Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Particles of ZnO
2.2.2. Preparation of Hybrid Membrane
2.2.3. Sorption Procedure
2.2.4. Simulated Wastewater
3. Results and Discussions
3.1. Characterization of the Hybrid Membrane
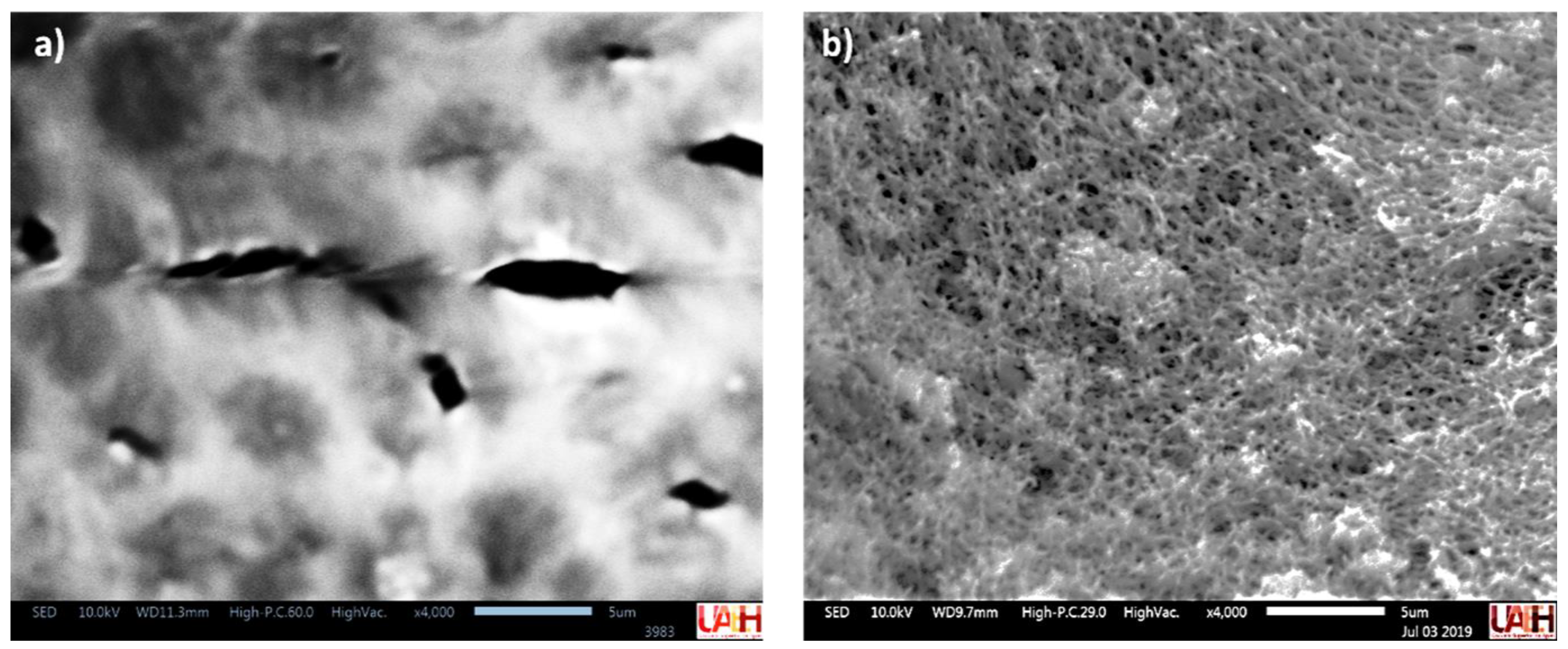
3.1.1. Scanning Electronic Microscopy (SEM)
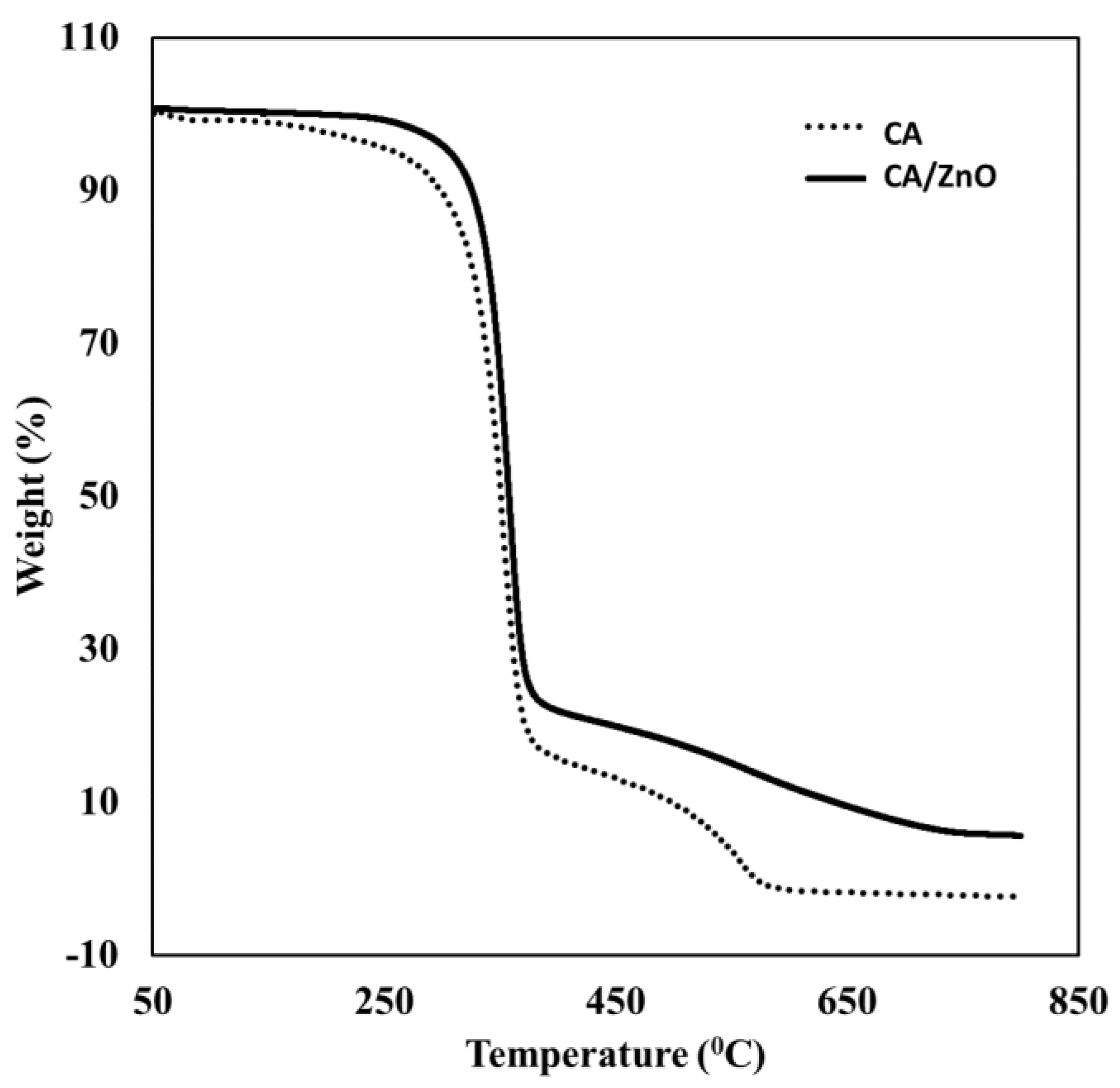
3.1.2. Thermogravimetric Analysis (TGA)
3.1.3. Fourier-Transform Infrared Spectroscopy (FTIR)
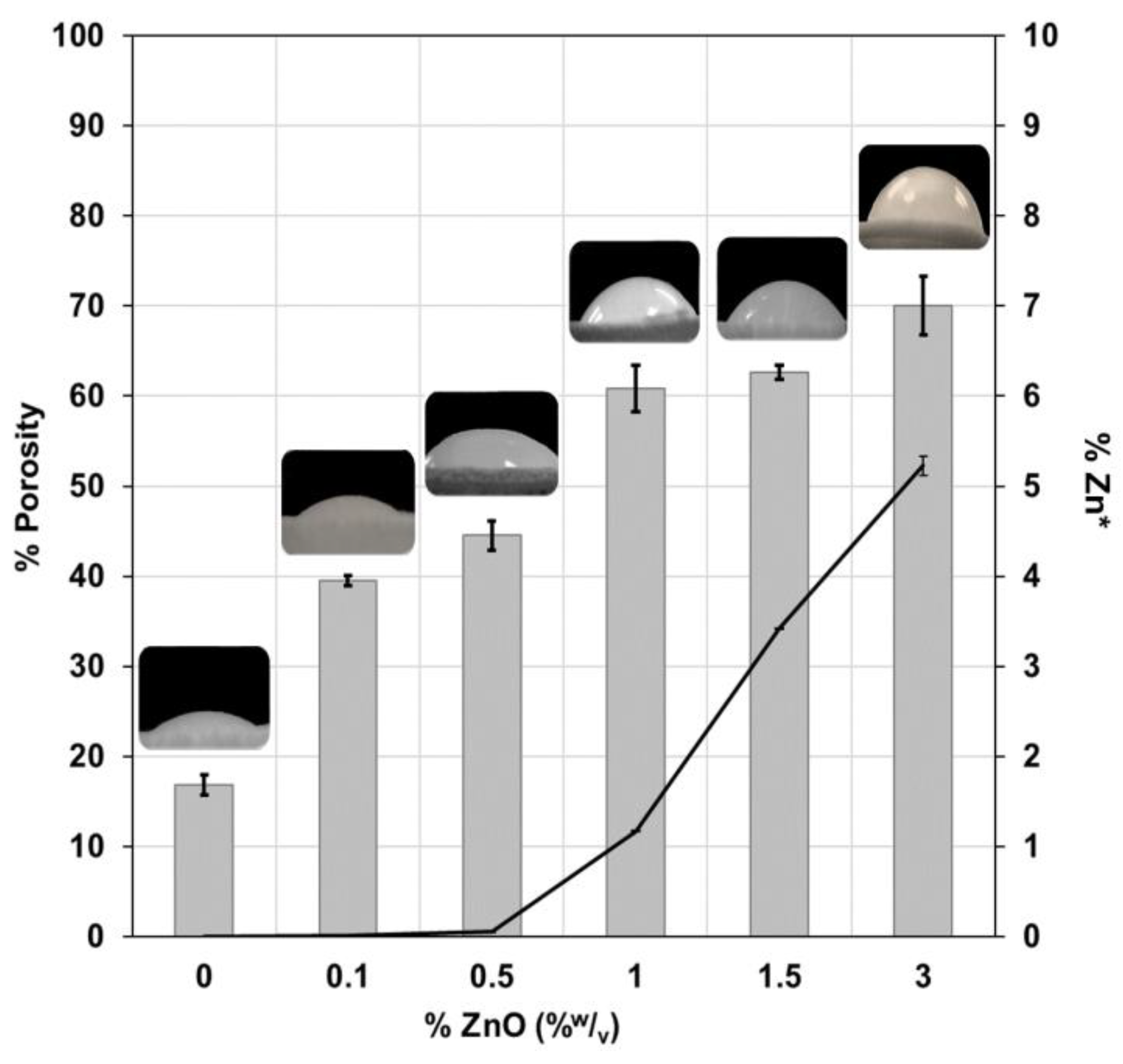
3.1.4. Porosity and Hydrophobicity
3.2. Amount of ZnO Nanoparticles
3.3. Evaluation pH in Aqueous Solution
3.4. Adsorption Isotherms
3.5. Thermodynamic Study
3.6. Simulated Wastewater
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2018, 17, 145–155. [Google Scholar] [CrossRef]
- Kapahi, M.; Sachdeva, S. Bioremediation Options for Heavy Metal Pollution. J. Health Pollut. 2019, 9, 191203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Ezugbe, E.O.; Rathilal, S. Membrane Technologies in Wastewater Treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef]
- Ahmad, H.; Kamarudin, S.K.; Hasran, U.A.; Daud, W.R.W. Overview of hybrid membranes for direct-methanol fuel-cell applications. Int. J. Hydrog. Energy 2010, 35, 2160–2175. [Google Scholar] [CrossRef]
- Souza, V.C.; Quadri, M.G.N. Organic-Inorganic Hybrid Membranes In Separation Processes: A 10-Year Review. Braz. J. Chem. Eng. 2013, 30, 683–700. [Google Scholar] [CrossRef]
- Jhaveri, J.H.; Murthy, Z.V.P. Nanocomposite membranes. Desalin Water Treat. 2015, 57, 26803–26819. [Google Scholar] [CrossRef]
- Ursino, C.; Castro-Muñoz, R.; Drioli, E.; Gzara, L.; Albeirutty, M.H.; Figoli, A. Progress of Nanocomposite Membranes for Water Treatment. Membranes 2018, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.; Yuan, J.; Ma, X.; Wang, S.; Liu, Y. Polymeric nanocomposite membranes for water treatment: A review. Environ. Chem. Lett. 2019, 17, 1539–1551. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, S.; Wu, Y.; Yan, F.; Qin, S.; Yang, J.; Li, J.; Cui, Z. Fabrication of polymer@TiO2 NPs hybrid membrane based on covalent bonding and coordination and its mechanism of enhancing photocatalytic performance. J. Alloys Compd. 2022, 910, 164887. [Google Scholar] [CrossRef]
