Pretransplant BK Virus-Specific T-Cell-Mediated Immunity and Serotype Specific Antibodies May Have Utility in Identifying Patients at Risk of BK Virus-Associated Haemorrhagic Cystitis after Allogeneic HSCT
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients, Donors and Sample Collection in PBIHC Study (Pretransplant BKPyV—Specific Immune Response for HC Risk Assessment)
2.2. Haemorrhagic Cystitis Diagnosis
2.3. Algorithm for Assessment of HC Risk upon Patient Demographic and Clinical Pretransplant Characteristics
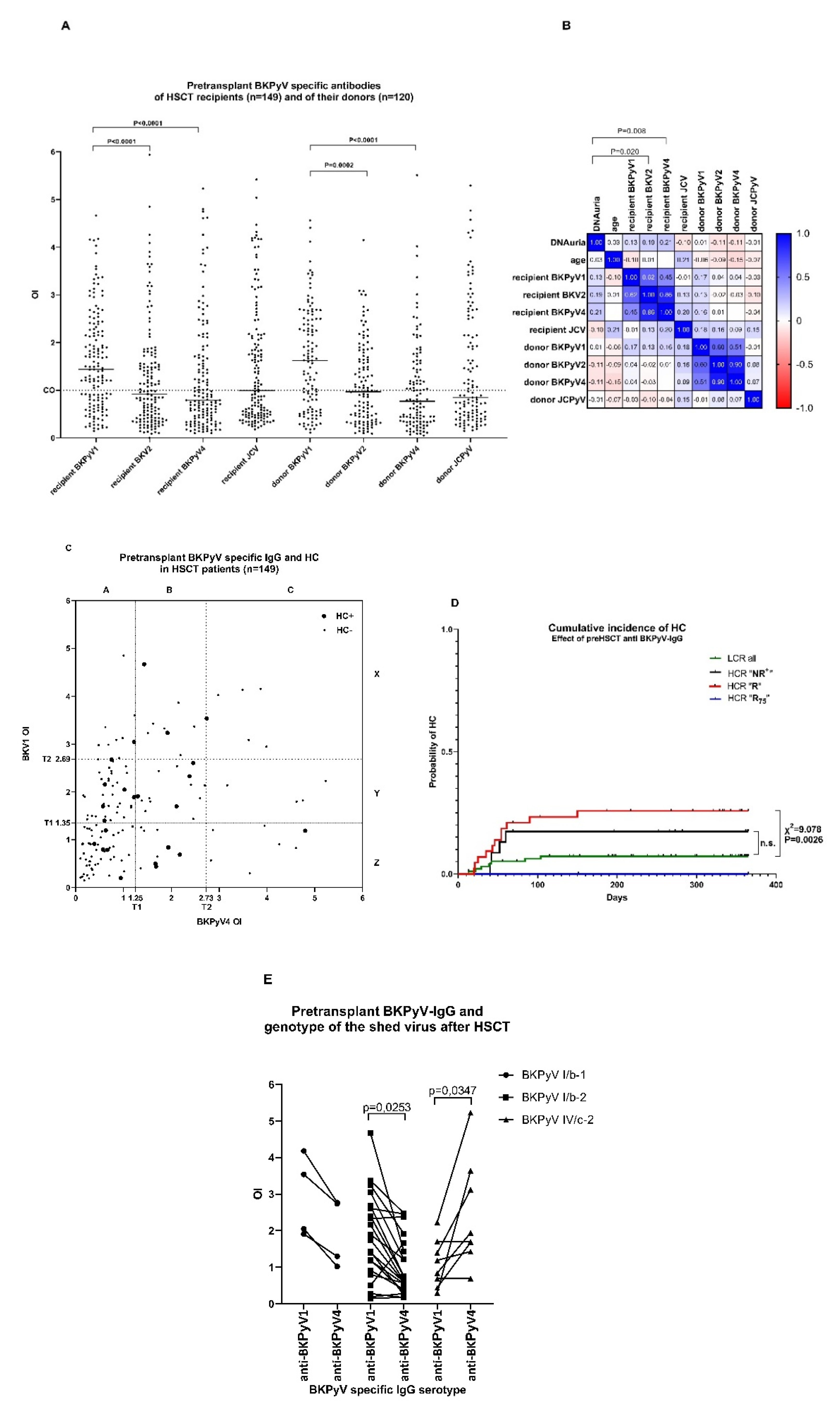
| PBIHC Study | Risk of HC | |||||
|---|---|---|---|---|---|---|
| Univariate Associations | Multivariate Associations | |||||
| Parameter | HC | Non HC | HC Rate [%] | ap Value | b χ2 Test | Logistic Regression |
| HSCT recipients (n = 149) | 22 | 127 | 14.8 | |||
| Maximum post HSCT BKPyV viruria | ||||||
| ref < 107 copies/mL | 0 | 90 | 0 | OR = 9.149 95%IC = 2.717–62.75 | ||
| >107 copies/mL | 22 | 37 | 37.2.0 | p < 0.0001 HR = 4.233 95%IC = 4.233–5.709 | ||
| Pretransplant conditioning | ||||||
| MAC | 19 | 92 | 17.1 | p = 0.1963 | n.s. | |
| RIC | 3 | 35 | 7.9 | |||
| Gender | ||||||
| Male | 18 | 74 | 19.5 | p = 0.055 | n.s. | |
| Female | 4 | 53 | 7.0 | |||
| Clinical risk group | ||||||
| HCR (male MAC + MUD + MMRD) | 15 | 43 | 25.8 | p = 0.0038 HR = 3.362 95%IC = 1.500–7.613 | ||
| LCR (all RIC + all MRD female MAC + MUD + MMRD) | 7 | 84 | 7.6 | |||
| Pretransplant anti BKPyV1,4 IgG ref = NR+ level | ||||||
| 1 “NR+” levels anti-BKPyV IgG | 5 | 50 | 9.0 | p = 0.2411 | ||
| 2 “R” levels anti-BKPyV IgG | 14 | 57 | 19.7 | p = 0.1878 | ||
| 3 “R75”anti-BKPyV IgG | 3 | 20 | 13.0 | p = 0.4878 | ||
| Combined risk HC ref = LCR “NR+” BKPyV IgG | ||||||
| LCR 1 “NR+” anti-BKPyV IgG | 1 | 34 | 2.8 | p = 0.1603 | ||
| LCR 2 “R” anti-BKPyV IgG | 4 | 36 | 10.0 | p = 0.3636 | ||
| LCR 3 “R75” anti-BKPyV IgG | 2 | 14 | 12.5 | p = 0.2286 | ||
| HCR 1 “NR+” anti-BKPyV IgG | 4 | 16 | 20.0 | p = 0.0532 | ||
| HCR 2 “R” anti-BKPyV IgG | 11 | 21 | 34.7 | p = 0.0009 HR = 0.4165 95%CI = 0.2832–0.6466 | ||
| HCR 3 “R75” anti-BKPyV IgG | 0 | 6 | 0.0 | p > 0.999 | ||

2.4. BK and JC Virus-Like Particles
2.5. Measurement of BK and JC Virus Specific Antibodies
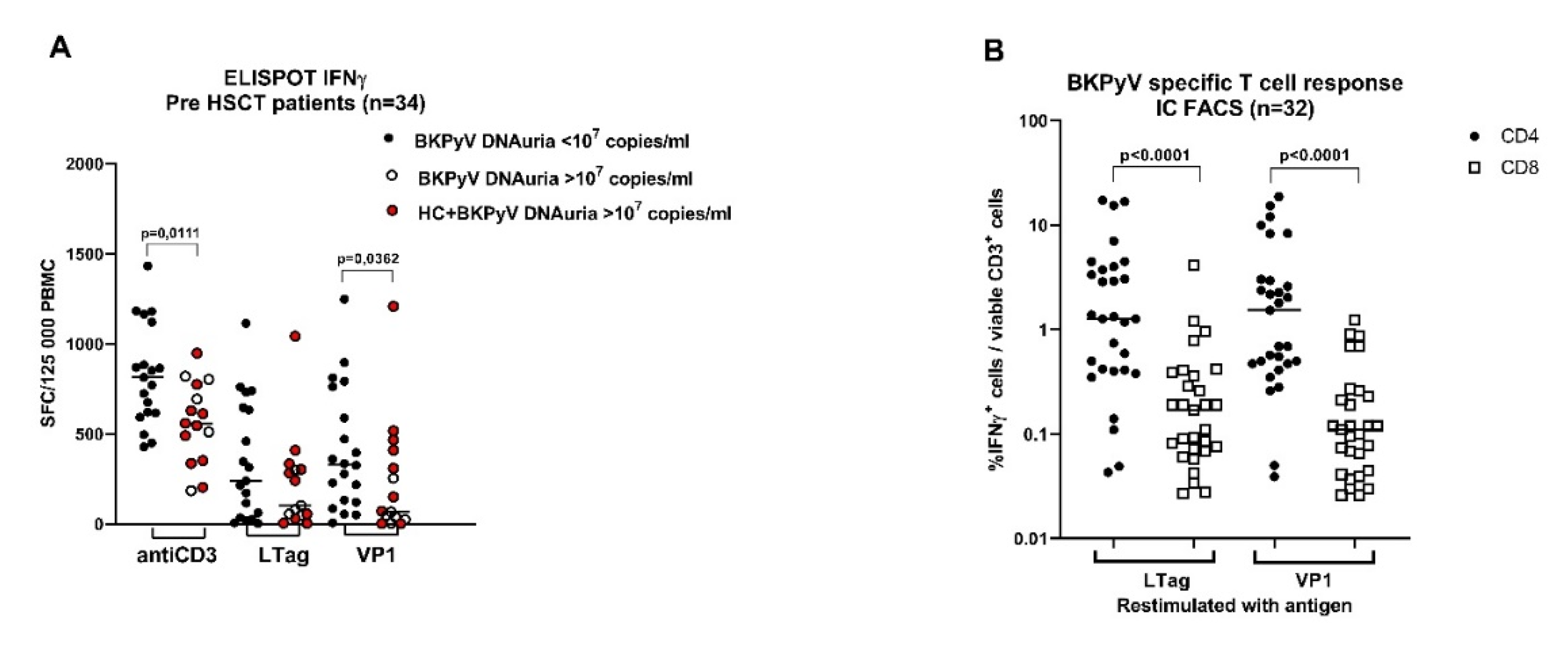
2.6. Detection of BKPyV-Specific T-cells
2.7. ELISPOT-IFNγ
2.8. Intracellular Cytokine Detection by Flow Cytometry (IC FACS)
2.9. BKPyV Genotyping
2.10. Statistics
3. Results
3.1. Polyomavirus-Specific Antibodies before HSCT in Recipients and Donors
