Distinct African Swine Fever Virus Shedding in Wild Boar Infected with Virulent and Attenuated Isolates
Abstract
:1. Introduction
2. Materials and Methods
2.1. ASFV Isolates
2.2. Animals
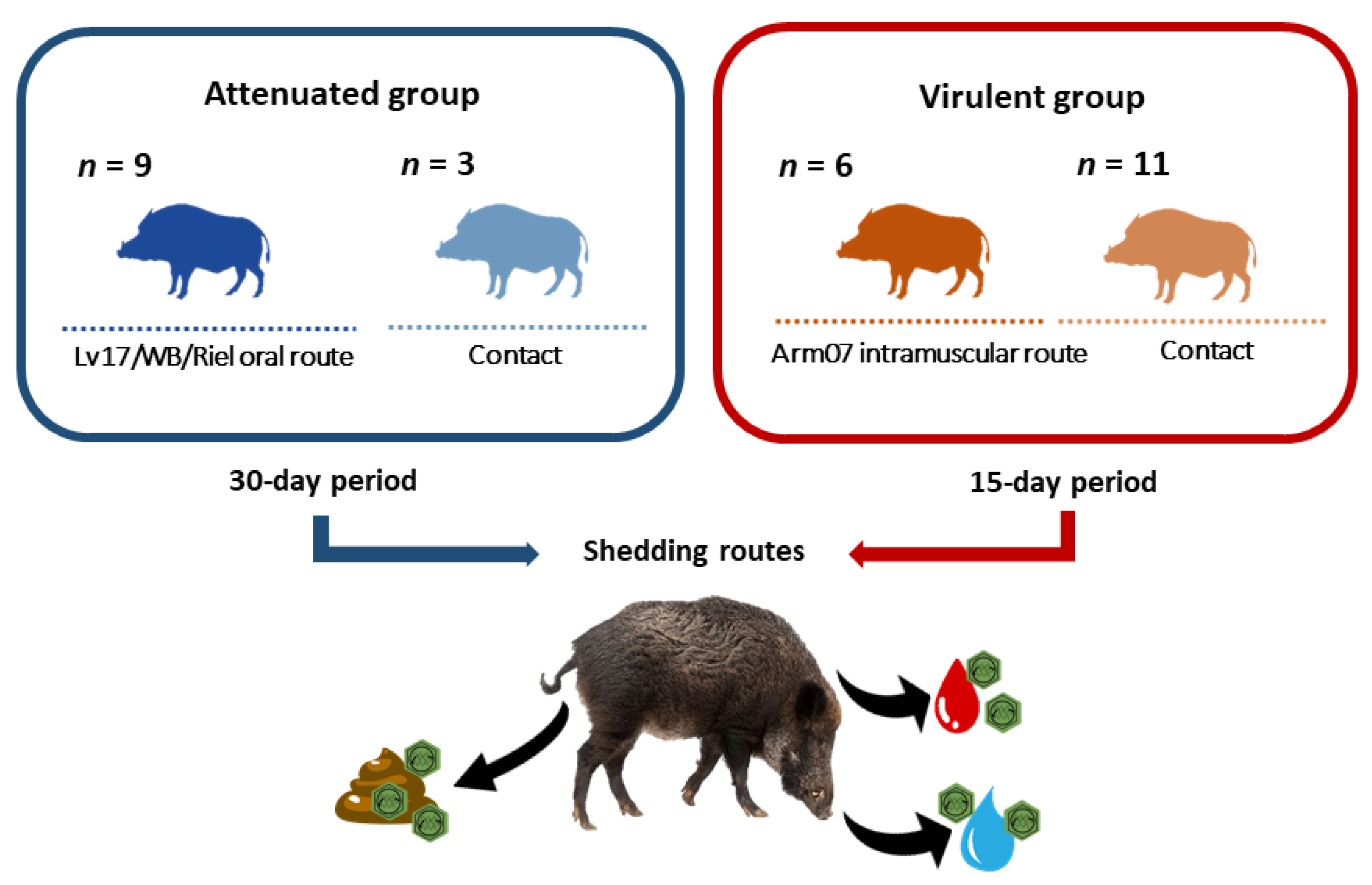
2.3. Study Design
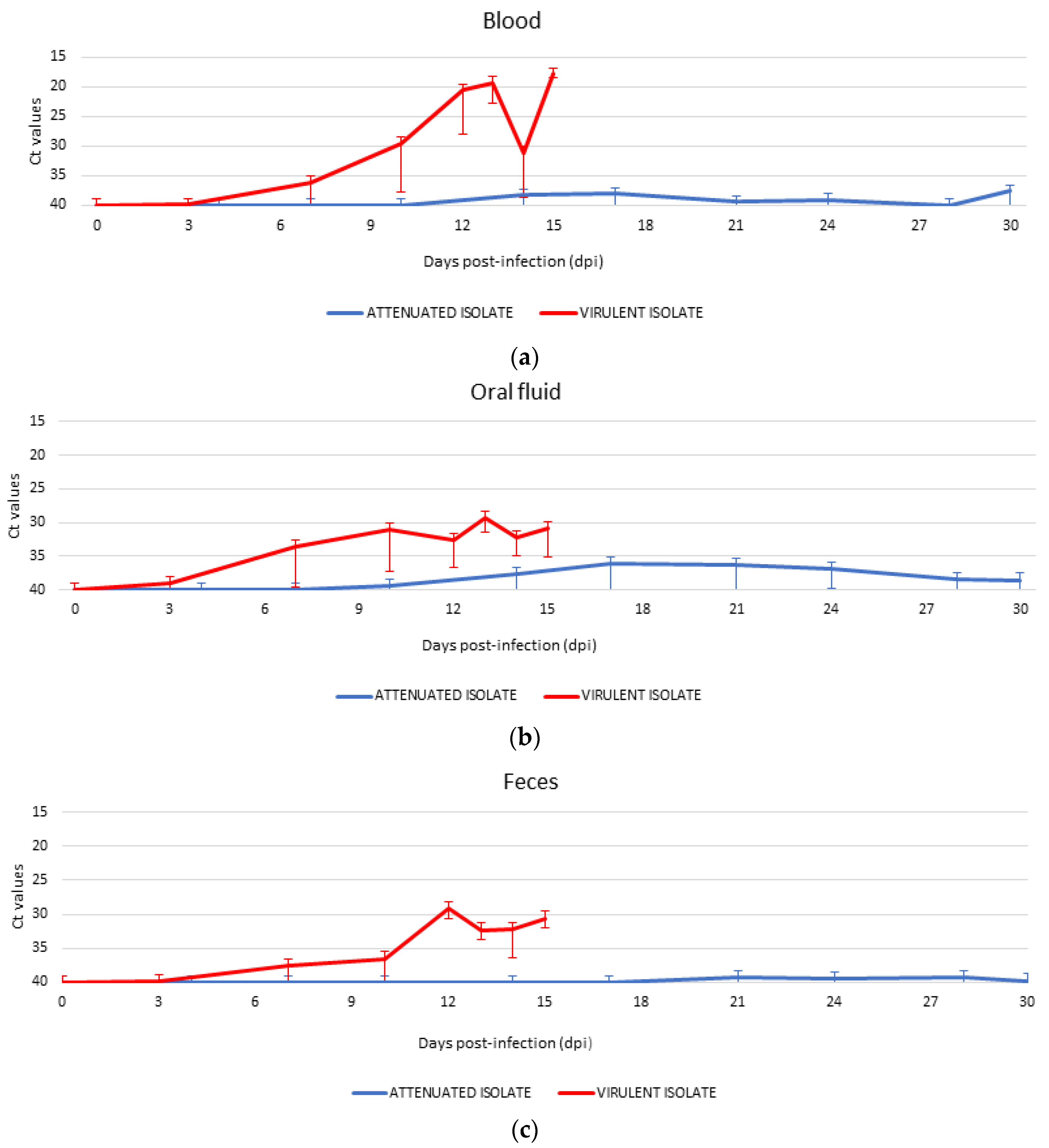
2.4. Sampling and ASFV DNA Detection
2.5. Statistical Analysis
3. Results
3.1. Clinical Signs
3.2. Inoculation and Contact Infection
3.3. Detection of ASFV Genome and Starting Time of Shedding in Different Routes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arias, M.; Sánchez-Vizcaíno, J.M. African swine fever. In Diseases of Swine, 10th ed.; Zimmerman, J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; John Wiley and Sons: New York, NY, USA, 2012; pp. 396–404. [Google Scholar]
- OIE. 2020: WAHID Database. Available online: http://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseaseoutbreakmaps?disease_type_hidden=&disease_id_hidden=&selected_disease_name_hidden=&disease_type=0&disease_id_terrestrial=12&disease_id_aquatic=-999&selected_start_day=1&selected_start_month=1&selected_start_year=2012&selected_end_day=1&selected_end_month=12&selected_end_year=2013&submit2=OK (accessed on 29 April 2020).