- Manikandan, S.; Subbaiya, R.; Saravanan, M.; Ponraj, M.; Selvam, M.; Pugazhendhi., A. A critical review of advanced nanotechnology and hybrid membrane based water recycling, reuse, and wastewater treatment processes. Chemosphere 2022, 289, 132867. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Monti, S. Microscopic Hybrid Membranes Made of Cellulose-Based Materials Tuned for Removing Metal Ions from Industrial Effluents. ACS Appl. Polym. Mater. 2021, 3, 3733–3746. [Google Scholar] [CrossRef]
- Iehi, A.Y.; Mousavirad, S.J.; Akbari, A. Pre-treatment of textile wastewaters containing Chrysophenine using hybrid membranes. Membr. Water Treat. 2017, 8, 89–112. [Google Scholar]
- Pal, M.; Malhotra, M.; Mandal, M.K.; Paine, T.K.; Pal, P. Recycling of wastewater from tannery industry through membrane-integrated hybrid treatment using a novel graphene oxide nanocomposite. J. Water Process. Eng. 2020, 36, 101324. [Google Scholar] [CrossRef]
- Sun, Y.; Yi, F.; Li, R.; Min, X.; Qin, H.; Cheng, S.; Liu, Y. Inorganic–Organic Hybrid Membrane Based on Pillararene-Intercalated MXene Nanosheets for Efficient Water Purification. Angew. Chem. 2022, 61, e202200482. [Google Scholar]
- Kamran, U.; Rhee, K.Y.; Lee, S.; Park, S. Innovative progress in graphene derivative-based composite hybrid membranes for the removal of contaminants in wastewater: A review. Chemosphere 2022, 306, 135590. [Google Scholar] [CrossRef]
- Ohshima, T.; Matsumoto, M.; Miyata, T.; Uragami, T. Organic-Inorganic Hybrid Membranes for Removal of Benzene from an Aqueous Solution by Pervaporation. Macromol. Chem. Phys. 2005, 2006, 473–483. [Google Scholar] [CrossRef]
- Weerasinghe, A.M.J.S.; Dissanayake, M.A.K.L.; Senadeera, G.K.R.; Senaviratne, V.A.; Thotawatthage, C.A.; Kumari, J.M.K.W. Application of electrospun cellulose acetate nanofibre membrane based quasi-solid state electrolyte for dye sensitized solar cells. Ceylon J. Sci. 2017, 46, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Toxicological Profile for Lead; 2020, ATSDR, Agency for Toxic Substances and Disease Registry; U.S. Departament of Health and Human Services. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp13.pdf (accessed on 1 September 2022).