3.2. “R” Levels of Pretransplant BKPyV-Specific IgG of Patients at High Clinical Risk (HCR) Are Associated with Increased Risk of HC
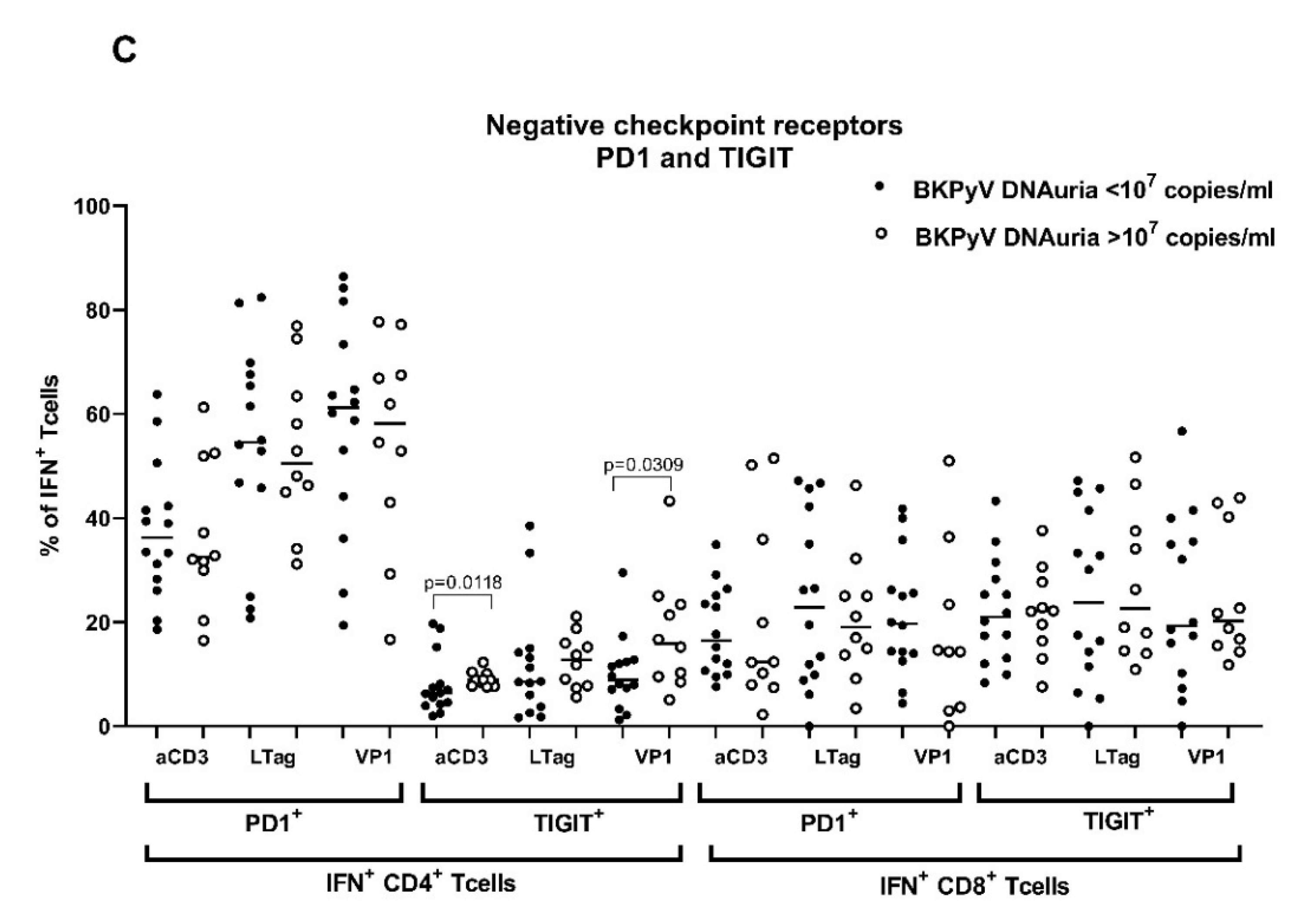
3.3. Pretransplant Non-Specific and BKPyV-Specific T-Cell Response Is Low in Patients with High BKPyV Viruria after HSCT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, C. Evolution and molecular epidemiology of polyomaviruses. Infect. Genet. Evol. 2020, 79, 104150. [Google Scholar] [CrossRef] [PubMed]
- Krumbholz, A.; Bininda-Emonds, O.R.; Wutzler, P.; Zell, R. Evolution of four BK virus subtypes. Infect. Genet. Evol. 2008, 8, 632–643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolt, A.; Sasnauskas, K.; Koskela, P.; Lehtinen, M.; Dillner, J. Seroepidemiology of the human polyomaviruses. J. Gen. Virol. 2003, 84, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Kean, J.M.; Rao, S.; Wang, M.; Garcea, R.L. Seroepidemiology of human polyomaviruses. PLoS Pathog. 2009, 5, e1000363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wunderink, H.F.; de Brouwer, C.S.; van der Meijden, E.; Pastrana, D.V.; Kroes, A.C.M.; Buck, C.B.; Feltkamp, M.C.W. Development and evaluation of a BK polyomavirus serotyping assay using Luminex technology. J. Clin. Virol. 2019, 110, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Sroller, V.; Hamsikova, E.; Ludvikova, V.; Vochozkova, P.; Kojzarova, M.; Fraiberk, M.; Salakova, M.; Moravkova, A.; Forstova, J.; Nemeckova, S. Seroprevalence rates of BKV, JCV, and MCPyV polyomaviruses in the general Czech Republic population. J. Med. Virol. 2014, 86, 1560–1568. [Google Scholar] [CrossRef]
- Cesaro, S.; Dalianis, T.; Hanssen, R.C.; Koskenvuo, M.; Pegoraro, A.; Einsele, H.; Cordonnier, C.; Hirsch, H.H. ECIL guidelines for the prevention, diagnosis and treatment of BK polyomavirus-associated haemorrhagic cystitis in haematopoietic stem cell transplant recipients. J. Antimicrob. Chemother. 2018, 73, 12–21. [Google Scholar] [CrossRef]
- Lunde, L.E.; Dasaraju, S.; Cao, Q.; Cohn, C.S.; Reding, M.; Bejanyan, N.; Trottier, B.; Rogosheske, J.; Brunstein, C.; Warlick, E.; et al. Hemorrhagic cystitis after allogeneic hematopoietic cell transplantation: Risk factors, graft source and survival. Bone Marrow Transplant. 2015, 50, 1432–1437. [Google Scholar] [CrossRef] [Green Version]
- Gorczynska, E.; Turkiewicz, D.; Rybka, K.; Toporski, J.; Kalwak, K.; Dyla, A.; Szczyra, Z.; Chybicka, A. Incidence, clinical outcome, and management of virus-induced hemorrhagic cystitis in children and adolescents after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 2005, 11, 797–804. [Google Scholar] [CrossRef] [Green Version]
