The Escape of Cancer from T Cell-Mediated Immune Surveillance: HLA Class I Loss and Tumor Tissue Architecture
Abstract
:1. Introduction
2. Tumor Immune Escape and HLA Class I Loss
3. T Cell Immune Selection of MHC Class I Negative Tumor Variants
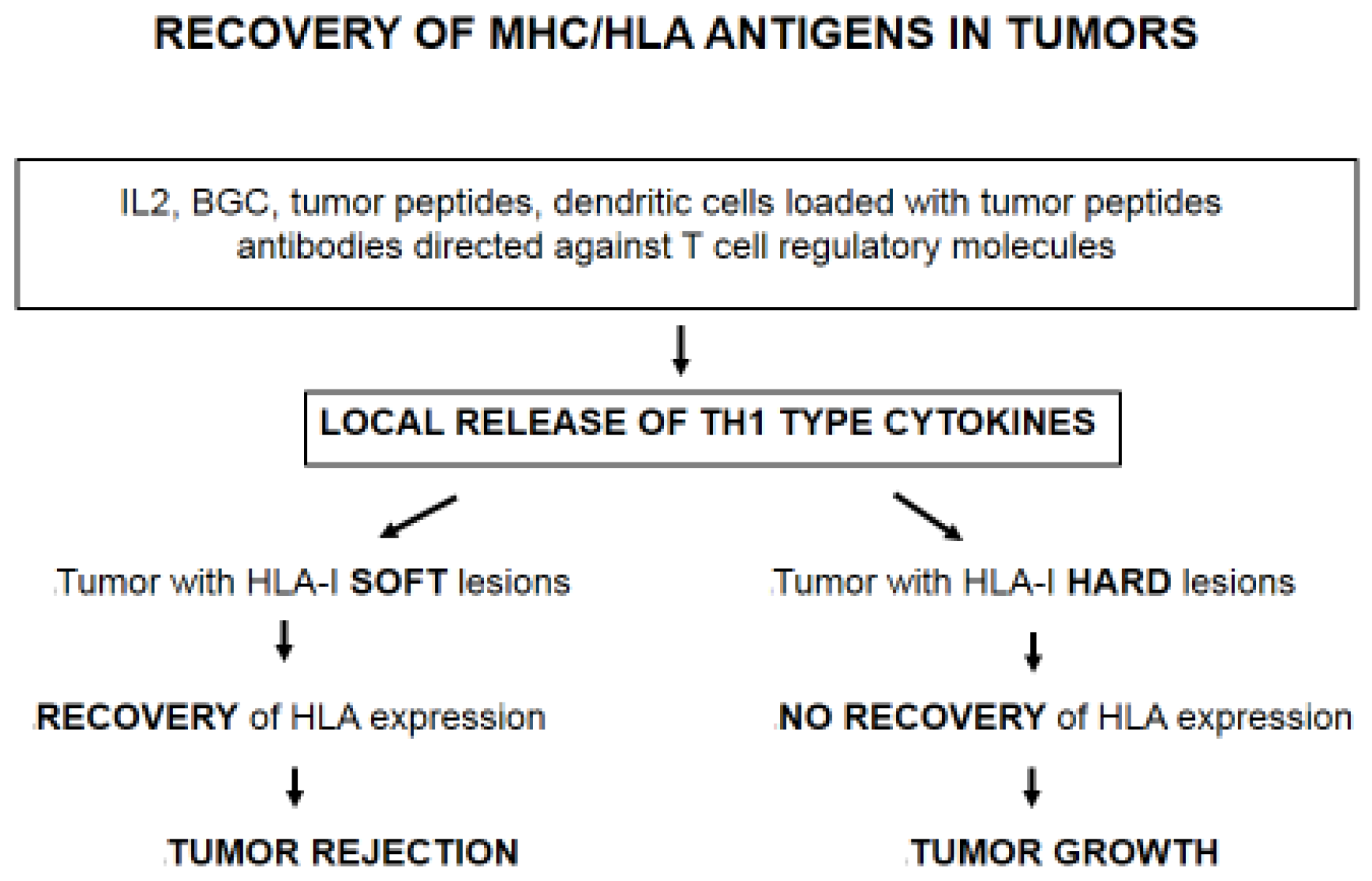
4. Immunotherapy Selects Tumor Cells with Irreversible/“Hard” HLA-I Lesions
5. Changes in Stromal/Tumor Tissue Organization and HLA Class I Loss
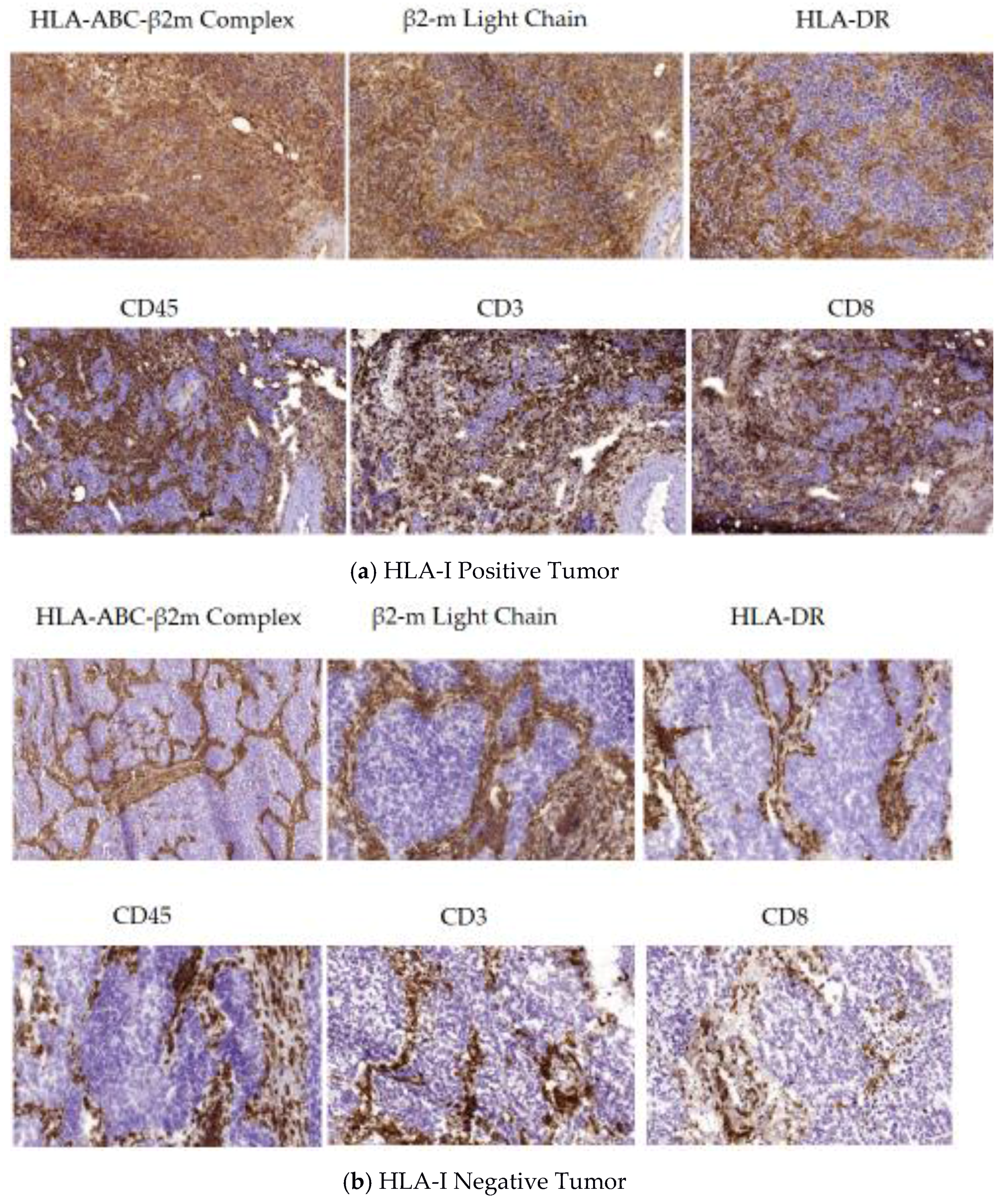
5.1. A Permissive Tumor Tissue Structure Observed in HLA-I Positive Tumors (Phase I)
5.2. Encapsulated Tumor Nodes Observed in HLA-I Negative Tumors (Phase II)
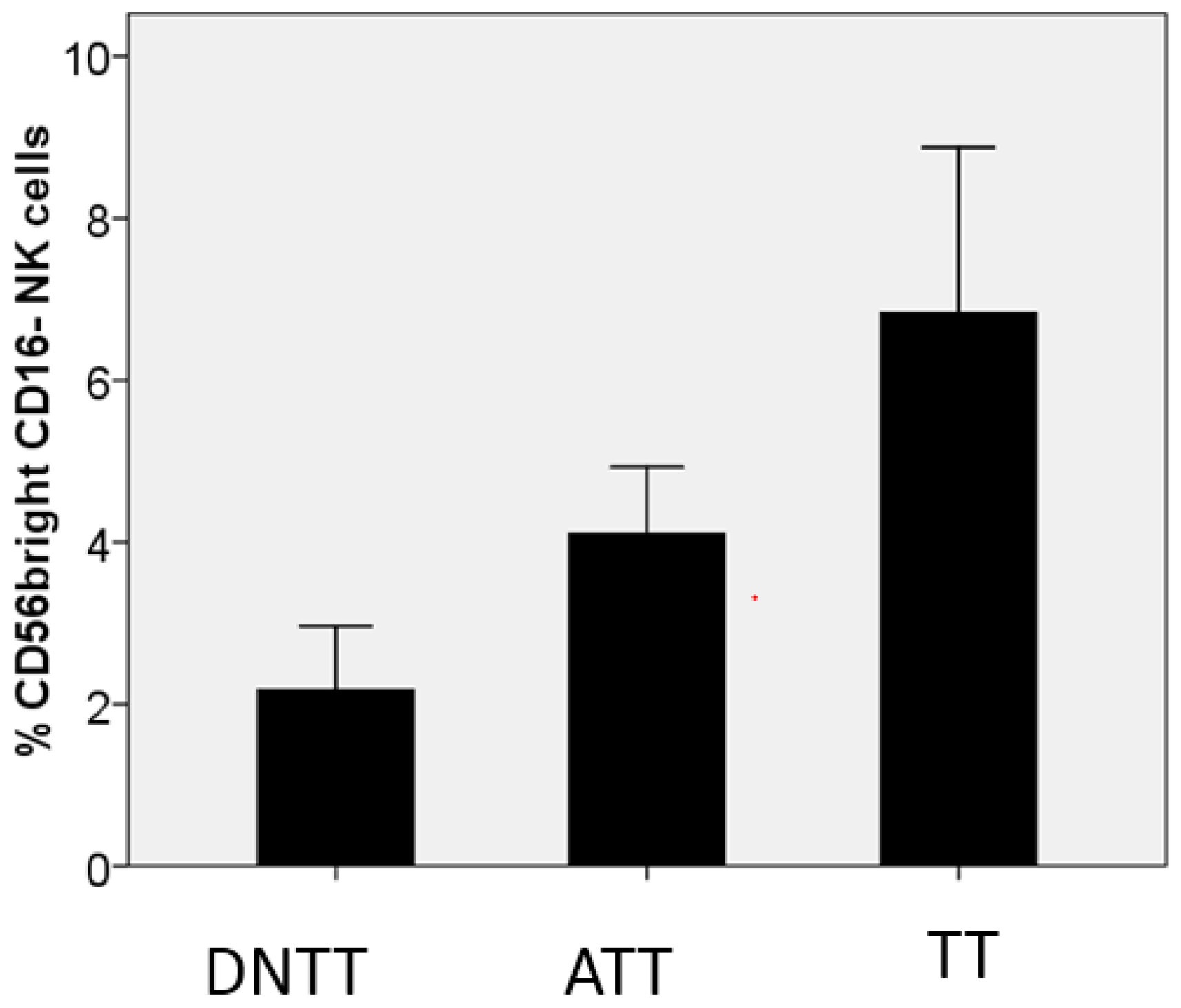
6. Progressive Exclusion and/or Inactivation of NK and CD8+ T Cells from the Distant Non-Tumor Tissue (DNTT) to the Tumor Tissue (TT)
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HLA | human leukocyte antigen |
| β2m | beta-2-microglobulin |
| CTL | cytotoxic T lymphocyte |
| IFN | Interferon |
| LOH | loss of heterozygosity |
| TIL | tumor infiltrating lymphocyte |
| TME | tumor microenvironment |
| NSCLC | non-small-cell lung carcinoma |
| MHC | major histocompatibility complex |
References
- Boesen, M.; Svane, I.M.; Engel, A.M.; Rygaard, J.; Thomsen, A.R.; Werdelin, O. CD8+ T cells are crucial for the ability of congenic normal mice to reject highly immunogenic sarcomas induced in nude mice with 3-methylcholantrene. Clin. Exp. Immunol. 2000, 121, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Boon, T.; Coulie, P.; Van Den Eynde, B.J.; Van Der Bruggen, P. Human T cell responses against melanoma. Annu. Rev. Immunol. 2006, 24, 175–208. [Google Scholar] [CrossRef] [PubMed]
- Romero, I.; Garrido, C.; Algarra, I.; Collado, A.; Garrido, F.; Garcia-Lora, A.M. T lymphocytes restrain spontaneous metastasis in permanent dormancy. Cancer Res. 2014, 74, 1958–1968. [Google Scholar] [CrossRef] [PubMed]