- Lu, G.; Pan, J.; Zhang, G. African Swine Fever Virus in Asia: Its Rapid Spread and Potential Threat to Unaffected Countries. J. Infect. 2020, 80, 350–371. [Google Scholar] [CrossRef]
- Gallardo, C.; Soler, A.; Rodze, I.; Nieto, R.; Jovita, C.C.; Arias, F.M. Attenuated and non-haemadsorbing (non-HAD) genotype II African swine fever virus (ASFV) isolated in Europe, Latvia 2017. Transbound. Emerg. Dis. 2019, 66, 1399–1404. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernández, E.; Jurado, C.; Rivera, B.; Rodríguez-Bertos, A.; Arias, M.; Sánchez-Vizcaíno, J.M. First Oral Vaccination of Eurasian Wild Boar against African Swine Fever Virus Genotype II. Front. Vet. Sci. 2019, 6, 1–10. [Google Scholar] [CrossRef]
- Arias, M.; de la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.; et al. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef]
- Mur, L.; Martínez-López, B.; Sánchez-Vizcaíno, J.M. Risk of African swine fever introduction into the European Union through transport-associated routes: Returning trucks and waste from international ships and planes. BMC Vet. Res. 2012, 8, 149. [Google Scholar] [CrossRef] [Green Version]
- McCallum, H.; Barlow, N.; Hone, J. How should pathogen transmission be modelled? Trends Ecol. Evol. 2001, 16, 295–300. [Google Scholar] [CrossRef]
- Neill, X.O.; White, A.; Ruiz-Fons, F.; Gortázar, C. Modelling the transmission and persistence of African swine fever in wild boar in contrasting European scenarios. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Saito, A.; Saito, M.; Koike, F.; Momose, H.; Mihira, T.; Uematsu, S.; Ohtani, T. Forecasting the range expansion of a recolonising wild boar Sus scrofa population. Wildl. Biol. 2012, 18, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Massei, G.; Kindberg, J.; Licoppe, A.; Gačić, D.; Šprem, N.; Kamler, J.; Baubet, E.; Hohmann, U.; Monaco, A.; Ozoliņš, J.; et al. Wild boar populations up, numbers of hunters down? A review of trends and implications for Europe. Pest Manag. Sci. 2015, 71, 492–500. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.J.; Lindsay, D.S. Wild boars as sources for infectious diseases in livestock and humans. Philosophical Transactions of the Royal Society B: Biological Sciences. R. Soc. 2009, 364, 2697–2707. [Google Scholar] [CrossRef] [Green Version]
- Cadenas-Fernández, E.; Sánchez-Vizcaíno, J.M.; Pintore, A.; Denurra, D.; Cherchi, M.; Jurado, C.; Vicente, J.; Barasona, J.A. Free-Ranging Pig and Wild Boar Interactions in an Endemic Area of African Swine Fever. Front. Vet. Sci. 2019, 6, 376. [Google Scholar] [CrossRef] [PubMed]
- Blome, S.; Gabriel, C.; Dietze, K.; Breithaupt, A.; Beer, M. High virulence of African swine fever virus caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012, 18, 708. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Ferreira, H.C.; Weesendorp, E.; Elbers, A.R.W.; Bouma, A.; Quak, S.; Stegeman, J.A.; Loeffen, W.L.A. African swine fever virus excretion patterns in persistently infected animals: A quantitative approach. Vet. Microbiol. 2012, 160, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Ekue, N.F.; Wilkinson, P.J.; Wardley, R.C. Infection of pigs with the Cameroon isolate (Cam/82) of African swine fever virus. J. Comp. Pathol. 1989, 100, 145–154. [Google Scholar] [CrossRef]
- Greig, A.; Plowright, W. The excretion of two virulent strains of African swine fever virus by domestic pigs. J. Hyg. (Lond.) 1970, 68, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kittawornrat, A.; Zimmerman, J.J. Toward a Better Understanding of Pig Behavior and Pig Welfare. Anim. Health Res. Rev. 2011, 12, 25–32. [Google Scholar] [CrossRef] [Green Version]
- de Carvalho Ferreira, H.C.; Weesendorp, E.; Quak, S.; Stegeman, J.A.; Loeffen, W.L.A. Suitability of faeces and tissue samples as a basis for non-invasive sampling for African swine fever in wild boar. Vet. Microbiol. 2014, 172, 449–454. [Google Scholar] [CrossRef]
