The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Membrane Preparation
2.4. Preparation of AuNPs on the Surface of PIMs
2.5. Extraction of Nitrate
2.6. Recovery of Au from the AuNP-Coated PIMs
2.7. Initial Flux Calculation
3. Results and Discussion
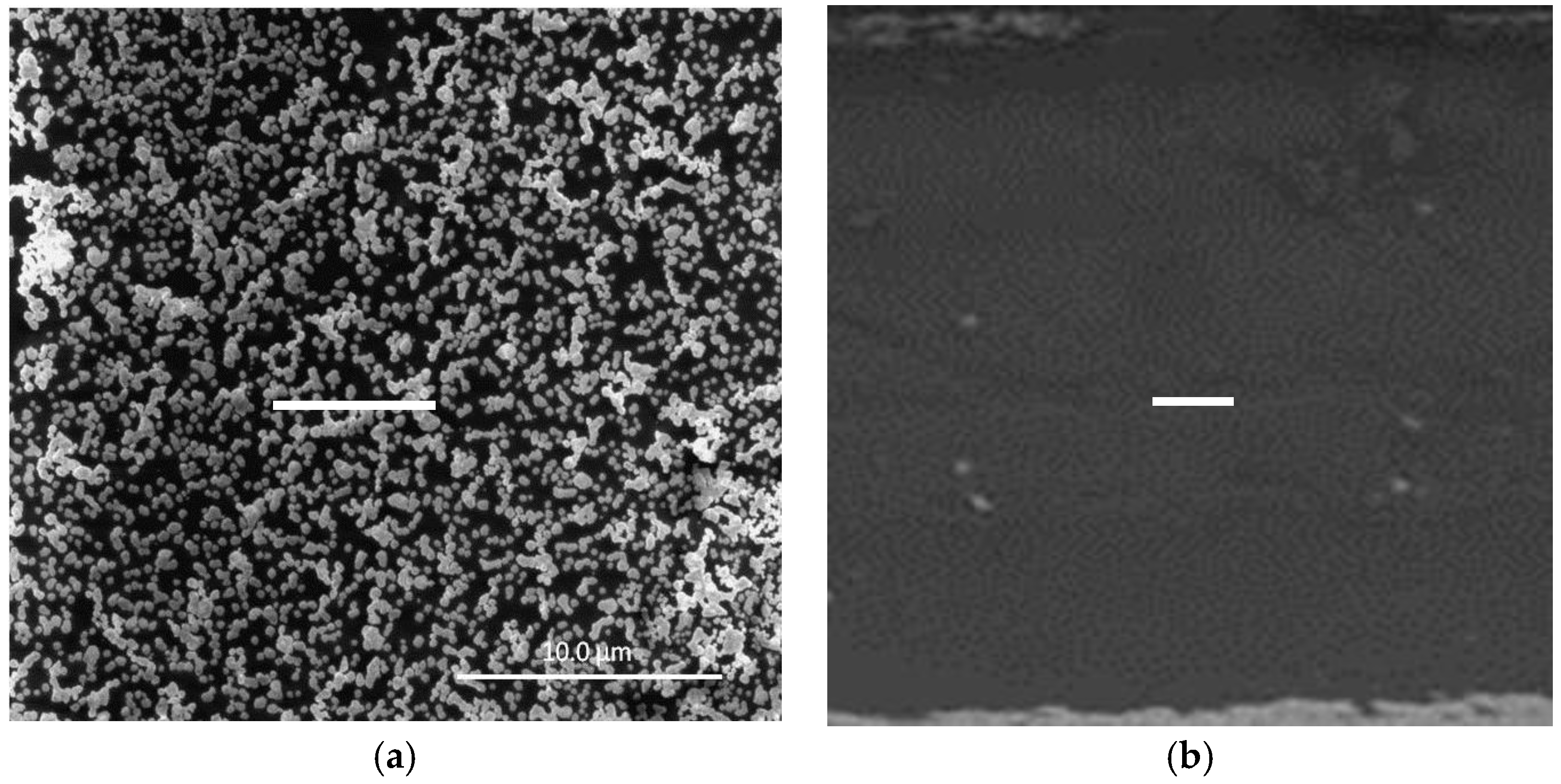
3.1. Formation of AuNPs on the PIM Surface
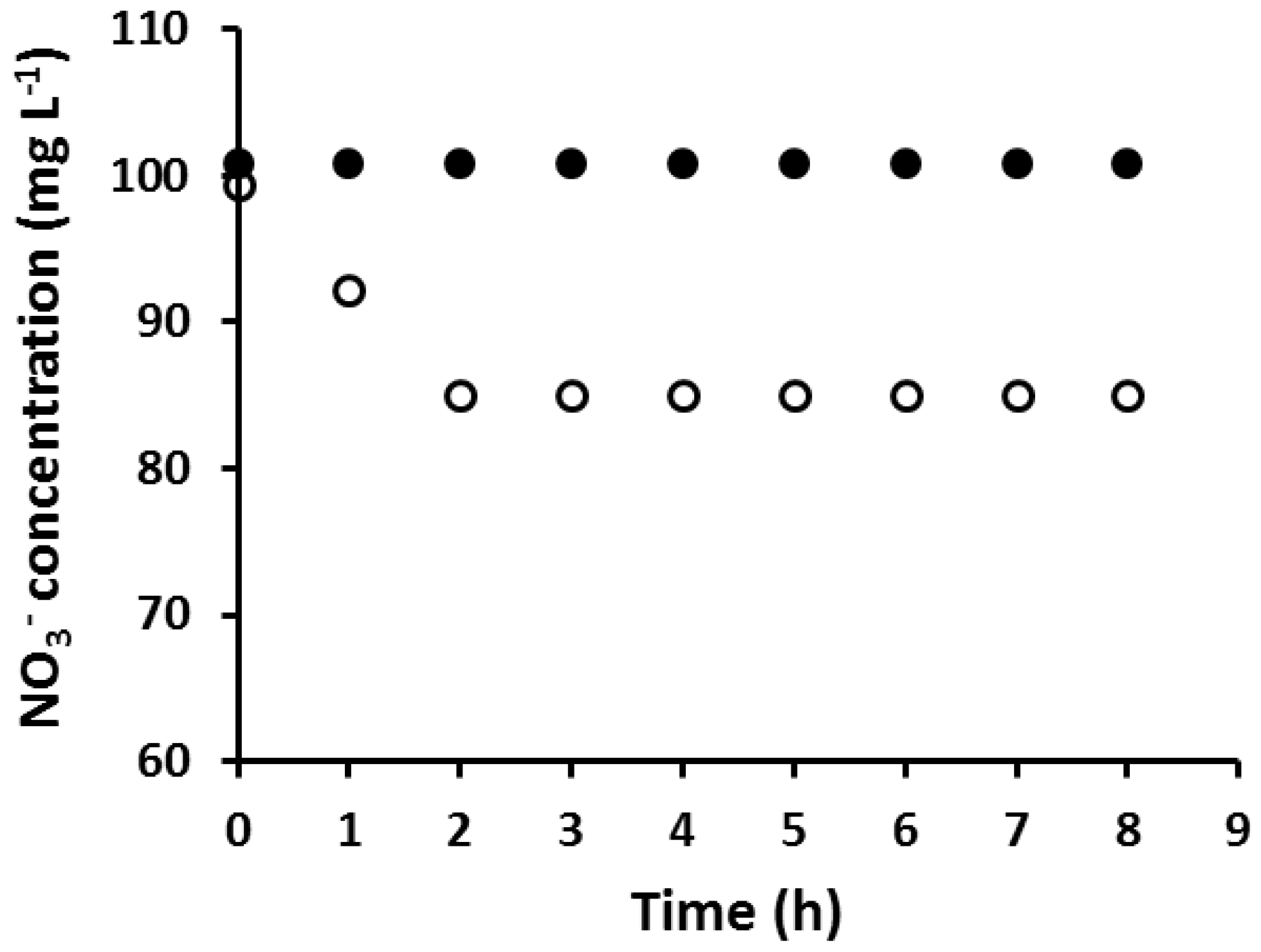
3.2. Extraction of NO3− Using an AuNP-Coated PIM
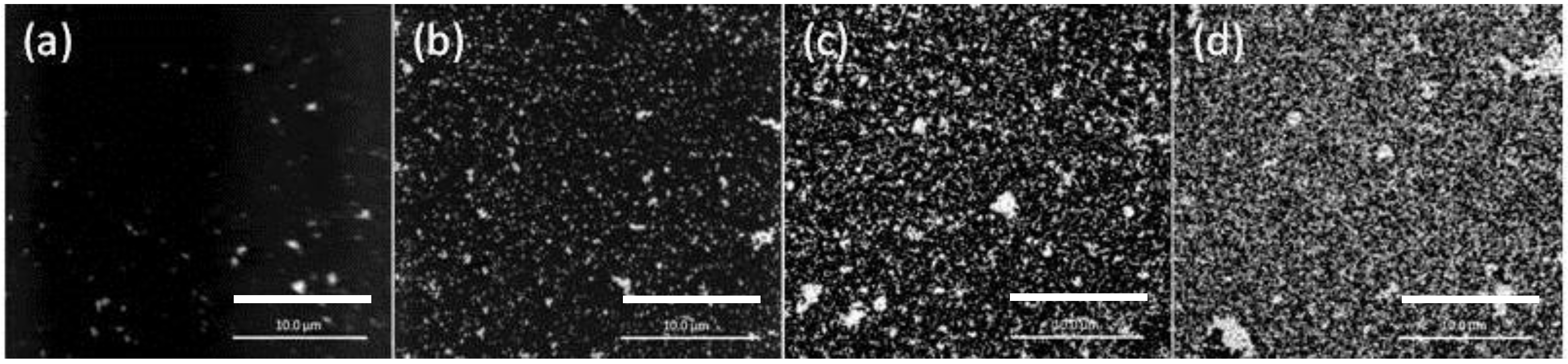
3.3. Quantitative Production of AuNPs on the PIM Surface
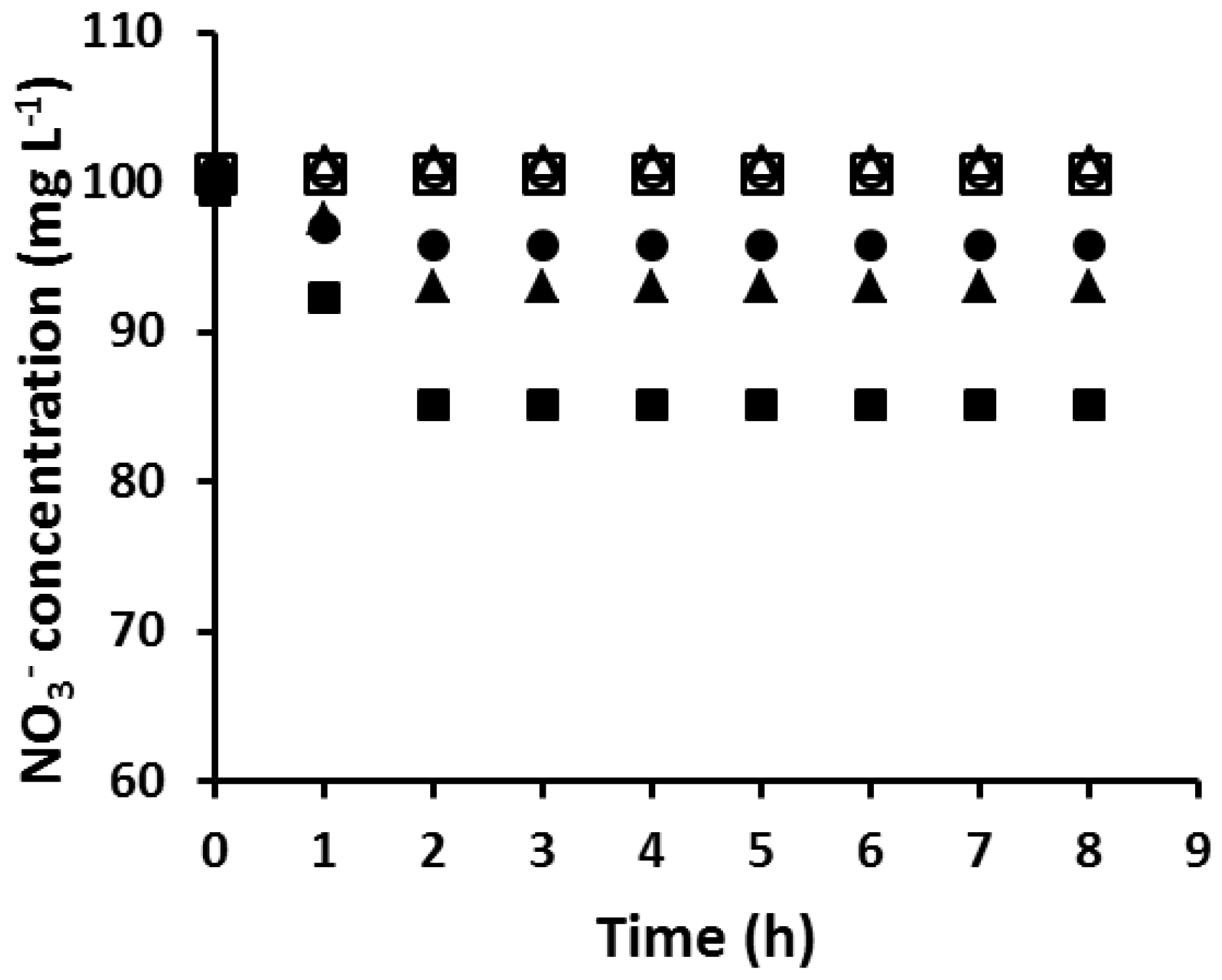
3.4. Effect of the Aliquat 336 Concentration on the “Critical AuNP Mass”
3.5. Effect of DD and NPOE