- Kessler, B.M.; Glas, R.; Ploegh, H.L. MHC class I antigen processing regulated by cytosolic proteolysis-short cuts that alter peptide generation. Mol. Immunol. 2012, 39, 171–179. [Google Scholar] [CrossRef]
- Romero, P.; Coulie, P. Adaptive T cell immunity and tumor antigen recognition. In Tumor Immunology and Immunotherapy; Rees, R., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 1–14. [Google Scholar]
- Drake, C.G.; Jaffee, E.M.; Pardoll, D.M. Mechanism of immune evasion by tumors. Adv. Immunol. 2006, 90, 51–81. [Google Scholar] [PubMed]
- Bodmer, W.; Browning, M.J.; Krausa, P.; Rowan, A.; Bicknell, D.C.; Bodmer, J. Tumour escape from immune response by variation in HLA expression and other mechanism. Ann. N. Y. Acad. Sci. 2001, 690, 42–49. [Google Scholar]
- Garrido, F.; Ruiz-Cabello, F.; Aptsiauri, N. Rejection versus escape: The tumor MHC dilemma. Cancer Immunol. Immunother. 2016, 66, 259–271. [Google Scholar] [CrossRef] [PubMed]
- Koopman, L.A.; Corver, W.E.; Van Der Slik, A.R.; Giphart, M.J.; Fleuren, G.J. Multiple genetic alterations at chromosome 6p cause frequent and heterogeneous HLA class I antigen loss in cervical cancer. J. Exp. Med. 2000, 191, 961–976. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Cabrera, T.; Accolla, R.S.; Bensa, J.C.; Bodmer, W.; Dohr, G.; Drouet, M.; Fauchet, R.; Ferrara, G.B.; Ferrone, S.; et al. HLA and Cancer. In HLA, Genetic Diversity of HLA. Functional and Medical Implications; Charron, D., Ed.; EDK Medical and Scientific International Publisher: University of Western Australia, Perth, Australia, 1997; pp. 445–452. [Google Scholar]
- Garrido, F.; Ruiz-Cabello, F.; Cabrera, T.; Perez-Villar, J.J.; Lopez-Botet, M.; Duggan-Keen, M.; Stern, P.L. Implications for immunosurveillance of altered HLA class I phenotypes in human tumours. Immunol. Today 1997, 18, 89–95. [Google Scholar] [CrossRef]
