ZIF-8 Nanoparticles Based Electrochemical Sensor for Non-Enzymatic Creatinine Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
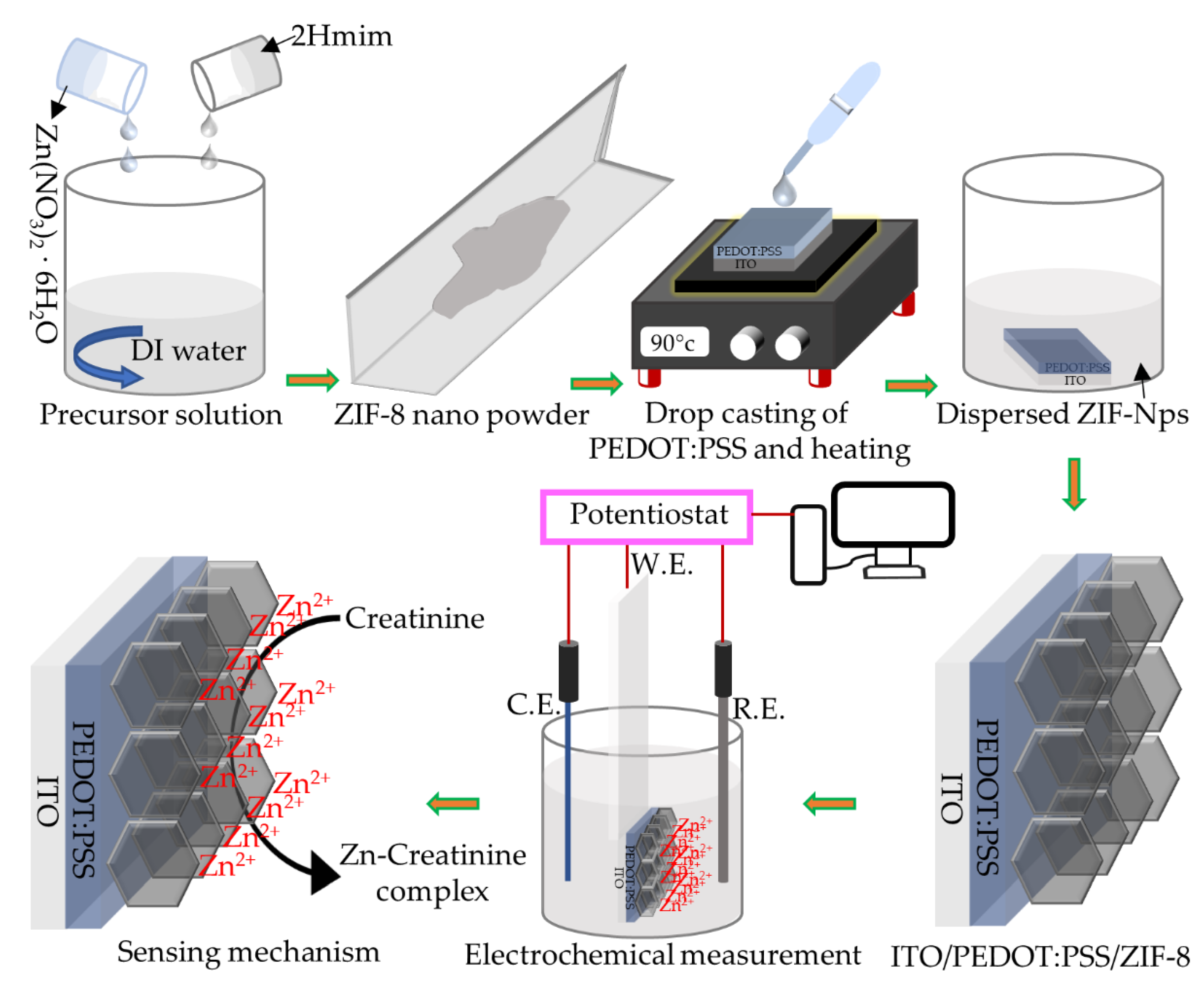
2.2. Synthesis of ZIF-8 NPs
2.3. Device Fabrication
2.4. Instrumentation for Physical and Electrochemical Characterization
3. Results
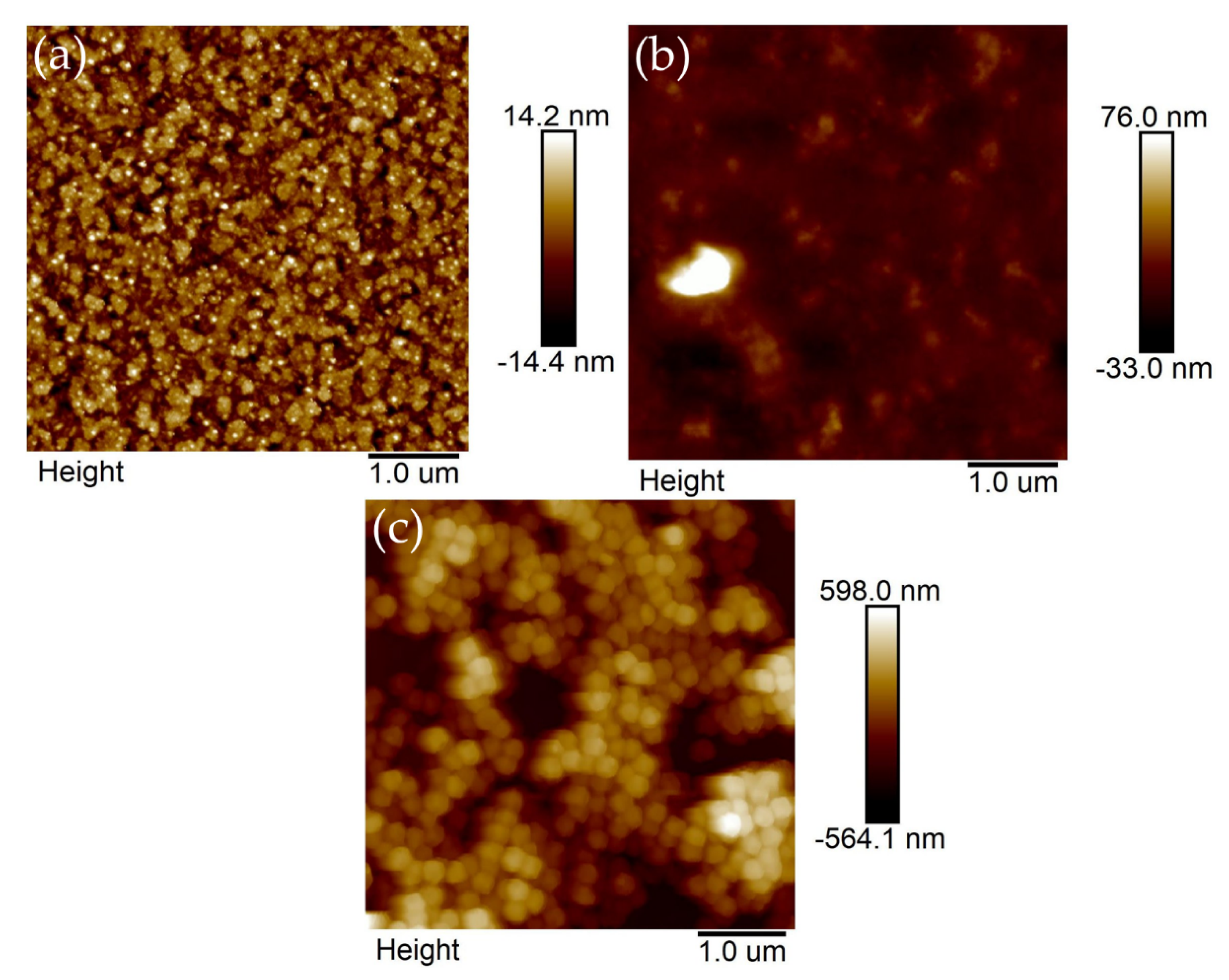
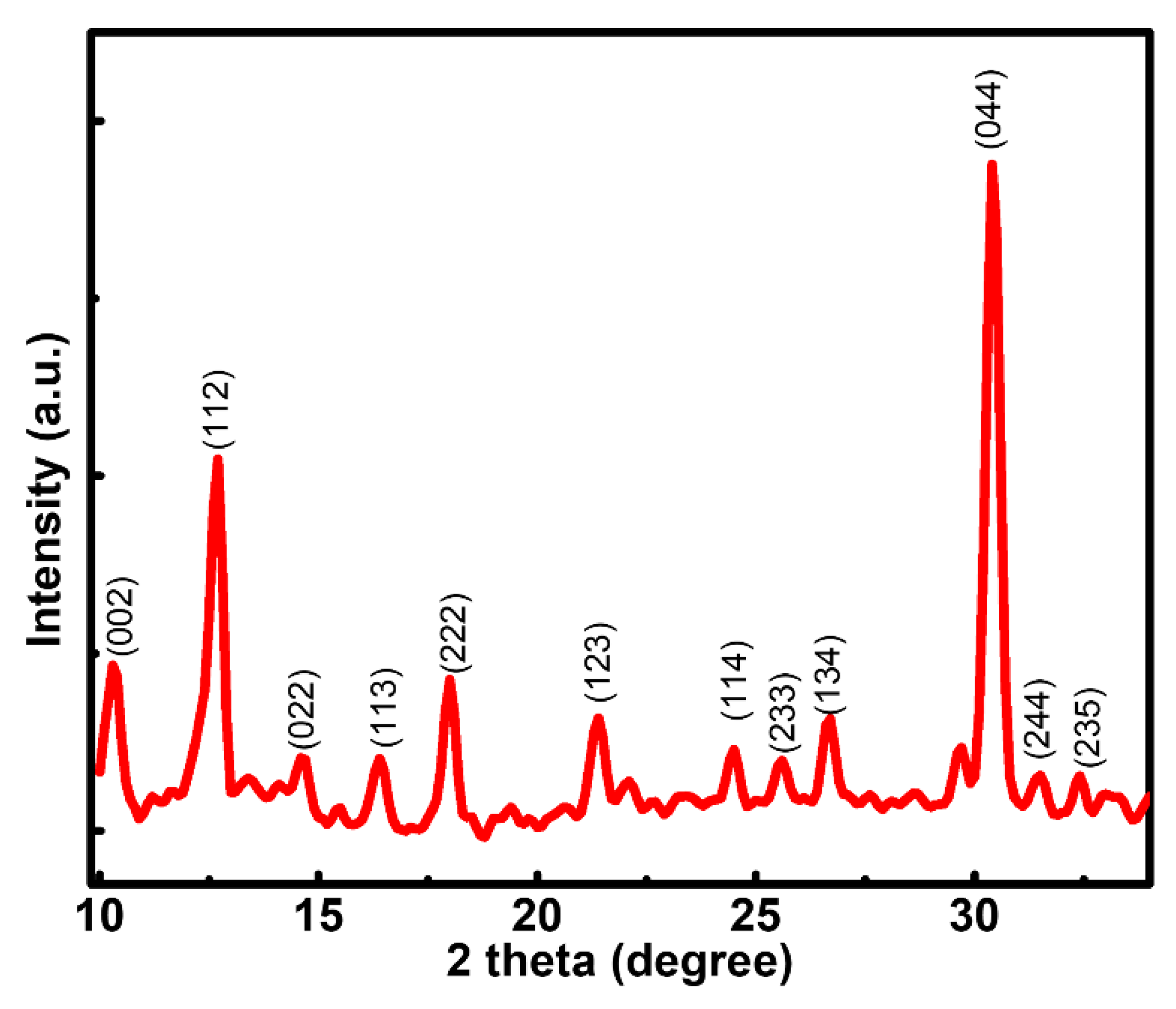
3.1. Morphological, Structural, and Stoichiometric Characterization
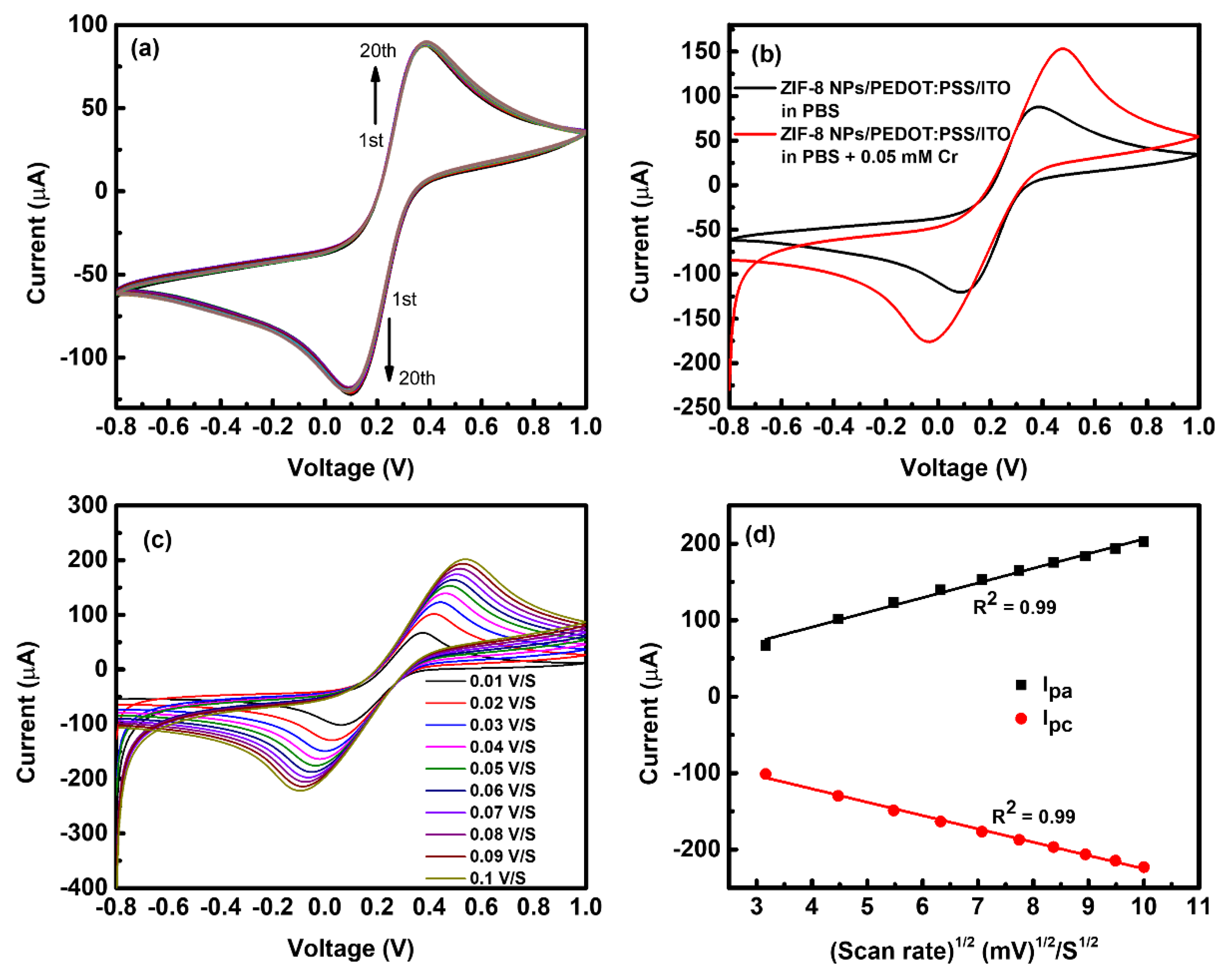
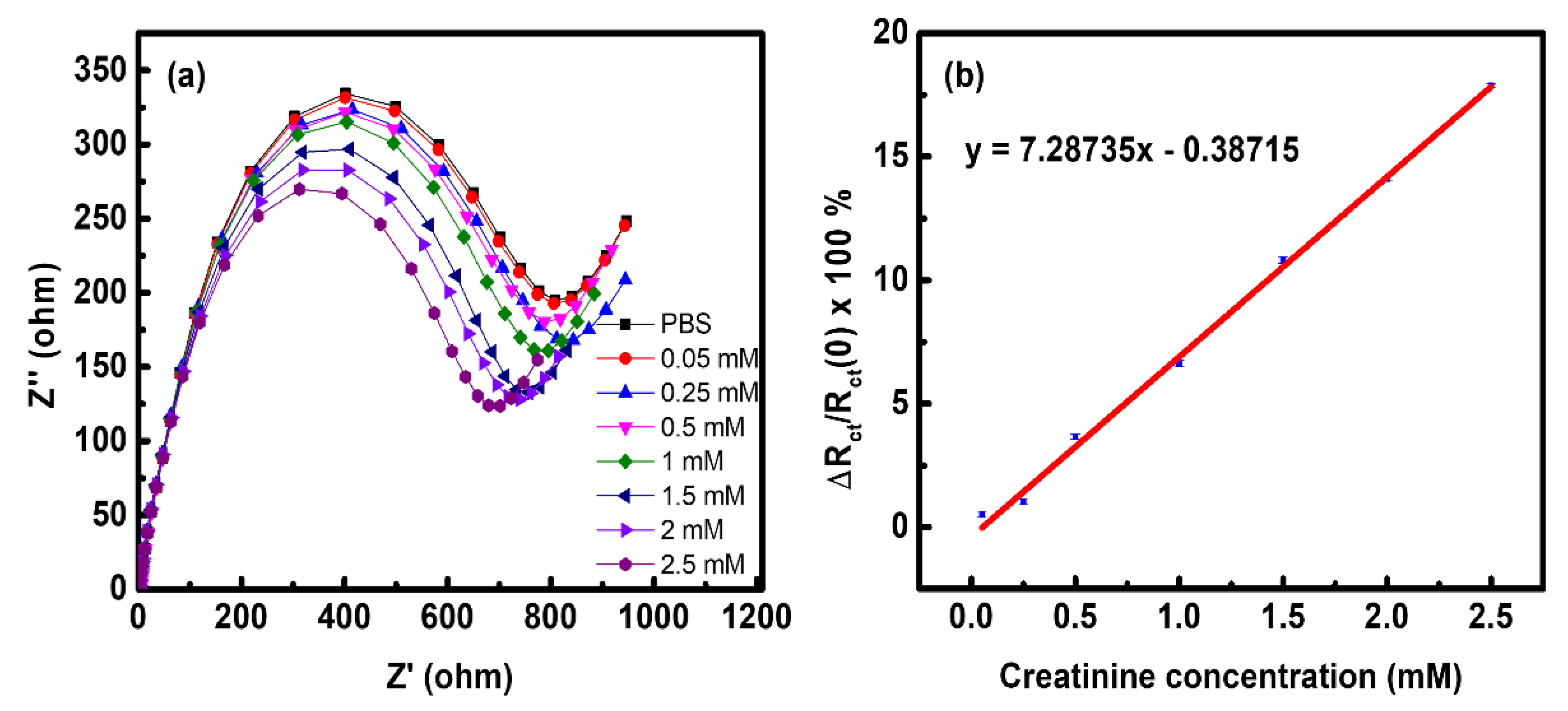
3.2. Electrochemical Characterization of the ZIF-8 NPs/PEDOT:PSS/ITO Sensing Electrode
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dasgupta, P.; Kumar, V.; Krishnaswamy, P.R.; Bhat, N. Biochemical assay for serum creatinine detection through a 1-methylhydantoin and cobalt complex. RSC Adv. 2020, 10, 39092–39101. [Google Scholar] [CrossRef]
- Raveendran, J.; Resmi, P.E.; Ramachandran, T.; Nair, B.G.; Babu, T.S. Fabrication of a disposable non-enzymatic electrochemical creatinine sensor. Sens. Actuators B Chem. 2017, 243, 589–595. [Google Scholar] [CrossRef]
