Immun. 2012, 80, 1510–1518. [Google Scholar] [CrossRef] [PubMed]
- Maxion, H.K.; Kelly, K.A. Differential chemokine expression in distinct regions of the murine genital tract during Chlamydia trachomatis infection. Infect. Immun. 2002, 70, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Kawana, K.; Matsumoto, J.; Miura, S.; Shen, L.; Kawana, Y.; Nagamatsu, T.; Yasugi, T.; Fujii, T.; Yang, H.; Quayle, A.J.; et al. Expression of CD1d and Ligand-Induced Cytokine Production Are Tissue Specific in Mucosal Epithelia of the Human Lower Reproductive Tract. Infect. Immun. 2008, 76, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Frazer, L.C.; O’Connell, C.M.; Andrews, C.W.; Zurenski, M.A.; Darville, T. Enhanced neutrophil longevity and recruitment contribute to the severity of oviduct pathology during Chlamydia muridarum infection. Infect. Immun. 2011, 79, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Murthy, A.K.; Li, W.; Chaganty, B.K.; Kamalakaran, S.; Guentzel, M.N.; Seshu, J.; Forsthuber, T.G.; Zhong, G.; Arulanandam, B.P. Tumor necrosis factor alpha production from CD8+ T cells mediates oviduct pathological sequelae following primary genital Chlamydia muridarum infection. Infect. Immun. 2011, 79, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.K.; Rank, R.G. Role of NK cells in the early host response to chlamydial genital infection. Infect. Immun. 1998, 66, 5867–5875. [Google Scholar] [PubMed]
- Nagarajan, U.M.; Sikes, J.; Prantner, D.; Andrews, C.W.; Frazer, L.; Goodwin, A.; Snowden, J.N.; Darville, T. MyD88 deficiency leads to decreased NK cell gamma interferon production and T cell recruitment during Chlamydia muridarum genital tract infection, but a predominant Th1 response and enhanced monocytic inflammation are associated with infection resolution. Infect. Immun. 2011, 79, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Coler, R.N.; Bhatia, A.; Maisonneuve, J.F.; Probst, P.; Barth, B.; Ovendale, P.; Fang, H.; Alderson, M.; Lobet, Y.; Cohen, J.; et al. Identification and characterization of novel recombinant vaccine antigens for immunization against genital Chlamydia trachomatis. FEMS Immunol. Med. Microbiol. 2009, 55, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Ifere, G.O.; He, Q.; Igietseme, J.U.; Ananaba, G.A.; Lyn, D.; Lubitz, W.; Kellar, K.L.; Black, C.M.; Eko, F.O. Immunogenicity and protection against genital Chlamydia infection and its complications by a multisubunit candidate vaccine. J. Microbiol. Immunol. Infect. 2007, 40, 188–200. [Google Scholar] [PubMed]
- Yu, H.; Jiang, X.; Shen, C.; Karunakaran, K.P.; Jiang, J.; Rosin, N.L.; Brunham, R.C. Chlamydia muridarum T-Cell Antigens Formulated with the Adjuvant DDA/TDB Induce Immunity against Infection That Correlates with a High Frequency of Gamma Interferon (IFN-2)/Tumor Necrosis Factor Alpha and IFN-2/Interleukin-17 Double-Positive CD4+ T Cells. Infect. Immun. 2010, 78, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- Belay, T.; Eko, F.O.; Ananaba, G.A.; Bowers, S.; Moore, T.; Lyn, D. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 2002, 70, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Patton, D.L.; Kuo, C.-C. Histopathology of Chlamydia trachomatis salpingitis after primary and repeated reinfections in the monkey subcutaneous pocket model. J. Reprod. Fertil. 1989, 85, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Bingaman, A.W.; Patke, D.S.; Mane, V.R.; Ahmadzadeh, M.; Ndejembi, M.; Bartlett, S.T.; Farber, D.L. Novel phenotypes and migratory properties distinguish memory CD4 T cell subsets in lymphoid and lung tissue. Eur. J. Immunol. 