- Cesaro, S.; Facchin, C.; Tridello, G.; Messina, C.; Calore, E.; Biasolo, M.A.; Pillon, M.; Varotto, S.; Brugiolo, A.; Mengoli, C.; et al. A prospective study of BK-virus-associated haemorrhagic cystitis in paediatric patients undergoing allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Leung, A.Y.; Suen, C.K.; Lie, A.K.; Liang, R.H.; Yuen, K.Y.; Kwong, Y.L. Quantification of polyoma BK viruria in hemorrhagic cystitis complicating bone marrow transplantation. Blood 2001, 98, 1971–1978. [Google Scholar] [CrossRef] [Green Version]
- Giraud, G.; Bogdanovic, G.; Priftakis, P.; Remberger, M.; Svahn, B.M.; Barkholt, L.; Ringden, O.; Winiarski, J.; Ljungman, P.; Dalianis, T. The incidence of hemorrhagic cystitis and BK-viruria in allogeneic hematopoietic stem cell recipients according to intensity of the conditioning regimen. Haematologica 2006, 91, 401–404. [Google Scholar]
- Hassan, Z.; Remberger, M.; Svenberg, P.; Elbander, M.; Omazic, B.; Mattsson, J.; Conrad, R.; Svahn, B.M.; Ahlgren, A.; Sairafi, D.; et al. Hemorrhagic cystitis: A retrospective single-center survey. Clin. Transplant. 2007, 21, 659–667. [Google Scholar] [CrossRef]
- Arai, Y.; Maeda, T.; Sugiura, H.; Matsui, H.; Jo, T.; Ueda, T.; Okada, K.; Kawata, T.; Onishi, T.; Mizutani, C.; et al. Risk factors for and prognosis of hemorrhagic cystitis after allogeneic stem cell transplantation: Retrospective analysis in a single institution. Hematology 2012, 17, 207–214. [Google Scholar] [CrossRef]
- Ringden, O.; Erkers, T.; Aschan, J.; Garming-Legert, K.; Le, B.K.; Hagglund, H.; Omazic, B.; Svenberg, P.; Dahllof, G.; Mattsson, J.; et al. A prospective randomized toxicity study to compare reduced-intensity and myeloablative conditioning in patients with myeloid leukaemia undergoing allogeneic haematopoietic stem cell transplantation. J. Intern. Med. 2013, 274, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Uhm, J.; Hamad, N.; Michelis, F.V.; Shanavas, M.; Kuruvilla, J.; Gupta, V.; Lipton, J.H.; Messner, H.A.; Seftel, M.; Kim, D.D. The risk of polyomavirus BK-associated hemorrhagic cystitis after allogeneic hematopoietic SCT is associated with myeloablative conditioning, CMV viremia and severe acute GVHD. Bone Marrow Transplant. 2014, 49, 1528–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzannou, I.; Papadopoulou, A.; Naik, S.; Leung, K.; Martinez, C.A.; Ramos, C.A.; Carrum, G.; Sasa, G.; Lulla, P.; Watanabe, A.; et al. Off-the-Shelf Virus-Specific T Cells to Treat BK Virus, Human Herpesvirus 6, Cytomegalovirus, Epstein-Barr Virus, and Adenovirus Infections After Allogeneic Hematopoietic Stem-Cell Transplantation. J. Clin. Oncol. 2017, 35, 3547–3557. [Google Scholar] [CrossRef] [PubMed]