- Garrido, F.; Cabrera, T.; Aptsiauri, N. “Hard” and “Soft” lesions underlying the HLA class I alterations in cancer cells: Implications for immunotherapy. Int. J. Cancer 2010, 127, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Aptsiauri, N.; Doorduijn, E.; Garcia-Lora, A.; van Hall, T. The urgent need to recover MHC class I in cancers for effective immunotherapy. Curr. Opin. Immunol. 2016, 39, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Khong, H.T.; Restifo, N.P. Natural selection of tumor variants in the generation of “tumor escape” phenotypes. Nat. Immunol. 2002, 3, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Angell, T.E.; Lechner, M.G.; Jang, J.K.; LoPresti, J.S.; Epstein, A.L. MHC class I loss is a frequent mechanism of immune escape in papillary thyroid cancer that is reversed by interferon and selumetinib treatment in vitro. Clin. Cancer Res. 2014, 20, 6034–6044. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Campoli, M.; Restifo, N.P.; Wang, X.; Ferrone, S. Immune selection of hot-spot beta 2-microglobulin gene mutations, HLA-A2 allospecificity loss, and antigen-processing machinery component down regulation in melanoma cells derived from recurrent metastases following immunotherapy. J. Immunol. 2005, 174, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Sucker, A.; Zhao, F.; Real, B.; Heeke, C.; Bielefeld, N.; Maβen, S.; Horn, S.; Moll, I.; Maltaner, R.; Horn, P.A.; et al. Genetic evolution of T-cell resistance in the course of melanoma progression. Clin. Cancer Res. 2014, 20, 6593–6604. [Google Scholar] [CrossRef] [PubMed]
- Perea, F.; Bernal, M.; Sánchez-Palencia, A.; Carretero, J.; Torres, C.; Bayarri, C.; Gómez-Morales, M.; Garrido, F.; Ruiz-Cabello, F. The absence of HLA class I expression in non-small cell lung cancer correlates with the tumor tissue structure and the pattern of T cell infiltration. Int. J. Cancer 2017, 140, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Ryschich, E.; Notzel, T.; Hinz, U.; Autschbach, F.; Ferguson, J.; Simon, I.; Weitz, J.; Fröhlich, B.; Klar, E.; Büchler, M.W.; et al. Control of T cell mediated immune response by HLA class I in human pancreatic carcinoma. Clin. Can. Res. 2005, 11, 498–504. [Google Scholar]
