Preparation of Porous Stainless Steel Hollow-Fibers through Multi-Modal Particle Size Sintering towards Pore Engineering
Abstract
:1. Introduction
2. Results and Discussion
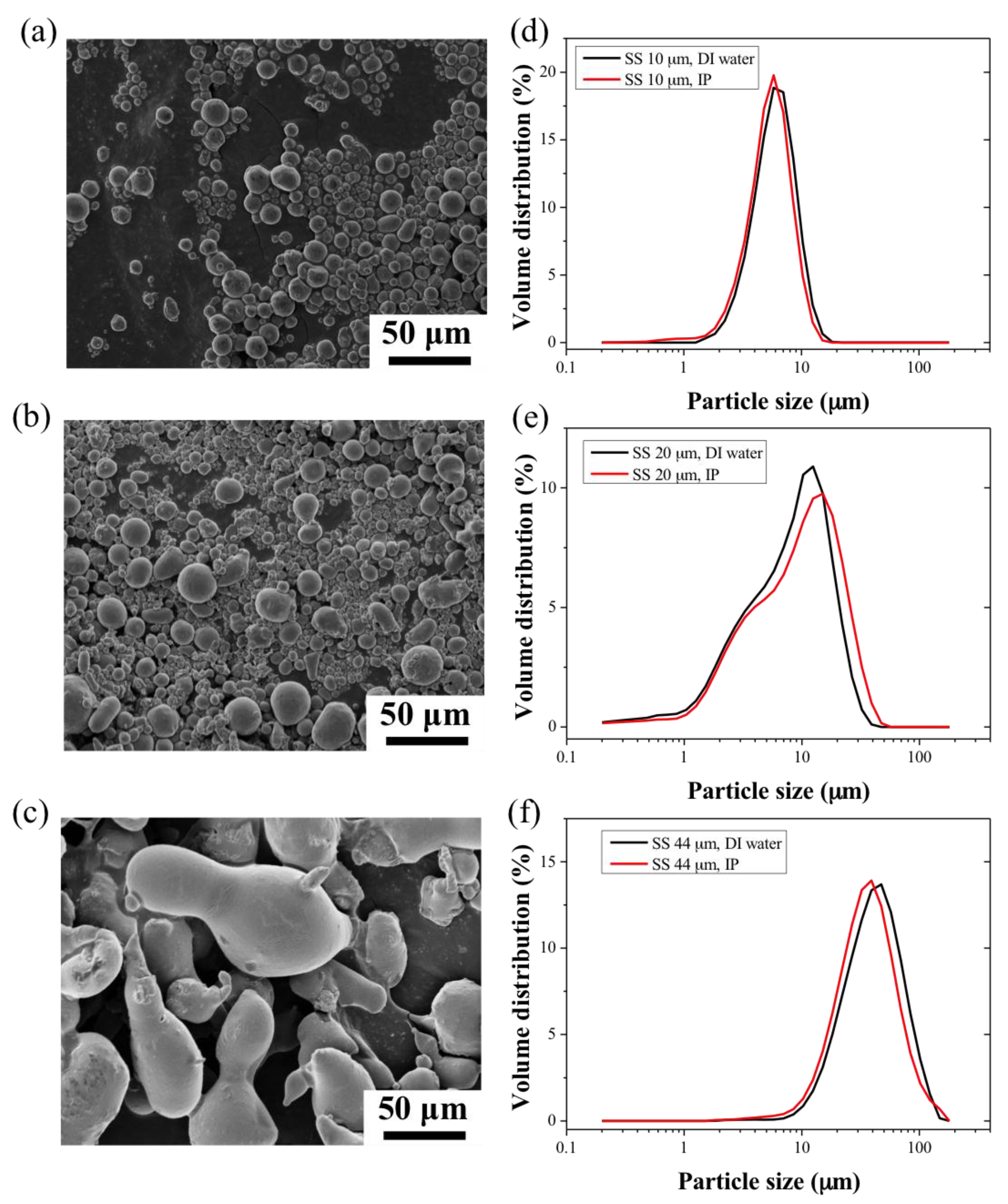
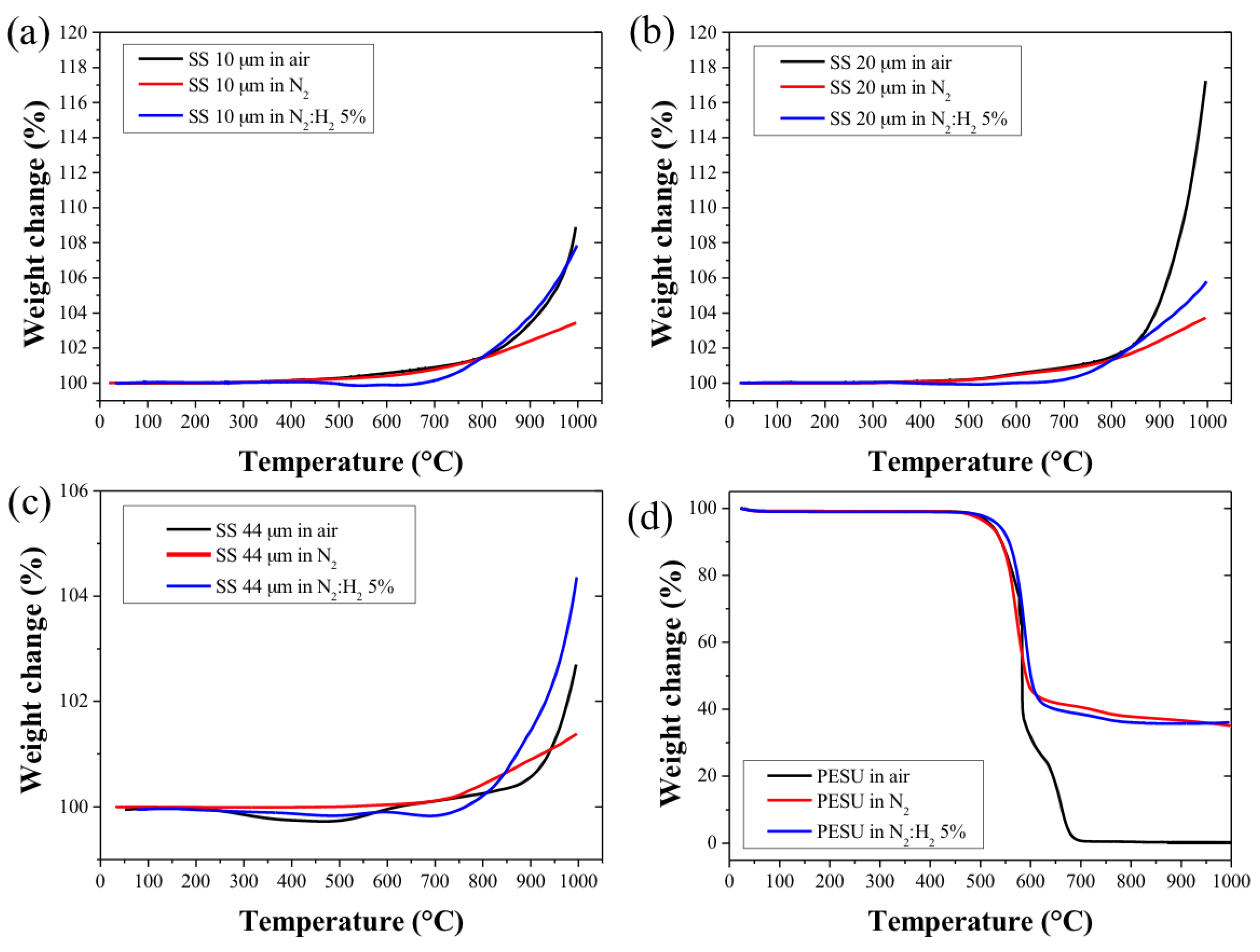
2.1. Metals Powders and Polymer Binder Characterisation
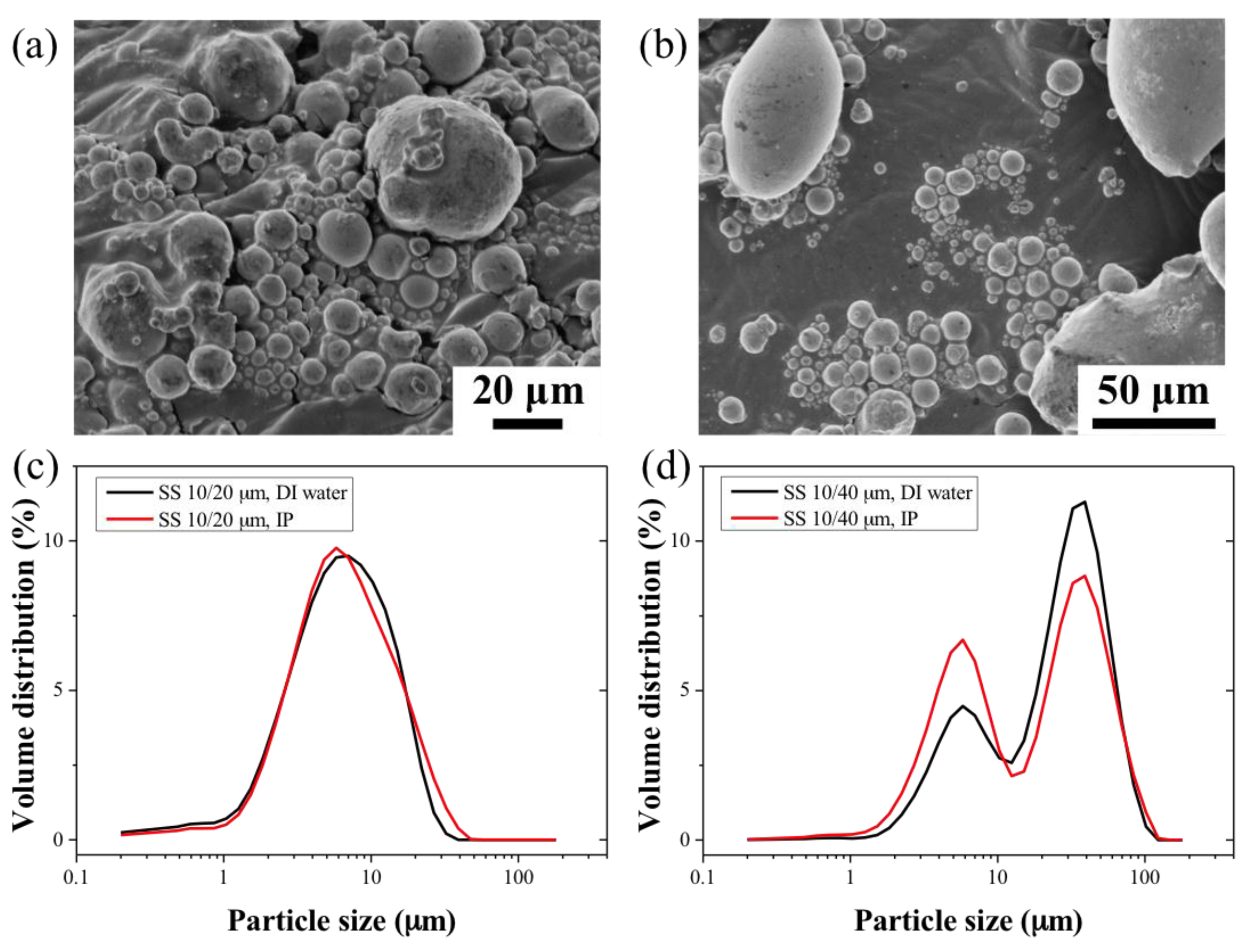
2.2. Green and Sintered HF Morphologies
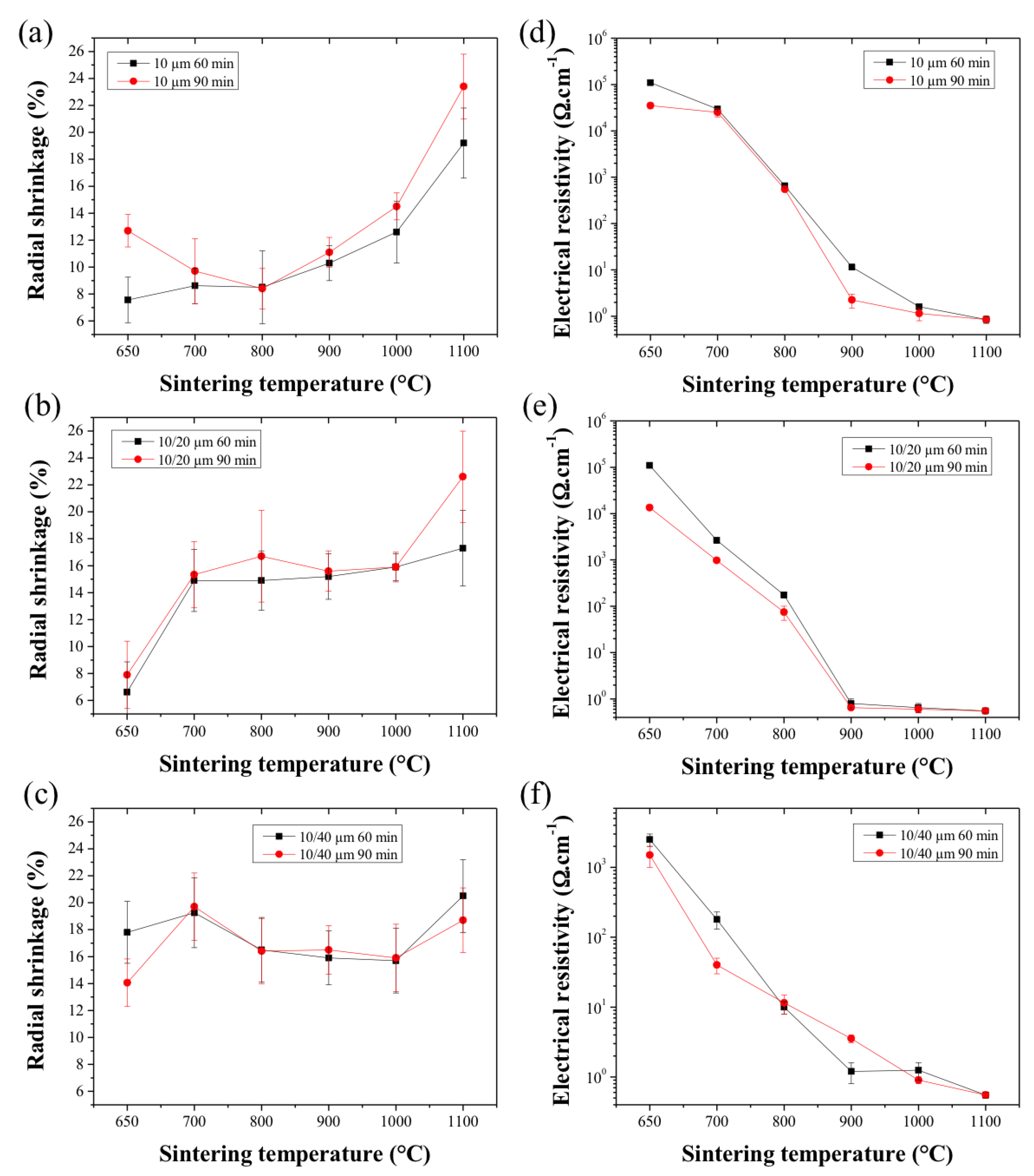
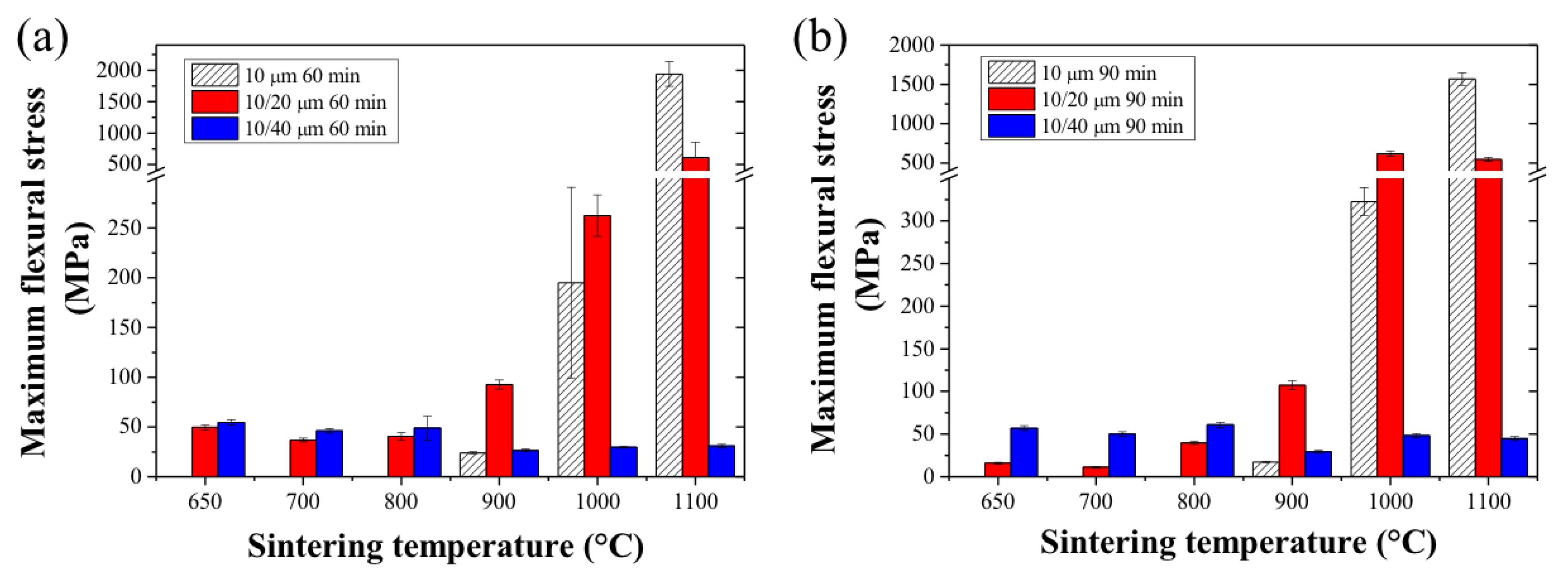
2.3. Sintered HF Properties
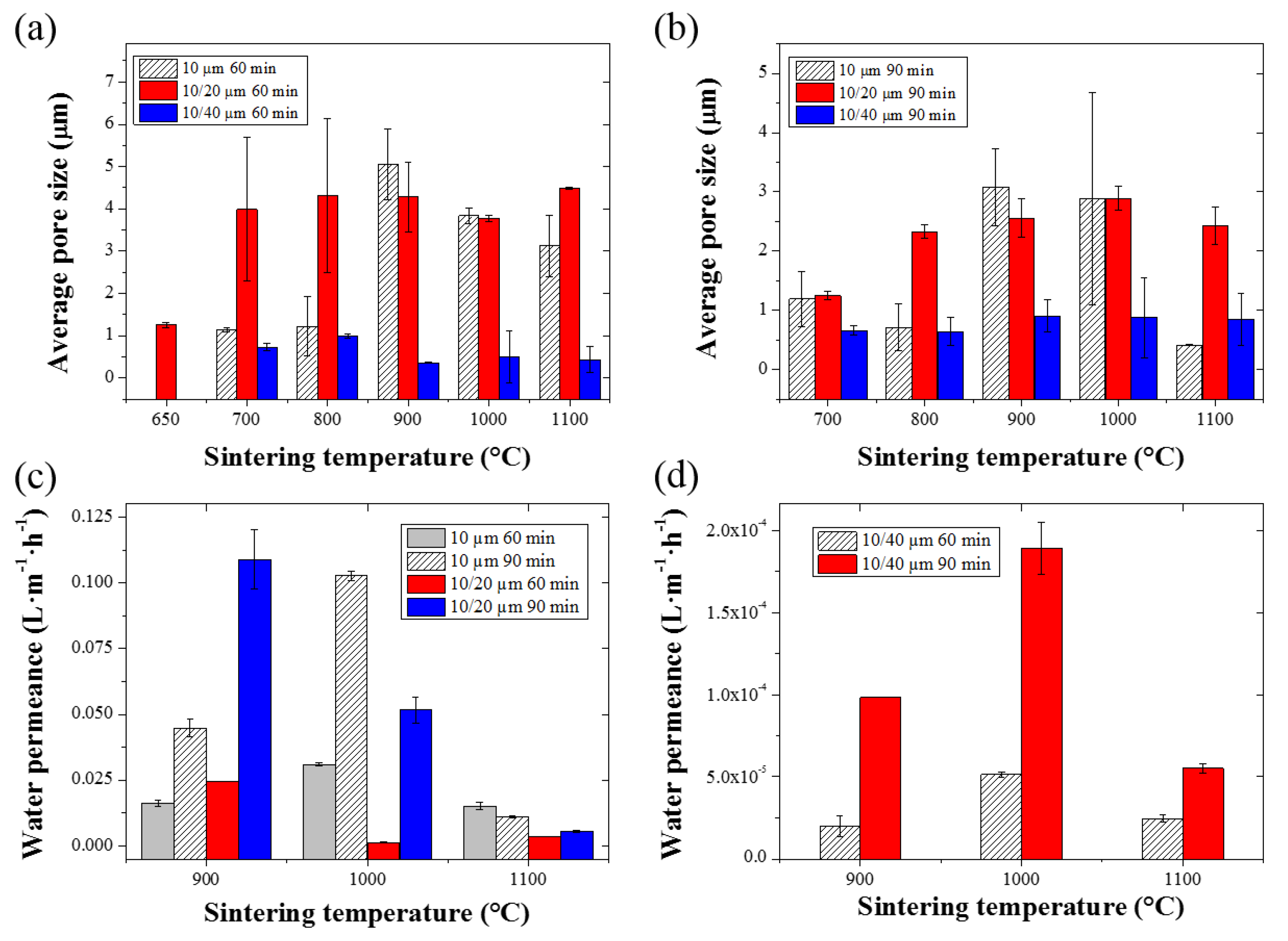
2.4. Pore Size Distribution and Water Permeance
3. Materials and Methods
3.1. Metal Particles and Chemicals
3.2. Preparation of the Green HFs
3.3. Drying and Thermal Treatments of Green HFs
3.4. Materials Characterization
4. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Li, N.N.; Fane, A.G.; Ho, W.S.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Mat, N.C.; Lou, Y.; Lipscomb, G.G. Hollow fiber membrane modules. Curr. Opin. Chem. Eng. 2014, 4, 18–24. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.C.; Matsuura, T.; Ismail, A.F. Recent progresses in polymeric hollow fiber membrane preparation, characterization and applications. Sep. Purif. Technol. 