- Bahadir, T.; Bakan, G.; Altas, L.; Buyukgungor, H. The investigation of lead removal by biosorption: An application at storage battery industry wastewaters. Enzyme Microb. Technol. 2007, 41, 98–102. [Google Scholar] [CrossRef]
- Mohammadian, M.; Es’haghi, Z.; Hooshmand, S. Green and chemical synthesis of zinc oxide nanoparticles and size evaluation by UV–vis spectroscopy. J. Nanomed Res. 2018, 7, 52–58. [Google Scholar]
- Yang, Y.; Wang, P. Preparation and characterizations of a new PS/TiO2 hybrid membranes by sol–gel process. Polymer 2006, 47, 2683–2688. [Google Scholar] [CrossRef]
- Dada, A.O.; Ojediran, J.O.; Okunola, A.A.; Dada, F.E.; Lawal, A.I.; Olalekan, A.P.; Dada, O. Modeling of biosorption of Pb(II) and Zn(II) ions onto PaMRH: Langmuir, Freundlich, Temkin, Dubinin-Raduskevich, Jovanovic, Flory-Huggins, Fowler-Guggenheim and Kiselev comparative isotherm studies. IJMET 2019, 10, 1048–1058. [Google Scholar]
- Ho, Y.S.; Chiu, W.T.; Wang, C.C. Regression analysis for the sorption isotherms of basic dyes on sugarcane dust. Bioresour. Technol. 2005, 96, 1285–1291. [Google Scholar] [CrossRef]
- Kalavathy, M.H.; Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Kinetic and isotherm studies of Cu(II) adsorption onto H3PO4 activated rubber wod sawdust. J. Colloid Interface Sci. 2005, 292, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Vergili, I.; Gönder, Z.B.; Kaya, Y.; Gürdağ, G.; Çavuş, S. Sorption of Pb (II) from battery industry wastewater using a weak acid cation exchange resin. Process. Saf. Environ. Prot. 2017, 107, 498–507. [Google Scholar] [CrossRef]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Thanikaivelan, P.; Srinivasn, K.; Mohan, D.; Rajendran, M. Synthesis, characterization and thermal studies on cellulose acetate membranes with additive. Eur. Polym. J. 2004, 40, 2153–2159. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.S.; Ismail, A.F.; Susanti, R. Effect of binary zinc-magnesium oxides on polyphenylsulfone /cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water. Chem. Eng. J. 2021, 405, 126809. [Google Scholar] [CrossRef]
