Protective Potential of an Autogenous Vaccine in an Aerogenous Model of Escherichia coli Infection in Broiler Breeders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals, Housing and Experimental Design
2.2. Inoculum
2.3. E. coli Aerosol-Challenge
2.4. Euthanasia and Gross Pathology
2.5. Microbiology
2.6. Histopathology
2.7. Ethics
2.8. Statistics
3. Results
3.1. Exposure Levels and Inoculation Conditions
3.2. Clinical Signs
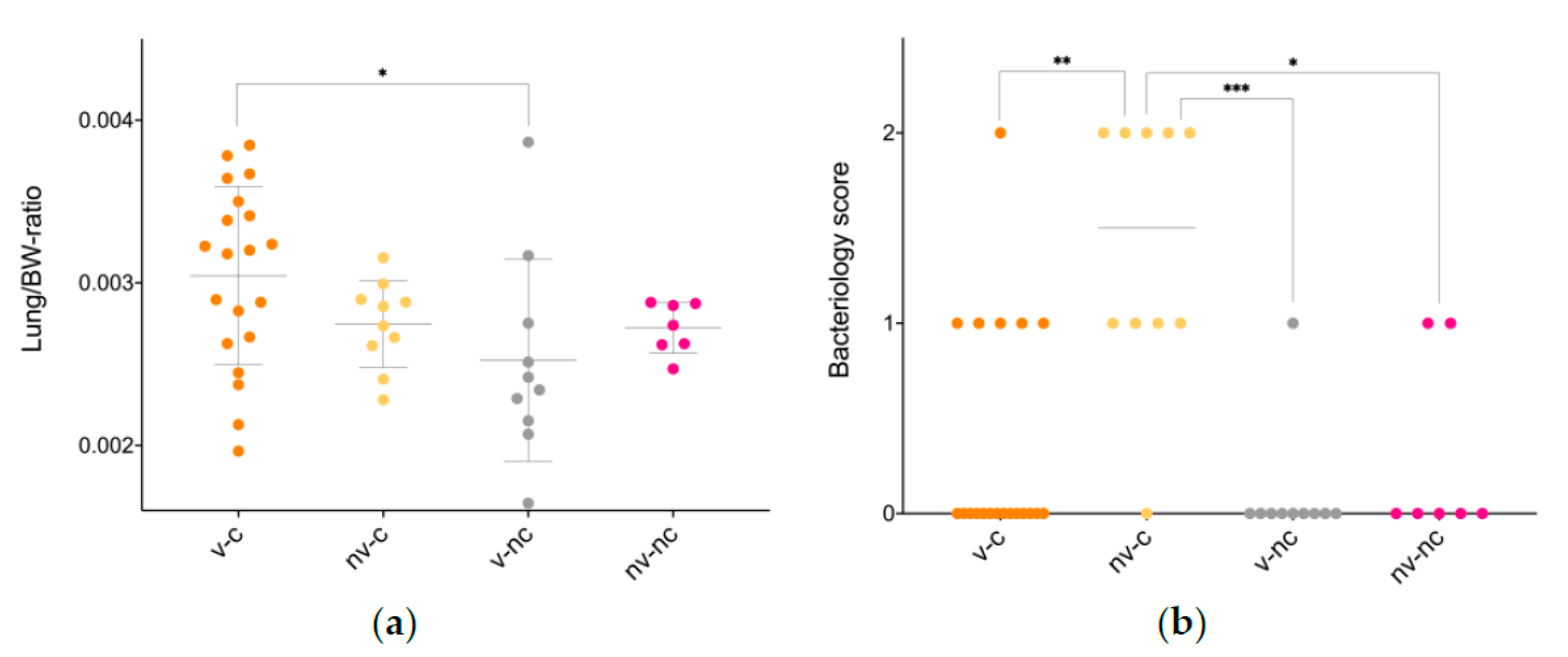
3.3. Gross Pathology
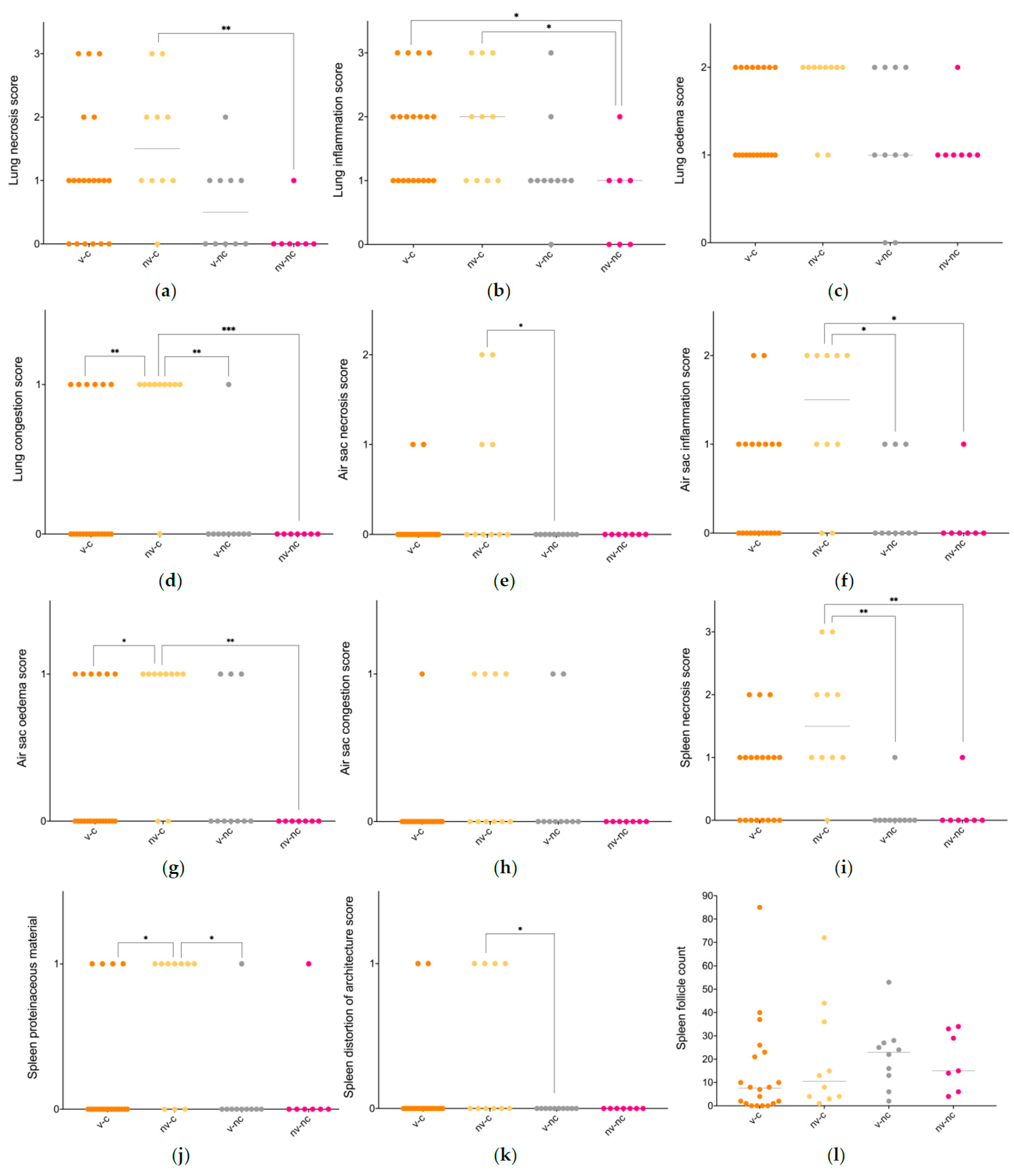
3.4. Histopathology
3.5. Microbiology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Vaccinated-Challenged (n = 20) | Not Vaccinated-Challenged (n = 10) | Vaccinated-Not Challenged (n = 10) | Not Vaccinated-Not Challenged (n = 7) | |
|---|---|---|---|---|
| Lung/BW-ratio | 3 × 10−3 ± 5.5 × 10−4 * | 2.7 × 10−3 ± 2.7 × 10−4 | 2.5 10−3 ± 6.2 × 10−4 | 2.7 × 10−3 ± 1.6 × 10−3 |
| Liver/BW-ratio | 2.4 × 10−2 ± 3.9 × 10−3 | 2.3 × 10−2 ± 3.7 × 10−3 | 2.2 × 10−2 ± 2.8 × 10−3 | 2.3 × 10−2 ± 3.8 × 10−3 |
| Spleen/BW-ratio | 9.2 × 10−4 ± 1.8 × 10−4 | 8.9 × 10−4 ± 1.7 × 10−4 | 7.6 × 10−4 ± 1.6 × 10−4 | 8.2 × 10−4 ± 1.5 × 10−4 |
Appendix B
References
- Nolan, L.K.; Vaillancourt, J.-P.; Barbieri, N.L.; Logue, C.M. Colibacillosis. In Diseases of Poultry; Wiley Blackwell, John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 770–830. [Google Scholar]
- Ewers, C.; Antao, E.-M.; Diehl, I.; Philipp, H.-C.; Wieler, L.H. Intestine and environment of the chicken as reservoirs for extraintestinal pathogenic Escherichia coli strains with zoonotic potential. Appl. Environ. Microbiol. 2009, 75, 184–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.J.; Kariyawasam, S.; Wannemuehler, Y.; Mangiamele, P.; Johnson, S.J.; Doetkott, C.; Skyberg, J.A.; Lynne, A.M.; Johnson, J.R.; Nolan, L.K. The genome sequence of avian pathogenic Escherichia coli strain O1:K1:H7 shares strong similarities with human extraintestinal pathogenic E. coli genomes. J. Bacteriol. 