2013, 111, 43–71, Repeated with 10. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology; Wiley Online Library: Hoboken, NJ, USA, 2000. [Google Scholar]
- Luiten-Olieman, M. Inorganic porous Hollow Fiber Membranes: With Tunable Small Radial Dimensions; Gildeprint Drukkerijen: Enschede, The Netherlands, 2012. [Google Scholar]
- Wickramasinghe, S.; Semmens, M.J.; Cussler, E. Mass transfer in various hollow fiber geometries. J. Membr. Sci. 1992, 69, 235–250. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Kas, R.; Hummadi, K.K.; Kortlever, R.; de Wit, P.; Milbrat, A.; Luiten-Olieman, M.W.J.; Benes, N.E.; Koper, M.T.M.; Mul, G. Three-dimensional porous hollow fibre copper electrodes for efficient and high-rate electrochemical carbon dioxide reduction. Nat. Commun. 2016, 7, 10748. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Y.; Groth, A.; Gray, S.; Duke, M. Enhanced abrasion resistant PVDF/nanoclay hollow fibre composite membranes for water treatment. J. Membr. Sci. 2014, 449, 146–157. [Google Scholar] [CrossRef]
- Irfan, M.; Idris, A. Overview of PES biocompatible/hemodialysis membranes: PES–blood interactions and modification techniques. Mater. Sci. Eng. 2015, 56, 574–592. [Google Scholar] [CrossRef] [PubMed]
- Dumee, L.F.; He, L.; Lin, B.; Ailloux, F.-M.; Lemoine, J.-B.; Velleman, L.; She, F.; Duke, M.C.; Orbell, J.D.; Erskine, G.; et al. The fabrication and surface functionalization of porous metal frameworks—A review. J. Mater. Chem. A 2013, 1, 15185–15206. [Google Scholar] [CrossRef] [Green Version]
- Dumée, L.F.; He, L.; Wang, Z.; Sheath, P.; Xiong, J.; Feng, C.; Tan, M.Y.; She, F.; Duke, M.; Gray, S. Growth of nano-textured graphene coatings across highly porous stainless steel supports towards corrosion resistant coatings. Carbon 2015, 87, 395–408. [Google Scholar] [CrossRef]
- Gitis, V.; Rothenberg, G. Ceramic Membranes: New Opportunities and Practical Applications; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Cassano, A.; Rastogi, N.K.; Basile, A. 18-Membrane technologies for water treatment and reuse in the food and beverage industries. In Advances in Membrane Technologies for Water Treatment; Woodhead Publishing: Oxford, UK, 2015; pp. 551–580. [Google Scholar]