- Pello, O.M.; Innes, A.J.; Bradshaw, A.; Finn, S.A.; Uddin, S.; Bray, E.; Olavarria, E.; Apperley, J.F.; Pavlu, J. BKV-specific T cells in the treatment of severe refractory haemorrhagic cystitis after HLA-haploidentical haematopoietic cell transplantation. Eur. J. Haematol. 2017, 98, 632–634. [Google Scholar] [CrossRef] [PubMed]
- Solis, M.; Velay, A.; Porcher, R.; Domingo-Calap, P.; Soulier, E.; Joly, M.; Meddeb, M.; Kack-Kack, W.; Moulin, B.; Bahram, S.; et al. Neutralizing Antibody-Mediated Response and Risk of BK Virus-Associated Nephropathy. J. Am. Soc. Nephrol. 2018, 29, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arthur, R.R.; Shah, K.V.; Baust, S.J.; Santos, G.W.; Saral, R. Association of BK viruria with hemorrhagic cystitis in recipients of bone marrow transplants. N. Engl. J. Med. 1986, 315, 230–234. [Google Scholar] [CrossRef]
- Wong, A.S.; Chan, K.H.; Cheng, V.C.; Yuen, K.Y.; Kwong, Y.L.; Leung, A.Y. Relationship of pretransplantation polyoma BK virus serologic findings and BK viral reactivation after hematopoietic stem cell transplantation. Clin. Infect. Dis. 2007, 44, 830–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Zheng, J.; Kolitsopoulos, Y.; Chung, D.; Amigues, I.; Son, T.; Choo, K.; Hester, J.; Giralt, S.A.; Glezerman, I.G.; et al. Relationship of BK polyoma virus (BKV) in the urine with hemorrhagic cystitis and renal function in recipients of T Cell-depleted peripheral blood and cord blood stem cell transplantations. Biol. Blood Marrow Transplant. 2014, 20, 1204–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskin, B.L.; Sullivan, K.E.; Hester, J.; Goebel, J.; Davies, S.M.; Jodele, S. Antibodies to BK virus in children prior to allogeneic hematopoietic cell transplant. Pediatr. Blood Cancer 2015, 62, 1670–1673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drummond, J.E.; Shah, K.V.; Saral, R.; Santos, G.W.; Donnenberg, A.D. BK virus specific humoral and cell mediated immunity in allogeneic bone marrow transplant (BMT) recipients. J. Med. Virol. 1987, 23, 331–344. [Google Scholar] [CrossRef]
- Schneidawind, D.; Schmitt, A.; Wiesneth, M.; Mertens, T.; Bunjes, D.; Freund, M.; Schmitt, M. Polyomavirus BK-specific CD8+ T cell responses in patients after allogeneic stem cell transplant. Leuk. Lymphoma 2010, 51, 1055–1062. [Google Scholar] [CrossRef]