- Lad, U.; Khokhar, S.; Kale, G.M. Electrochemical creatinine biosensors. Anal. Chem. 2008, 80, 7910–7917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cánovas, R.; Cuartero, M.; Crespo, G.A. Modern creatinine (bio)sensing: Challenges of point-of-care platforms. Biosens. Bioelectron. 2019, 130, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.; Kumar, V.; Krishnaswamy, P.R.; Bhat, N. Serum creatinine electrochemical biosensor on printed electrodes using monoenzymatic pathway to 1-methylhydantoin detection. ACS Omega 2020, 5, 22459–22464. [Google Scholar] [CrossRef]
- Perrone, R.D.; Madias, N.E.; Levey, A.S. Serum creatinine as an index of renal function: New insights into old concepts. Clin. Chem. 1992, 38, 1933–1953. [Google Scholar] [CrossRef] [PubMed]
- Sreeramareddygari, M.; Devaramani, S.; Thippeswamy, R.; Kempahanumakkagari, S.; Surareungchai, W. Trending approaches in electrochemical sensing. Electrochemistry 2021, 16, 1–43. [Google Scholar]
- Khue, N.V.; Wolff, C.M.; Seris, J.L.; Schwing, J.P. Immobilized enzyme electrode for creatinine determination in serum. Anal. Chem. 1991, 63, 611–614. [Google Scholar] [CrossRef]