- Luiten-Olieman, M.W.J.; Winnubst, L.; Nijmeijer, A.; Wessling, M.; Benes, N.E. Porous stainless steel hollow fiber membranes via dry–wet spinning. J. Membr. Sci. 2011, 370, 124–130. [Google Scholar] [CrossRef]
- David, O.; Gendel, Y.; Wessling, M. Tubular macro-porous titanium membranes. J. Membr. Sci. 2014, 461, 139–145. [Google Scholar] [CrossRef]
- Lee, S.-M.; Choi, I.-H.; Myung, S.-W.; Park, J.-Y.; Kim, I.-C.; Kim, W.-N.; Lee, K.-H. Preparation and characterization of nickel hollow fiber membrane. Desalination 2008, 233, 32–39. [Google Scholar] [CrossRef]
- Meng, B.; Tan, X.; Meng, X.; Qiao, S.; Liu, S. Porous and dense Ni hollow fibre membranes. J. Alloys Compd. 2009, 470, 461–464. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Coenen, K.; Melendez, J.; Viviente, J.L.; Tanaka, D.A.P.; van Sint Annaland, M.; Gallucci, F. Development of thin Pd–Ag supported membranes for fluidized bed membrane reactors including WGS related gases. Int. J. Hydrogen Energy 2015, 40, 3506–3519. [Google Scholar] [CrossRef]