- Ibrahim, M.; Osman, O. Spectroscopic Analyses of Cellulose: Fourier Transform Infrared and Molecular Modelling Study. J. Comput. Theor. Nanosci. 2009, 6, 1054–1058. [Google Scholar] [CrossRef]
- Nagaraju, G.; Udayabhanu; Shivaraj; Prashanth, S.A.; Shastri, M.; Yathish, K.V.; Anupama, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Smolders, C.A.; Reuvers, A.J.; Boom, R.M.; Wienk, I.M. Microstructures in phase-inversion membranes. Part 1. Formation of macrovoids. J. Membr. Sci. 1992, 73, 259–275. [Google Scholar] [CrossRef] [Green Version]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Bruggen, B.V.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Abu-Dalo, M.A.; Al-Rosan, S.A.; Albiss, B.A. Photocatalytic Degradation of Methylene Blue Using Polymeric Membranes Based on Cellulose Acetate Impregnated with ZnO Nanostructures. Polymers 2021, 13, 3451. [Google Scholar] [CrossRef] [PubMed]
- Ibupoto, A.S.; Qureshi, U.A.; Arain, M.; Ahmed, F.; Khatri, Z.; Brohi, R.Z.; Kim, I.S.; Ibupoto, Z. ZnO/Carbon nanofibers for efficient adsorption of lead from aqueous solutions. Environ. Technol. 2019, 41, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Salem, I.A.; Salem, M.A.; El-Ghobashy, M.A. The dual role of ZnO nanoparticles for efficient capture of heavy metals and Acid blue 92 from water. J. Mol. Liq. 2017, 248, 527–538. [Google Scholar] [CrossRef]
- Huang, J.; Yuan, F.; Zeng, G.; Li, X.; Gu, Y.; Shi, L.; Liu, W.; Shi, Y. Influence of pH on heavy metal speciation and removal from wastewater using micellar-enhanced ultrafiltration. Chemosphere 2017, 173, 199–206. [Google Scholar]
- Azizi, S.; Shahri, M.M.; Mohamad, R. Green Synthesis of Zinc Oxide Nanoparticles for Enhanced Adsorption of Lead Ions from Aqueous Solutions: Equilibrium, Kinetic and Thermodynamic Studies. Molecules 2017, 22, 831. [Google Scholar] [CrossRef]