- Husdan, H.; Rapoport, A. Estimation of creatinine by the Jaffe reaction. Clin. Chem. 1968, 14, 222–238. [Google Scholar] [CrossRef]
- Mitewa, M. Coordination properties of the bioligands creatinine and creatine in various reaction media. Coord. Chem. Rev. 1995, 140, 1–25. [Google Scholar] [CrossRef]
- Peedikakkal, A.M.; Adarsh, N.N. Porous coordination polymers. In Polymers and Polymeric Composites: A Reference Series; Springer: Berlin/Heidelberg, Germany, 2019; pp. 181–223. [Google Scholar]
- Wu, M.; Ye, H.; Zhao, F.; Zeng, B. High-quality metal–organic framework zif-8 membrane supported on electrodeposited ZnO/2-methylimidazole nanocomposite: Efficient adsorbent for the enrichment of acidic drugs. Sci. Rep. 2017, 7, 39778. [Google Scholar] [CrossRef]
- García-Palacín, M.; Martínez, J.I.; Paseta, L.; Deacon, A.; Johnson, T.; Malankowska, M.; Téllez, C.; Coronas, J. Sized-controlled zif-8 nanoparticle synthesis from recycled mother liquors: Environmental impact assessment. ACS Sustain. Chem. Eng. 2020, 8, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Hummel, M.; Gu, Z. Synthesis of au@zif-8 nanocomposites for enhanced electrochemical detection of dopamine. ECS Meet. Abstr. 2020, MA2020-01, 2585. [Google Scholar] [CrossRef]
- Bergaoui, M.; Khalfaoui, M.; Awadallah-F, A.; Al-Muhtaseb, S. A review of the features and applications of zif-8 and its derivatives for separating CO2 and isomers of C3- and C4- hydrocarbons. J. Nat. Gas Sci. Eng. 2021, 96, 104289. [Google Scholar] [CrossRef]
- Karaderi, S.; Bilgic, D. Zinc(ii) and cadmium(ii) binary complexes with creatinine and their mixed-ligand complexes with L-asparagine or L-glutamic acid: Potentiometrie studies. Main Group Met. Chem. 2006, 29, 145–156. [Google Scholar] [CrossRef]
- Chappanda, K.N.; Tchalala, M.R.; Shekhah, O.; Surya, S.G.; Eddaoudi, M.; Salama, K.N. A comparative study of interdigitated electrode and quartz crystal microbalance transduction techniques for metal–organic framework-based acetone sensors. Sensors 2018, 18, 3898. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, N.; Fazaeli, R.; Aliyan, H. One-pot hydrothermal synthesis of H3pw12o40 supported on zeolite imidazolate frameworks (zif-8): A highly efficient heterogeneous catalyst for oxidation of sulfides to sulfoxides and sulfones. Z. Fuer Nat. B 2016, 71, 211–217. [Google Scholar] [CrossRef]
- Tian, F.; Cerro, A.M.; Mosier, A.M.; Wayment-Steele, H.K.; Shine, R.S.; Park, A.; Webster, E.R.; Johnson, L.E.; Johal, M.S.; Benz, L. Surface and stability characterization of a nanoporous zif-8 thin film. J. Phys. Chem. C 2014, 118, 14449–14456. [Google Scholar] [CrossRef]
- Si, Y.; Li, X.; Yang, G.; Mie, X.; Ge, L. Fabrication of a novel core–shell CQDS@ZIF-8 composite with enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 13049–13061. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, M.S. A novel structural specific creatinine sensing scheme for the determination of the urine creatinine. Biosens. Bioelectron. 2012, 31, 90–94. [Google Scholar] [CrossRef]
- Kamel, A.H. Solid contact potentiometric sensors based on host-tailored molecularly imprinted polymers for Creatine Assessment. Int. J. Electrochem. Sci. 2016, 11, 8938–8949. [Google Scholar] [CrossRef]