- Cukor, J.; Linda, R.; Václavek, P.; Mahlerová, K.; Šatrán, P.; Havránek, F. Confirmed cannibalism in wild boar and its possible role in African swine fever transmission. Transbound. Emerg. Dis. 2020, 67, 1068–1073. [Google Scholar] [CrossRef]
- Gabriel, C.; Blome, S.; Malogolovkin, A.; Parilov, S.; Kolbasov, D.; Teifke, J.P.; Beer, M. Characterization of African Swine Fever Virus Caucasus Isolate in European Wild Boars. Emerg. Infect. Dis. 2011, 17, 2342–2345. [Google Scholar] [CrossRef]
- Rodríguez-Bertos, A.; Cadenas-Fernández, E.; Rebollada-Merino, A.; Porras-González, N.; Mayoral-Alegre, F.J.; Barreno, L.; Kosowska, A.; Tomé-Sánchez, I.; Barasona, J.A.; Sánchez-Vizcaíno, J.M. Clinical Course and Gross Pathological Findings in Wild Boar Infected with a Highly Virulent Strain of African Swine Fever Virus Genotype II. Pathogens 2020, 9, 688. [Google Scholar] [CrossRef]
- Carrascosa, A.L.; Bustos, M.J.; de Leon, P. Methods for growing and titrating African swine fever virus: Field and laboratory samples. Curr. Protoc. Cell Biol. 2011, 53, 26.14.1–26.14.25. [Google Scholar] [CrossRef]
- Gallardo, C.; Sánchez, E.G.; Pérez-Núñez, D.; Nogal, M.; de León, P.; Carrascosa, Á.L.; Nieto, R.; Soler, A.; Arias, M.L.; Revilla, Y. African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses. Vaccine 2018, 36, 2694–2704. [Google Scholar] [CrossRef] [PubMed]
- Arenas-Montes, A.; García-Bocanegra, I. Blood sampling by puncture in the cavernous sinus from hunted wild boar. Eur. J. Wildl. Res. 2013, 59, 299–303. [Google Scholar] [CrossRef]
- King, D.P.; Reid, S.M.; Hutchings, G.H.; Grierson, S.S.; Wilkinson, P.J.; Dixon, L.K.; Bastos, A.D.S.; Drew, T.W. Development of a TaqMan® PCR assay with internal amplification control for the detection of African swine fever virus. J. Virol. Methods 2003, 107, 53–61. [Google Scholar] [CrossRef]
- Fernández-Pinero, J.; Gallardo, C.; Elizalde, M.; Robles, A.; Gómez, C.; Bishop, R.; Heath, L.; Couacy-Hymann, E.; Fasina, F.O.; Pelayo, V.; et al. Molecular Diagnosis of African Swine Fever by a New Real-Time PCR Using Universal Probe Library. Transbound. Emerg. Dis. 2013, 60, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Blome, S.; Gabriel, C.; Beer, M. Pathogenesis of African Swine Fever in Domestic Pigs and European Wild Boar. Virus Res. (Elsevier) 2013, 173, 122–130. [Google Scholar] [CrossRef]
- Pikalo, J.; Zani, L.; Hühr, J.; Beer, M.; Blome, S. Pathogenesis of African swine fever in domestic pigs and European wild boar—Lessons learned from recent animal trials. Virus Res. 2019, 271, 197614. [Google Scholar] [CrossRef]
- Guinat, C.; Reis, A.L.; Netherton, C.L.; Goatley, L.; Pfeiffer, D.U.; Dixon, L. Dynamics of African swine fever virus shedding and excretion in domestic pigs infected by intramuscular inoculation and contact transmission. Vet. Res. 2014, 45, 1–9. [Google Scholar] [CrossRef]
- Oura, C.A.L.; Edwards, L.; Batten, C.A. Virological Diagnosis of African Swine Fever-Comparative Study of Available Tests. Virus Res. 2013, 173, 150–158. [Google Scholar] [CrossRef]
- McVicar, J.W. Quantitative aspects of the transmission of African swine fever. Am. J. Vet. Res. 1984, 45, 1535–1541. [Google Scholar]
- Davies, K.; Goatley, L.C.; Guinat, C.; Netherton, C.L.; Gubbins, S.; Dixon, L.K.; Reis, A.L. Survival of African Swine Fever Virus in Excretions from Pigs Experimentally Infected with the Georgia 2007/1 Isolate. Transbound. Emerg. Dis. 2017, 64, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.; Jurado, C.; Gallardo, C.; Fernández-Pinero, J.; Sánchez-Vizcaíno, J.M. Gaps in African Swine Fever: Analysis and Priorities. Transbound. Emerg. Dis. 2018, 65, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Gethmann, J.; Amler, S. The potential role of scavengers in spreading African swine fever among wild boar. Sci. Rep. 2019, 9, 11450. [Google Scholar] [CrossRef] [PubMed]
- OIE. 2019: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. African Swine Fever. Available online: https://www.oie.int/en/standard-setting/terrestrial-manual/access-online/ (accessed on 10 December 2020).