- Kikuchi, E.; Yamazaki, K.; Torigoe, T.; Cho, Y.; Miyamoto, M.; Oizumi, S.; Hommura, F.; Dosaka-Akita, H.; Nishimura, M. HLA class I antigen expression is associated with a favorable prognosis in early stage non-small cell lung cancer. Cancer Sci. 2007, 98, 1424–1430. [Google Scholar] [CrossRef] [PubMed]
- Paschen, A.; Méndez, R.M.; Jiménez, P.; Sucker, A.; Ruiz-Cabello, F.; Song, M.; Garrido, F.; Schadendorf, D. Complete loss of HLA class I antigen expression on melanoma cells: A result of successive mutation events. Int. J. Cancer 2003, 103, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Lora, A.; Martinez, M.; Algarra, I.; Gaforio, J.J.; Garrido, F. MHC class I deficient metastatic tumor variants immunoselected by T lymphocytes originate from the coordinated downregulation of APM components. Int. J. Cancer 2003, 106, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Lechner, M.G.; Harimi, S.S.; Barry-Holson, K.; Angel, T.E.; Murphy, K.A.; Church, C.H.; Ohlfest, J.R.; Hu, P.; Epstein, A.L. Immunogenicity of murine solid tumor models as a defining feature of in vivo behavior and response to immunotherapy. J. Immunother. 2013, 36, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Marincola, F.M.; Kawakami, Y.; Taubenberger, J.; Yannelli, J.R.; Rosenberg, S.A. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl. Cancer Inst. 1996, 88, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Aptsiauri, N.; Carretero, R.; Garcia-Lora, A.; Real, L.M.; Cabrera, T.; Garrido, F. Regressing and progressing metastatic lesions: Resistance to immunotherapy is predetermined by irreversible HLA class I antigen alterations. Cancer Immunol. Immunother. 