- Magalhães, J.M.; Machado, A.A. Array of potentiometric sensors for the analysis of creatinine in urine samples. Analyst 2002, 127, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Fava, E.L.; Prado, T.M.; Garcia-Filho, A.; Silva, T.A.; Cincotto, F.H.; Cruz de Moraes, F.; Faria, R.C.; Fatibello-Filho, O. Non-enzymatic electrochemical determination of creatinine using a novel screen-printed microcell. Talanta 2020, 207, 120277. [Google Scholar] [CrossRef] [PubMed]
- Dal Dosso, F.; Decrop, D.; Pérez-Ruiz, E.; Daems, D.; Agten, H.; Al-Ghezi, O.; Bollen, O.; Breukers, J.; De Rop, F.; Katsafadou, M.; et al. Creasensor: Simple technology for creatinine detection in plasma. Anal. Chim. Acta 2018, 1000, 191–198. [Google Scholar] [CrossRef] [PubMed]






| Sensing Material | Method of Detection | Measurement Technique | LOD (µM) | Linear Range |
|---|---|---|---|---|
| Β-cyclodextrin incorporated poly-3,4-ethylene dioxythiophene mofified glassy carbon electrode [21] | Non-enzymatic | Potentiometric | 50 | 100–100,000 |
| PANi [22] | Enzymatic | Amperometric | 50 | 50–1000 |
| Poly venyle chloride [23] | Enzymatic | Potentiometric | 44–88 | 88–4400 |
| Paper absorbed Fe (III) ions [24] Microfluidic cartridge [25] | Non-enzymatic Enzymatic | Amperometric Colorimetric | 43 60 | 100–6500 67.2–1768 |
| This work | Non-enzymatic | Impedemetric | 30 | 50–2500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakraborty, T.; Das, M.; Lin, C.-Y.; Su, Y.; Yuan, B.; Kao, C.-H. ZIF-8 Nanoparticles Based Electrochemical Sensor for Non-Enzymatic Creatinine Detection. Membranes 2022, 12, 159. https://doi.org/10.3390/membranes12020159
Chakraborty T, Das M, Lin C-Y, Su Y, Yuan B, Kao C-H. ZIF-8 Nanoparticles Based Electrochemical Sensor for Non-Enzymatic Creatinine Detection. Membranes. 2022; 12(2):159. https://doi.org/10.3390/membranes12020159
Chicago/Turabian StyleChakraborty, Titisha, Munmun Das, Chan-Yu Lin, Yen Su, Bing Yuan, and Chyuan-Haur Kao. 2022. "ZIF-8 Nanoparticles Based Electrochemical Sensor for Non-Enzymatic Creatinine Detection" Membranes 12, no. 2: 159. https://doi.org/10.3390/membranes12020159