- Liu, L.; Luo, X.B.; Ding, L.; Luo, S.L. Application of Nanotechnology in the Removal of Heavy Metal From Water. In Nanomaterials for the Removal of Pollutants and Resource Reutilization; A Volume in Micro and Nano Technologies; Luo, X., Deng, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 83–147. [Google Scholar]
- Sani, H.A.; Ahmad, M.B.; Hussein, M.Z.; Ibrahim, N.A.; Musa, A.; Saleh, T.A. Nanocomposite of ZnO with montmorillonite for removal of lead and copper ions from aqueous solutions. Process. Saf. Environ. Prot. 2017, 109, 97–105. [Google Scholar]
- Lee, S.M.; Laldawngliana, C.; Tiwari, D. Iron oxide nano-particles-immobilized-sand material in the treatment of Cu(II), Cd(II) and Pb(II) contaminated waste waters. Chem. Eng. J. 2012, 195–196, 103–111. [Google Scholar] [CrossRef]
- Abdullah, N.; Gohari, R.J.; Yusof, N.; Ismail, A.F.; Juhana, J.; Lau, W.J.; Matsuura, T. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: Preparation, characterization and its adsorptive removal of lead (II) from aqueous solution. Chem. Eng. J. 2016, 289, 28–37. [Google Scholar] [CrossRef]
- Yin, J.; Jiang, Z.; Chang, G.; Hu, B. Simultaneous on-line preconcentration and determination of trace metals in environmental samples by flow injection combined with inductively coupled plasma mass spectrometry using a nanometer-sized alumina packed micro-column. Anal. Chim. Acta 2005, 540, 333–339. [Google Scholar] [CrossRef]
- Eren, E. Removal of lead ions by Unye (Turkey) bentonite in iron and magnesium oxide-coated forms. J. Hazard Mater. 2009, 165, 63–70. [Google Scholar] [CrossRef]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Experiment and modeling for flux and permeate concentration of heavy metal ion in adsorptive membrane filtration using a metal-organic framework incorporated nanofibrous membrane. Chem. Eng. J. 2018, 352, 737–744. [Google Scholar] [CrossRef]
- Yu, Y.; Zhuang, Y.Y.; Wang, Z.H.; Qiu, M.Q. Adsorption of water-soluble dyes onto modified resin. Chemosphere 2004, 54, 425–430. [Google Scholar] [CrossRef]
- Seki, Y.; Yurdakoç. Adsorption of Promethazine hydrochloride with KSF Montmorillonite. Adsorption 2006, 12, 89–100. [Google Scholar] [CrossRef]