2007, 189, 3228–3236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeniyi, A.A. Direct transmission of Escherichia coli from poultry to humans. Epidemiol. Infect. 1989, 103, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Linton, A.H.; Howe, K.; Bennett, P.M.; Richmond, M.H.; Whiteside, E.J. Colonization of human gut by antibiotic resistant Escherichia coli from chickens. J. Appl. Bacteriol. 1977, 43, 465–469. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, L.; Spangholm, D.J.; Pedersen, K.; Jensen, L.B.; Emborg, H.D.; Agerso, Y.; Aarestrup, F.M.; Hammerum, A.M.; Frimodt-Moller, N. Broiler chickens, broiler chicken meat, pigs and pork as sources of ExPEC related virulence genes and resistance in Escherichia coli isolates from community-dwelling humans and UTI patients. Int. J. Food Microbiol. 2010, 142, 264–272. [Google Scholar] [CrossRef]
- Kluytmans, J.; Overdevest, I.; Willemsen, I.; Kluytmans-van den Bergh, M.F.Q.; van der Zwaluw, K.; Heck, M.; Rijnsburger, M.; Vandenbroucke-Grauls, C.; Savelkoul, P.H.M.; Johnston, B.D.; et al. Extended-spectrum beta-lactamase-producing Escherichia coli from retail chicken meat and humans: Comparison of strains, plasmids, resistance genes, and virulence factors. Clin. Infect. Dis. 2013, 56, 478–487. [Google Scholar] [CrossRef] [Green Version]
- Overdevest, I.; Willemsen, I.; Rijnsburger, M.; Eustace, A.; Xu, L.; Hawkey, P.; Heck, M.; Savelkoul, P.; Vandenbroucke-Grauls, C.; van der Zwaluw, K.; et al. Extended-spectrum beta-lactamase genes of Escherichia coli in chicken meat and humans, the Netherlands. Emerg. Infect. Dis. 2011, 17, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Cortes, P.; Blanc, V.; Mora, A.; Dahbi, G.; Blanco, J.E.; Blanco, M.; Lopez, C.; Andreu, A.; Navarro, F.; Pilar Alonso, M.; et al. Isolation and characterization of potentially pathogenic antimicrobial-resistant Escherichia coli strains from chicken and pig farms in Spain. Appl. Environ. Microbiol. 