- Leung, A.Y.; Chan, M.; Tang, S.C.; Liang, R.; Kwong, Y.L. Real-time quantitative analysis of polyoma BK viremia and viruria in renal allograft recipients. J. Virol. Methods 2002, 103, 51–56. [Google Scholar] [CrossRef]
- McNees, A.L.; White, Z.S.; Zanwar, P.; Vilchez, R.A.; Butel, J.S. Specific and quantitative detection of human polyomaviruses BKV, JCV, and SV40 by real time PCR. J. Clin. Virol. 2005, 34, 52–62. [Google Scholar] [CrossRef]
- Sroller, V.; Hamsikova, E.; Ludvikova, V.; Musil, J.; Nemeckova, S.; Salakova, M. Seroprevalence rates of HPyV6, HPyV7, TSPyV, HPyV9, MWPyV and KIPyV polyomaviruses among the healthy blood donors. J. Med. Virol. 2016, 88, 1254–1261. [Google Scholar] [CrossRef]
- Hrbacek, J.; Urban, M.; Hamsikova, E.; Tachezy, R.; Eis, V.; Brabec, M.; Heracek, J. Serum antibodies against genitourinary infectious agents in prostate cancer and benign prostate hyperplasia patients: A case-control study. BMC Cancer 2011, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Morel, V.; Martin, E.; Francois, C.; Helle, F.; Faucher, J.; Mourez, T.; Choukroun, G.; Duverlie, G.; Castelain, S.; Brochot, E. A Simple and Reliable Strategy for BK Virus Subtyping and Subgrouping. J. Clin. Microbiol. 2017, 55, 1177–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anoop, P.; Shaw, B.E.; Riley, U.; Ethell, M.E.; Taj, M.; Lancaster, D.L.; Atra, A.; Saso, R.; Littlewood, S.; Mohammed, K.; et al. Clinical profile and outcome of urotheliotropic viral haemorrhagic cystitis following haematopoietic stem cell transplantation: A 7-year tertiary centre analysis. Hematology 2011, 16, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bogdanovic, G.; Priftakis, P.; Giraud, G.; Kuzniar, M.; Ferraldeschi, R.; Kokhaei, P.; Mellstedt, H.; Remberger, M.; Ljungman, P.; Winiarski, J.; et al. Association between a high BK virus load in urine samples of patients with graft-versus-host disease and development of hemorrhagic cystitis after hematopoietic stem cell transplantation. J. Clin. Microbiol. 2004, 42, 5394–5396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Zimaity, M.; Saliba, R.; Chan, K.; Shahjahan, M.; Carrasco, A.; Khorshid, O.; Caldera, H.; Couriel, D.; Giralt, S.; Khouri, I.; et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplantation: Donor type matters. Blood 2004, 103, 4674–4680. [Google Scholar] [CrossRef]