2005, 35, 3173–3186. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.L.; Farber, D.L. Mucosal resident memory CD4 T cells in protection and immunopathology. Front. Immunol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.A.; Wiley, D.; Wiesmeier, E.; Briskin, M.; Butch, A.W.; Darville, T. The Combination of the Gastrointestinal Integrin (a4b7) and Selectin Ligand Enhances T-Cell Migration to the Reproductive Tract During Infection with Chlamydia trachomatis. Am. J. Reprod. Immunol. 2009, 61, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, R.A.; Kelly, K.A.; Rank, R.G. Expression of a mucosal-associated integrin may influence the trafficking of a protective MoPn-specific CD4+ clone to the GT during genital infection. In Proceedings of the Ninth International Symposium on Chlamydia Infection, Napa Valley, CA, USA, 21–26 June 1998; Stephens, R.S., Byrne, G.I., Christiansen, G., Clarke, I.N., Grayston, J.T., Rank, R.G., Eds.; International Chlamydia Symposium: San Francisco, CA, USA, 1998; pp. 395–398. [Google Scholar]
- Nagarajan, U.M.; Prantner, D.; Sikes, J.D.; Andrews, C.W., Jr.; Goodwin, A.M.; Nagarajan, S.; Darville, T. Type I IFN signaling exacerbates Chlamydia muridarum genital infection in a murine model. Infect. Immun. 2008, 76, 4642–4648. [Google Scholar] [CrossRef] [PubMed]
- Perry, L.L.; Feilzer, K.; Portis, J.L.; Caldwell, H.D. Distinct homing pathways direct T lymphocytes to the genital and intestinal mucosae in Chlamydia-infected mice. J. Immunol. 1998, 160, 2905–2914. [Google Scholar] [PubMed]
- Zaph, C.; Rook, K.A.; Goldschmidt, M.; Mohrs, M.; Scott, P.; Artis, D. Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth. J. Immunol. 2006, 177, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, K.H.; Newhall, W.J.; Rank, R.G. Humoral immune response to chlamydial genital infection of mice with the agent of mouse pneumonitis. Infect. Immun. 1989, 57, 2441–2446. [Google Scholar] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine delivery: A matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Jennings, G.T.; Bachmann, M.F. Designing recombinant vaccines with viral properties: A rational approach to more effective vaccines. Curr. Mol. Med. 2007, 7, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Sater, A.A.; Koo, E.; Häcker, G.; Ojcius, D.M. Inflammasome-dependent caspase-1 activation in cervical epithelial cells stimulates growth of the intracellular pathogen Chlamydia trachomatis. J. Biol. Chem. 2009, 284, 26789–26796. [Google Scholar] [CrossRef] [PubMed]
- Mikyas, Y.; Makabi, M.; Raval-Fernandes, S.; Harrington, L.; Kickhoefer, V.A.; Rome, L.H.; Stewart, P.L. Cryoelectron Microscopy Imaging of Recombinant and Tissue Derived Vaults: Localization of the MVP N Termini and VPARP. J. Mol. Biol. 2004, 344, 91–105. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Liu, G.; Kickhoefer, V.A.; Rome, L.H.; Li, L.-X.; McSorley, S.J.; Kelly, K.A. A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant. Vaccines 2017, 5, 3. https://doi.org/10.3390/vaccines5010003
Jiang J, Liu G, Kickhoefer VA, Rome LH, Li L-X, McSorley SJ, Kelly KA. A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant. Vaccines. 2017; 5(1):3. https://doi.org/10.3390/vaccines5010003
Chicago/Turabian StyleJiang, Janina, Guangchao Liu, Valerie A. Kickhoefer, Leonard H. Rome, Lin-Xi Li, Stephen J. McSorley, and Kathleen A. Kelly. 2017. "A Protective Vaccine against Chlamydia Genital Infection Using Vault Nanoparticles without an Added Adjuvant" Vaccines 5, no. 1: 3. https://doi.org/10.3390/vaccines5010003