2008, 57, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Carretero, R.; Wang, E.; Rodriguez, A.I.; Reinboth, J.; Ascierto, M.L.; Engle, A.M.; Liu, H.; Camacho, F.M.; Marincola, F.M.; Garrido, F.; et al. Regression of melanoma metastases after immunotherapy is associated with activation of antigen presentation and interferon-mediated rejection genes. Int. J. Cancer 2012, 131, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, J.M.; Angel Garcia-Diaz, B.S.; Shin, D.S.; Escuin-Ordinas, H.; Hugo, W.; Hu-Lieskovan, S.; Torrejon, D.Y.; Abril-Rodriguez, G.; Sandoval, S.; Barthly, L.; et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N. Engl. J. Med. 2016, 75, 819–829. [Google Scholar] [CrossRef] [PubMed]
- Dunn, G.P.; Koebel, C.M.; Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 2006, 11, 836–848. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The role of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Hams, E.; Aviello, G.; Fallon, P.G. The schistosoma granuloma: Friend or foe? Front. Immunol. 2013, 4, 89. [Google Scholar] [CrossRef] [PubMed]
- Del Mar Valenzuela-Membrives, M.; Perea-García, F.; Sanchez-Palencia, A.; Ruiz-Cabello, F.; Gómez-Morales, M.; Miranda-León, M.T.; Galindo-Angel, I.; Fárez-Vidal, M.E. Progressive changes in composition of lymphocytes in lung tissues from patients with non-small-cell lung cancer. Oncotarget 2016, 7, 71608–71619. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.Y.; Wang, X.F.; Li, J.; Dong, J.N.; Liu, J.Q.; Li, N.P.; Yun, B.; Xia, R.L. Overexpression of CD39 and high tumoral CD39(+)/CD8(+) ratio are associated with adverse prognosis in resectable gastric cancer. Int. J. Clin. Exp. Pathol. 2015, 8, 14757–14764. [Google Scholar] [PubMed]
- Sundström, P.; Stenstad, H.; Langenes, V.; Ahlmanner, F.; Ndah, T.G.; Fredin, K.; Börjesson, L.; Gustavsson, B.; Bastid, J.; Quiding-Järbrink, M. Regulatory T cells from colon cancer patients inhibit effector T-cell migration through an adenosine-dependent mechanism. Cancer Immunol. Res. 2016, 3, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Carrega, P.; Morandi, B.; Costa, R.; Frumento, G.; Forte, G.; Altavila, G.; Ratto, G.B.; Mingari, M.C.; Moretta, L.; Ferlazzo, G. Natural killer cells