- Dalianis, T.; Ljungman, P. Full myeloablative conditioning and an unrelated HLA mismatched donor increase the risk for BK virus-positive hemorrhagic cystitis in allogeneic hematopoetic stem cell transplanted patients. Anticancer Res. 2011, 31, 939–944. [Google Scholar]
- Rorije, N.M.; Shea, M.M.; Satyanarayana, G.; Hammond, S.P.; Ho, V.T.; Baden, L.R.; Antin, J.H.; Soiffer, R.J.; Marty, F.M. BK virus disease after allogeneic stem cell transplantation: A cohort analysis. Biol. Blood Marrow Transplant. 2014, 20, 564–570. [Google Scholar] [CrossRef] [Green Version]
- Oltolini, C.; Greco, R.; Galli, L.; Clerici, D.; Lorentino, F.; Xue, E.; Lupo Stanghellini, M.T.; Giglio, F.; Uhr, L.; Ripa, M.; et al. Infections after Allogenic Transplant with Post-Transplant Cyclophosphamide: Impact of Donor HLA Matching. Biol. Blood Marrow Transplant. 2020, 26, 1179–1188. [Google Scholar] [CrossRef]
- Silva Lde, P.; Patah, P.A.; Saliba, R.M.; Szewczyk, N.A.; Gilman, L.; Neumann, J.; Han, X.Y.; Tarrand, J.; Ribeiro, R.; Gulbis, A.; et al. Hemorrhagic cystitis after allogeneic hematopoietic stem cell transplants is the complex result of BK virus infection, preparative regimen intensity and donor type. Haematologica 2010, 95, 1183–1190. [Google Scholar] [CrossRef]
- Seber, A.; Shu, X.O.; Defor, T.; Sencer, S.; Ramsay, N. Risk factors for severe hemorrhagic cystitis following BMT. Bone Marrow Transplant. 1999, 23, 35–40. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, R.; Kusumi, E.; Kami, M.; Yuji, K.; Hamaki, T.; Saito, A.; Murasgihe, N.; Hori, A.; Kim, S.W.; Makimoto, A.; et al. Late hemorrhagic cystitis after reduced-intensity hematopoietic stem cell transplantation (RIST). Bone Marrow Transplant. 2003, 32, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Baldanti, F.; Lilleri, D.; Gerna, G. Monitoring human cytomegalovirus infection in transplant recipients. J. Clin. Virol. 2008, 41, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.; Dunne, J.; Crowley, B. Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-Cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital. J. Med. Virol. 2010, 82, 433–440. [Google Scholar] [CrossRef]
- Orti, G.; Iacoboni, G.; Barba, P.; Gimeno, R.; Roldan, E.; Fox, L.; Salamero, O.; Bosch, F.; Valcarcel, D. Donor lymphocyte infusion for BK virus hemorrhagic cystitis and nephropathy: A case report. Bone Marrow Transplant. 2019, 54, 772–774. [Google Scholar] [CrossRef]