- Schmeda-Lopez, D.R.; Smart, S.; Nunes, E.H.M.; Vasconcelos, D.; Vasconcelos, W.L.; Bram, M.; Meulenberg, W.A.; da Costa, J.C.D. Stainless steel hollow fibres—Sintering, morphology and mechanical properties. Sep. Purif. Technol. 2015, 147, 379–387. [Google Scholar] [CrossRef]
- Luiten-Olieman, M.W.J.; et al. Towards a generic method for inorganic porous hollow fibers preparation with shrinkage-controlled small radial dimensions, applied to Al2O3, Ni, SiC, stainless steel, and YSZ. J. Membr. Sci. 2012, 407–408, 155–163. [Google Scholar] [CrossRef]
- German, R.M. Chapter Four—Measurement Tools and Experimental Observations. In Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Boston, MA, USA, 2014; pp. 71–130. [Google Scholar]
- Dumée, L.F.; She, F.; Duke, M.; Gray, S.; Hodgson, P.; Kong, L. Fabrication of meso-porous sintered metal thin films by selective etching of silica based sacrificial template. Nanomaterials 2014, 4, 686–699. [Google Scholar] [CrossRef] [PubMed]
- German, R.M. Chapter Eleven—Mixed Powders and Composites. In Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Boston, MA, USA, 2014; pp. 335–385. [Google Scholar]
- Bouvard, D.; Lange, F.F. Relation between percolation and particle coordination in binary powder mixtures. Acta Metall. Mater. 1991, 39, 3083–3090. [Google Scholar] [CrossRef]