| Amount of ZnO Particles (% W/v) | Percentage of Adsorption ) * |
|---|---|
| 0.0 | 27.47 (17.41) |
| 0.1 | 45.18 (9.70) |
| 0.5 | 65.05 (8.83) |
| 1.0 | 81.02 (1.13) |
| 1.5 | 82.83 (5.34) |
| 3.0 | 84.81 (1.32) |
| pH | Percentage of Adsorption ) * |
|---|---|
| 1 | 27.47 (17.41) |
| 2 | 45.18 (9.70) |
| 3 | 65.05 (8.83) |
| 4 | 81.02 (1.13) |
| 5 | 84.81 (1.32) |
| 6 | 82.83 (5.34) |
| Langmuir | Freundlich | |||||||
|---|---|---|---|---|---|---|---|---|
| Qo (mg/g) | KL (L/ mg) | RL | R2 | χ2 | KF (mg/g) | no | R2 | χ2 |
| 15.55 | 0.035 | 0.06–0.97 | 0.992 | 4.304 | 1.77 | 2.76 | 0.980 | 0.819 |
| Thermodynamic parameters | ||||||||
| Temperature (K) | ∆G0 (KJ mol−1) | ∆H0 (KJ mol−1) | ∆S0 (KJ mol−1K−1) | |||||
| 291 | −21.52 | 119.41 | 0.48 | |||||
| 298 | −24.66 | |||||||
| 308 | −29.73 | |||||||
| Adsorbent | Qmax (mg·g−1) | Reference |
|---|---|---|
| Iron oxide nanoparticles immobilized in sand | 2.087 | [43] |
| Ultrafiltration membrane of polysulfone with hydrous ferric oxide | 13.20 | [44] |
| Hybrid membrane of cellulose acetate with zinc oxide | 15.55 | This work |
| Aluminum oxide | 17.50 | [45] |
| Iron-oxide-coated bentonite | 22.20 | [46] |
| Polyacrylonitrile with metal–organic framework (MOF-808) membrane | 23.98 | [47] |
| Magnesium-oxide-coated bentonite | 31.86 | [46] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Silva, I.; Páez-Hernández, M.E.; Ibarra, I.S.; Camacho-Mendoza, R.L. Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead. Membranes 2023, 13, 123. https://doi.org/10.3390/membranes13020123
Pérez-Silva I, Páez-Hernández ME, Ibarra IS, Camacho-Mendoza RL. Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead. Membranes. 2023; 13(2):123. https://doi.org/10.3390/membranes13020123
Chicago/Turabian StylePérez-Silva, Irma, Ma. Elena Páez-Hernández, Israel S. Ibarra, and Rosa Luz Camacho-Mendoza. 2023. "Evaluation of the Hybrid Membrane of ZnO Particles Supported in Cellulose Acetate for the Removal of Lead" Membranes 13, no. 2: 123. https://doi.org/10.3390/membranes13020123