- Chen, Y.; Trofe, J.; Gordon, J.; Du Pasquier, R.A.; Roy-Chaudhury, P.; Kuroda, M.J.; Woodle, E.S.; Khalili, K.; Koralnik, I.J. Interplay of cellular and humoral immune responses against BK virus in kidney transplant recipients with polyomavirus nephropathy. J. Virol. 2006, 80, 3495–3505. [Google Scholar] [CrossRef] [Green Version]
- Schachtner, T.; Muller, K.; Stein, M.; Diezemann, C.; Sefrin, A.; Babel, N.; Reinke, P. BK virus-specific immunity kinetics: A predictor of recovery from polyomavirus BK-associated nephropathy. Am. J. Transplant 2011, 11, 2443–2452. [Google Scholar] [CrossRef]
- Schachtner, T.; Stein, M.; Babel, N.; Reinke, P. The Loss of BKV-specific Immunity from Pretransplantation to Posttransplantation Identifies Kidney Transplant Recipients at Increased Risk of BKV Replication. Am. J. Transplant 2015, 15, 2159–2169. [Google Scholar] [CrossRef]
- Johnston, R.J.; Comps-Agrar, L.; Hackney, J.; Yu, X.; Huseni, M.; Yang, Y.; Park, S.; Javinal, V.; Chiu, H.; Irving, B.; et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014, 26, 923–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barber, D.L.; Wherry, E.J.; Masopust, D.; Zhu, B.; Allison, J.P.; Sharpe, A.H.; Freeman, G.J.; Ahmed, R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006, 439, 682–687. [Google Scholar] [CrossRef] [PubMed]
- Trandem, K.; Zhao, J.; Fleming, E.; Perlman, S. Highly activated cytotoxic CD8 T cells express protective IL-10 at the peak of coronavirus-induced encephalitis. J. Immunol. 2011, 186, 3642–3652. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Madan, R.; Karp, C.L.; Braciale, T.J. Effector T cells control lung inflammation during acute influenza virus infection by producing IL-10. Nat. Med. 2009, 15, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Philip, M.; Ferrell, P.B. Alterations of T-cell-mediated immunity in acute myeloid leukemia. Oncogene 2020, 39, 3611–3619. [Google Scholar] [CrossRef]
| Risk of HC | ||||||
|---|---|---|---|---|---|---|
| Univariate Associations | Multivariate Associations | |||||
| Parameter | HC | Non HC | HC Rate (%) | χ2 Test | Logistic Regression | |
| HSCT recipients (n = 524) | 66 | 458 | 12.6 | |||
| a Age. median. min-max (year) | 46 (20–65) | 52 (19–68) | p = 0.0057 | n.s. | ||
| b Gender | ||||||
| Male | 50 | 273 | 15.5 | p = 0.0143 HR = 1.666 95%CI = 1.1084–2.638 | OR = 0.4174 95%CI = 0.1868–0.8929 | |
| Female | 16 | 185 | 8.0 | |||
| aGvHD | ||||||
| ref = no aGvHD | 39 | 268 | 12.7 | n.s. | ||
| aGvHD grade 1 | 16 | 121 | 11.6 | p = 0.876 | ||
| aGvHD grade 2 | 10 | 52 | 16.1 | p = 0.5374 | ||
| aGvHD grade 3 | 1 | 17 | 5.5 | p = 0.7093 | ||
| b Pretransplant conditioning regimen | ||||||