- Spierings, A.; Levy, G. Comparison of density of stainless steel 316L parts produced with selective laser melting using different powder grades. In Proceedings of the Annual International Solid Freeform Fabrication Symposium, Austin, TX, USA, 3–5 August 2009. [Google Scholar]
- Higgins, P.; Munir, Z. Influence of Surface Oxide Layer on Sintering Process. of Lead. Powder Metall. 1978, 21, 188–194. [Google Scholar] [CrossRef]
- Lima, A.S.; Nascimento, A.M.; Abreu, H.F.G.; de Lima-Neto, P. Sensitization evaluation of the austenitic stainless steel AISI 304L, 316L, 321 and 347. J. Mater. Sci. 2005, 40, 139–144. [Google Scholar] [CrossRef]
- German, R.M. Sintering theory and practice. Solar-Terr. Phys. 1996, 568. [Google Scholar]
- Tarabay, J.; Peres, V.; Pijolat, M. Oxidation of Stainless Steel Powder. Oxid. Met. 2013, 80, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Bergman, O. Influence of Oxygen Partial Pressure in Sintering Atmosphere on Properties of Cr–Mo Prealloyed Powder Metallurgy Steel. Powder Metall. 2013, 50, 243–249. [Google Scholar] [CrossRef]
- Nayar, H.S.; Wasiczko, B. Nitrogen absorption control during sintering of stainless steel parts. Met. Powder Rep. 1990, 45, 611–614. [Google Scholar] [CrossRef]
- Karlsson, H.; Nyborg, L.; Frykholm, R. Surface Reactions During Fabrication and Sintering of 410 L Stainless Steel Powder. Powder Metall. Prog. 2005, 5, 220–233. [Google Scholar]
- Rui, W.; Zhang, C.; Cai, C.; Gu, X. Effects of sintering atmospheres on properties of stainless steel porous hollow fiber membranes. J. Membr. Sci. 2015, 489, 90–97. [Google Scholar] [CrossRef]
- Bordia, R.K.; Raj, R. Sintering Behavior of Ceramic Films Constrained by a Rigid Substrate. J. Am. Ceram. Soc. 1985, 68, 287–292. [Google Scholar] [CrossRef]
- Luiten-Olieman, M.W.J.; Raaijmakers, M.J.T.; Winnubst, L.; Wessling, M.; Nijmeijer, A.; Benes, N.E. Porous stainless steel hollow fibers with shrinkage-controlled small radial dimensions. Scr. Mater. 2011, 65, 25–28. [Google Scholar] [CrossRef]
- Dumee, L.; Velleman, L.; Sears, K.; Hill, M.; Schutz, J.; Finn, N.; Duke, M.; Gray, S. Control of porosity and pore size of metal reinforced carbon nanotube membranes. Membranes 2010, 1, 25–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.