infiltrating Human Nonsmall-Cell Lung Cancer are enriched in CD56brightCD16- cells and display an impaired capability to kill tumor cells. Cancer 2008, 112, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Bruno, A.; Focaccetti, C.; Pagani, A.; Imperatori, A.S.; Spagnoletti, M.; Rotolo, N.; Cantelmo, A.R.; Franzi, F.; Capella, C.; Ferlazzo, G.; et al. The proangiogenic phenotype of natural killer cells in patients with non-small cell lung cancer. Neoplaisa 2013, 15, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Jin, S.; Deng, Y.; Hao, J.W.; Li, Y.; Liu, B.; Yu, Y.; Shi, F.-D.; Zhou, Q.-H. NK cell phenotipic modulation in lung cáncer environment. PLoS ONE 2014, 9, e109976. [Google Scholar] [CrossRef] [PubMed]
- Garrido, F.; Algarra, I. MHC antigens and tumor escape from immune surveillance. Adv. Cancer Res. 2001, 83, 117–158. [Google Scholar] [PubMed]
- Aptsiauri, N.; Cabrera, T.; Garcia-Lora, A.; Lopez-Nevot, M.A.; Ruiz-Cabello, F.; Garrido, F. MHC class I antigens and immune surveillance in transformed cells. Int. Rev. Cytol. 2007, 256, 139–189. [Google Scholar] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garrido, F.; Perea, F.; Bernal, M.; Sánchez-Palencia, A.; Aptsiauri, N.; Ruiz-Cabello, F. The Escape of Cancer from T Cell-Mediated Immune Surveillance: HLA Class I Loss and Tumor Tissue Architecture. Vaccines 2017, 5, 7. https://doi.org/10.3390/vaccines5010007
Garrido F, Perea F, Bernal M, Sánchez-Palencia A, Aptsiauri N, Ruiz-Cabello F. The Escape of Cancer from T Cell-Mediated Immune Surveillance: HLA Class I Loss and Tumor Tissue Architecture. Vaccines. 2017; 5(1):7. https://doi.org/10.3390/vaccines5010007
Chicago/Turabian StyleGarrido, Federico, Francisco Perea, Mónica Bernal, Abel Sánchez-Palencia, Natalia Aptsiauri, and Francisco Ruiz-Cabello. 2017. "The Escape of Cancer from T Cell-Mediated Immune Surveillance: HLA Class I Loss and Tumor Tissue Architecture" Vaccines 5, no. 1: 7. https://doi.org/10.3390/vaccines5010007