| MAC | 56 | 271 | 17.1 | p < 0.0001 HR = 0.296 95%CI = 0.155–0.567 | OR = 6.099 95%CI = 2.623–15.50 | |
| RIC | 10 | 187 | 5.1 | |||
| b Donor HLA match | ||||||
| Ref = 10/10 MRD | 6 | 86 | 6.5 | p = 0.186 | n.s. | |
| 10/10 MUD with PtCy two doses-50 mg/kg | 6 | 51 | 9.6 | p = 0.0537 | ||
| 10/10 MUD with ATG 20–40 mg/kg | 30 | 152 | 16.5 | p = 0.0228 HR = 1.305 95%CI = 1.049–1.51 | ||
| Haploidentical | 14 | 103 | 12.0 | p = 0.238 | ||
| <10/10 MMRD | 10 | 66 | 13.3 | p = 0.188 | ||
| b Diagnosis | ||||||
| Ref = non Hodgkin lymphoma | 1 | 25 | 3.8 | p = 0.0217 RR = 1.919 95%CI = 1.139–3.881 | n.s. | |
| AML | 33 | 200 | 14.1 | p = 0.218 | ||
| MDS | 9 | 113 | 7.4 | p = 0.552 | ||
| ALL LBL | 9 | 65 | 12.2 | p = 0.446 | ||
| CLL | 3 | 14 | 17.6 | p = 0.284 | ||
| CML | 6 | 10 | 37.5 | p = 0.008 HR = 3.000 95%CI = 1.469–5.477 | ||
| c Other | 5 | 31 | 13.8 | p = 0.386 | ||
| Cummulated clinical risk groups HCR vs. LCR | ||||||
| Significant clinical risk (HCR) Male MAC+ 10/10 MUD Male MAC+ MMRD | 37 | 121 | 23.4 | p < 0.0001 HR = 2.955 95%CI = 1.890 to 4.607 | ||
| Low clinical risk (LCR) RIC+ All 10/10 MRD All female | 29 | 337 | 7.9 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šťastná-Marková, M.; Hamšíková, E.; Hainz, P.; Hubáček, P.; Kroutilová, M.; Kryštofová, J.; Ludvíková, V.; Musil, J.; Pecherková, P.; Saláková, M.; et al. Pretransplant BK Virus-Specific T-Cell-Mediated Immunity and Serotype Specific Antibodies May Have Utility in Identifying Patients at Risk of BK Virus-Associated Haemorrhagic Cystitis after Allogeneic HSCT. Vaccines 2021, 9, 1226. https://doi.org/10.3390/vaccines9111226
Šťastná-Marková M, Hamšíková E, Hainz P, Hubáček P, Kroutilová M, Kryštofová J, Ludvíková V, Musil J, Pecherková P, Saláková M, et al. Pretransplant BK Virus-Specific T-Cell-Mediated Immunity and Serotype Specific Antibodies May Have Utility in Identifying Patients at Risk of BK Virus-Associated Haemorrhagic Cystitis after Allogeneic HSCT. Vaccines. 2021; 9(11):1226. https://doi.org/10.3390/vaccines9111226
Chicago/Turabian StyleŠťastná-Marková, Markéta, Eva Hamšíková, Petr Hainz, Petr Hubáček, Marie Kroutilová, Jitka Kryštofová, Viera Ludvíková, Jan Musil, Pavla Pecherková, Martina Saláková, and et al. 2021. "Pretransplant BK Virus-Specific T-Cell-Mediated Immunity and Serotype Specific Antibodies May Have Utility in Identifying Patients at Risk of BK Virus-Associated Haemorrhagic Cystitis after Allogeneic HSCT" Vaccines 9, no. 11: 1226. https://doi.org/10.3390/vaccines9111226