2010, 76, 2799–2805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, D.; Vinue, L.; Poeta, P.; Coelho, A.C.; Matos, M.; Saenz, Y.; Somalo, S.; Zarazaga, M.; Rodrigues, J.; Torres, C. Prevalence of extended-spectrum beta-lactamase-producing Escherichia coli isolates in faecal samples of broilers. Vet. Microbiol. 2009, 138, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Landman, W.J.M.; van Eck, J.H.H. The efficacy of inactivated Escherichia coli autogenous vaccines against the E. coli peritonitis syndrome in layers. Avian Pathol. 2017, 46, 658–665. [Google Scholar] [CrossRef] [PubMed]
- DANMAP 2015–Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Foodanimals, Food and Humans in Denmark. ISSN 1600-2032. Available online: https://www.danmap.org/reports (accessed on 1 September 2021).
- Ronco, T.; Stegger, M.; Olsen, R.H.; Sekse, C.; Nordstoga, A.B.; Pohjanvirta, T.; Lilje, B.; Lyhs, U.; Andersen, P.S.; Pedersen, K. Spread of avian pathogenic Escherichia coli ST117 O78:H4 in Nordic broiler production. Bmc Genom. 2017, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, E.D.; Leitner, G.; Drabkin, N.; Melamed, D. Passive-immunization of chicks against Escherichia coli. Avian Pathol. 1990, 19, 345–354. [Google Scholar] [CrossRef]
- Ghunaim, H.; Abdelhamid, M.A.; Kariyawasam, S. Advances in vaccination against avian pathogenic Escherichia coli respiratory disease: Potentials and limitations. Vet. Microbiol. 2014, 172, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, J.K.; Fries, P.A.; Cloud, S.S. In vitro and in vivo characterization of avian Escherichia coli 3. immunization. Avian Dis. 1985, 29, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Thofner, I.; Christensen, J.P.; Ronco, T.; Pedersen, K.; Olsen, R.H. Evaluation of the efficacy of an autogenous Escherichia coli vaccine in broiler breeders. Avian Pathol. 2017, 46, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Gutman, N.; Thøfner, I.C.N.; Christensen, J.P.; Nielsen, S.S.; Bojesen, A.M. Experimental E. coli challenge of vaccinated broiler breeders indicate limited protection from lesions in the reproductive tract. 2018; unpublished work. [Google Scholar]