- Mazur-Panasiuk, N.; Woźniakowski, G.; Niemczuk, K. The First Complete Genomic Sequences of African Swine Fever Virus Isolated in Poland. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gallardo, C.; Nieto, R.; Soler, A.; Pelayo, V.; Fernández-Pinero, J.; Markowska-Daniel, I.; Pridotkas, G.; Nurmoja, I.; Granta, R.; Simón, A.; et al. Assessment of African Swine Fever Diagnostic Techniques as a Response to the Epidemic Outbreaks in Eastern European Union Countries: How to Improve Surveillance and Control Programs. J. Clin. Microbiol. 2015, 53, 2555–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Carvalho Ferreira, H.C.; Backer, J.A.; Weesendorp, E.; Klinkenberg, D.; Stegeman, J.A.; Loeffen, W.L.A. Transmission Rate of African Swine Fever Virus under Experimental Conditions. Vet. Microbiol. 2013, 165, 296–304. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A. Short Time Window for Transmissibility of African Swine Fever Virus from a Contaminated Environment. Transbound. Emerg. Dis. 2018, 65, 1024–1032. [Google Scholar] [CrossRef]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: A promising work still in progress. Porc. Heal. Manag. 2020, 6, 1–14. [Google Scholar] [CrossRef]
- Rock, D.L. Challenges for African swine fever vaccine development—“…perhaps the end of the beginning”. Vet. Microbiol. 2017, 206, 52–58. [Google Scholar] [CrossRef]
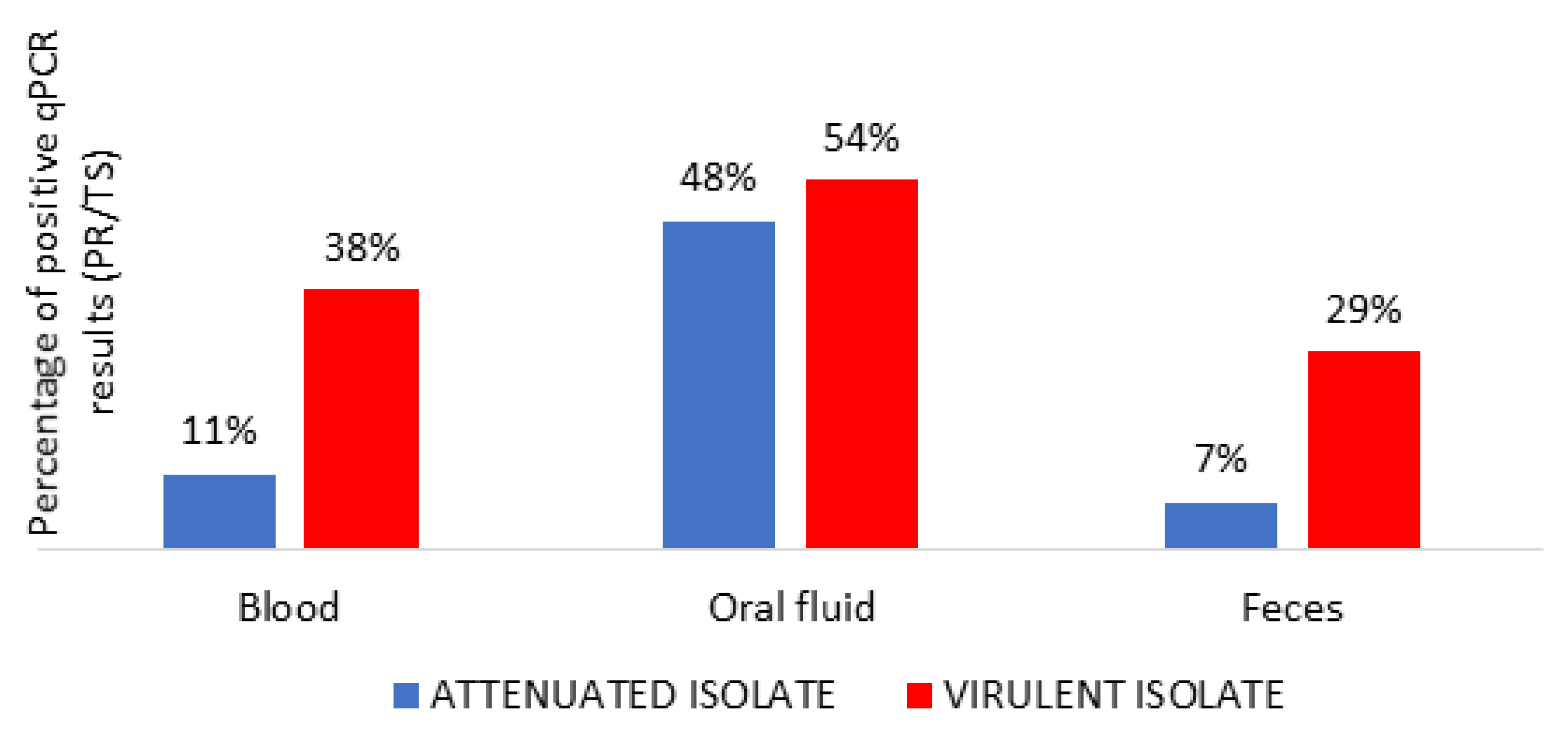
| Samples | Attenuated Group | Virulent Group |
|---|---|---|
| Blood | 66% | 100% |
| 8/12 | 17/17 | |
| Oral fluid | 83% | 88% |
| 10/12 | 15/17 | |
| Feces | 25% | 94% |
| 3/12 | 16/17 |