-J.L. Sintering: Densification, Grain Growth and Microstructure; Butterworth-Heinemann: Oxford, UK, 2004. [Google Scholar]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allioux, F.-M.; David, O.; Etxeberria Benavides, M.; Kong, L.; Pacheco Tanaka, D.A.; Dumée, L.F. Preparation of Porous Stainless Steel Hollow-Fibers through Multi-Modal Particle Size Sintering towards Pore Engineering. Membranes 2017, 7, 40. https://doi.org/10.3390/membranes7030040
Allioux F-M, David O, Etxeberria Benavides M, Kong L, Pacheco Tanaka DA, Dumée LF. Preparation of Porous Stainless Steel Hollow-Fibers through Multi-Modal Particle Size Sintering towards Pore Engineering. Membranes. 2017; 7(3):40. https://doi.org/10.3390/membranes7030040
Chicago/Turabian StyleAllioux, Francois-Marie, Oana David, Miren Etxeberria Benavides, Lingxue Kong, David Alfredo Pacheco Tanaka, and Ludovic F. Dumée. 2017. "Preparation of Porous Stainless Steel Hollow-Fibers through Multi-Modal Particle Size Sintering towards Pore Engineering" Membranes 7, no. 3: 40. https://doi.org/10.3390/membranes7030040