- Kromann, S.; Olsen, R.H.; Bojesen, A.M.; Jensen, H.E.; Thofner, I. Development of an aerogenous Escherichia coli infection model in adult broiler breeders. Sci. Rep. 2021, 11, 19556. [Google Scholar] [CrossRef]
- Landman, W.J.M.; Heuvelink, A.; van Eck, J.H.H. Reproduction of the Escherichia coli peritonitis syndrome in laying hens. Avian Pathol. 2013, 42, 157–162. [Google Scholar] [CrossRef]
- Chen, J.; Griffiths, M.W. PCR differentiation of Escherichia coli from other Gram-negative bacteria using primers derived from the nucleotide sequences flanking the gene encoding the universal stress protein. Lett. Appl. Microbiol. 1998, 27, 369–371. [Google Scholar] [CrossRef]
- Isling, L.K.; Aalbaek, B.; Schroder, M.; Leifsson, P.S. Pyelonephritis in slaughter pigs and sows: Morphological characterization and aspects of pathogenesis and aetiology. Acta Vet. Scand. 2010, 52. [Google Scholar] [CrossRef] [Green Version]
- Koutsianos, D.; Gantelet, H.; Franzo, G.; Lecoupeur, M.; Thibault, E.; Cecchinato, M.; Koutoulis, K.C. An assessment of the level of protection against colibacillosis conferred by several autogenous and/or commercial vaccination programs in conventional pullets upon experimental challenge. Vet. Sci. 2020, 7, 80. [Google Scholar] [CrossRef]
- Dho-Moulin, M.; Fairbrother, J.M. Avian pathogenic Escherichia coli (APEC). Vet. Res. 1999, 30, 299–316. [Google Scholar] [PubMed]
- Antao, E.-M.; Glodde, S.; Li, G.; Sharifi, R.; Homeier, T.; Laturnus, C.; Diehl, I.; Bethe, A.; Philipp, H.-C.; Preisinger, R.; et al. The chicken as a natural model for extraintestinal infections caused by avian pathogenic Escherichia coli (APEC). Microb. Pathog. 2008, 45, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Lara, L.J.; Rostagno, M.H. Impact of heat stress on poultry production. Animals 2013, 3, 356–369. [Google Scholar] [CrossRef] [PubMed]