| Samples 1 | Attenuated Group | Virulent Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Orally Inoculated | In-Contact | Intramuscularly Inoculated | In-Contact | |||||
| Ct Values of Real-Time PCR (±SD) | Days to Onset of ASFV DNA Detection 2 | Ct Values of Real-Time PCR (±SD) | Days to Onset of ASFV DNA Detection 2 | Ct Values of Real-Time PCR (±SD) | Days to Onset of ASFV DNA Detection 2 | Ct Values of Real-Time PCR (±SD) in Contacts | Days to Onset of ASFV DNA Detection 2 | |
| Blood | 39.32 (±2.64) | 23 (±6) | 38.87 (±2.51) | 24 | 27.91 (±8.70) | 8 (±3) | 31.05 (±9.30) | 10 (±2) |
| Oral fluid | 38.49 (±3.67) | 15 (±4) | 37.31 (±3.60) | 19 (±4) | 30.08 (±4.77) | 5 (±2) | 32.42 (±5.55) | 8 (±2) |
| Feces | 39.87 (±0.76) | 22 (±2) | 39.39 (±1.81) | 21 | 32.85 (±6.19) | 8 (±4) | 36.07 (±4.86) | 12 (±2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosowska, A.; Cadenas-Fernández, E.; Barroso, S.; Sánchez-Vizcaíno, J.M.; Barasona, J.A. Distinct African Swine Fever Virus Shedding in Wild Boar Infected with Virulent and Attenuated Isolates. Vaccines 2020, 8, 767. https://doi.org/10.3390/vaccines8040767
Kosowska A, Cadenas-Fernández E, Barroso S, Sánchez-Vizcaíno JM, Barasona JA. Distinct African Swine Fever Virus Shedding in Wild Boar Infected with Virulent and Attenuated Isolates. Vaccines. 2020; 8(4):767. https://doi.org/10.3390/vaccines8040767
Chicago/Turabian StyleKosowska, Aleksandra, Estefanía Cadenas-Fernández, Sandra Barroso, Jose M. Sánchez-Vizcaíno, and Jose A. Barasona. 2020. "Distinct African Swine Fever Virus Shedding in Wild Boar Infected with Virulent and Attenuated Isolates" Vaccines 8, no. 4: 767. https://doi.org/10.3390/vaccines8040767