| Vaccinated-Challenged (n = 20) | Not Vaccinated-Challenged (n = 10) | Vaccinated-Not Challenged (n = 10) | Not Vaccinated-Not Challenged (n = 7) | |
|---|---|---|---|---|
| In-lay, euthanasia 1 | 18/20 (90%) | 10/10 (100%) | 9/10 (90%) | 7/7 (100%) |
| Tracheal changes (hyperaemia, and/or mucoid, purulent and/or fibrinous tracheitis) | 7/20 (35%) | 5/10 (50%) | 2/10 (20%) | 0/7 (0%) |
| Pulmonary changes (consolidation, oedema and/or exudate) | 13/20 (65%) | 8/10 (80%) | 3/10 (30%) | 3/7 (42%) |
| Airsacculitis (hyperaemia, opaqueness and/or exudate) | 10/20 (50%) | 8/10 (80%) | 2/10 (20%) | 2/7 (28%) |
| Pericarditis (purulent and/or fibrinous) | 0/20 (0%) | 1/10 (10%) | 0/10 (0%) | 0/7 (0%) |
| Perihepatitis (purulent and/or fibrinous) | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/7 (0%) |
| Peritonitis (purulent and/or fibrinous) | 4/20 (20%) | 3/10 (30%) | 1/10 (10%) | 1/7 (14%) |
| Perioophoritis (purulent and/or fibrinous) | 5/20 (25%) | 3/10 (30%) | 1/10 (10%) | 1/7 (14%) |
| Overall lesion score | 1.95 | 2.8 | 0.9 | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kromann, S.; Olsen, R.H.; Bojesen, A.M.; Jensen, H.E.; Thøfner, I. Protective Potential of an Autogenous Vaccine in an Aerogenous Model of Escherichia coli Infection in Broiler Breeders. Vaccines 2021, 9, 1233. https://doi.org/10.3390/vaccines9111233
Kromann S, Olsen RH, Bojesen AM, Jensen HE, Thøfner I. Protective Potential of an Autogenous Vaccine in an Aerogenous Model of Escherichia coli Infection in Broiler Breeders. Vaccines. 2021; 9(11):1233. https://doi.org/10.3390/vaccines9111233
Chicago/Turabian StyleKromann, Sofie, Rikke Heidemann Olsen, Anders Miki Bojesen, Henrik Elvang Jensen, and Ida Thøfner. 2021. "Protective Potential of an Autogenous Vaccine in an Aerogenous Model of Escherichia coli Infection in Broiler Breeders" Vaccines 9, no. 11: 1233. https://doi.org/10.3390/vaccines9111233