4. Conclusions
- Individual AuNPs that aggregate into clusters are formed on the surface of the PIMs after the extraction of Au(III) and its subsequent reduction with EDTA.
- At a critical surface mass of the AuNPs, the PIM loses its ability to extract NO3−, which is consistent with AuNPs and clusters of those completely blocking the extraction sites on the PIM surface. At AuNP masses lower than the corresponding critical values, some sites are still available for the extraction of NO3−, but in such cases, the rate of extraction is reduced accordingly.
- The mass of AuNPs collected from PIMs after dissolution in THF equates exactly to the mass of Au(III) originally extracted. This demonstrates that all Au(III) extracted has been reduced to AuNPs on the PIM surface and the bulk of the PIM contains free Aliquat 336.
- The “critical AuNP mass”, and hence the population of extraction sites, is directly related to the PIM composition. Higher concentrations of Aliquat 336 result in higher Au(III) fluxes during Au(III) extraction and higher “critical AuNP mass” values. Additionally, the addition of increasing concentrations of DD or NPOE to the PIM formulation produces higher Au(III) fluxes and “critical AuNP mass” values.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nghiem, L.D.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar] [CrossRef]
- O′Rourke, M.; Cattrall, R.W.; Kolev, S.D.; Potter, I.D. The extraction and transport of organic molecules using polymer inclusion membranes. Solv. Extr. Res. Dev. Jpn. 2009, 16, 1–12. [Google Scholar]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415, 9–23. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A new generation of highly stable and permeable polymer inclusion membranes (PIMs) with their carrier immobilized in a crosslinked semi-interpenetrating polymer network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Guo, L.; Liu, Y.H.; Zhang, C.; Chen, J. Preparation of PVDF-based polymer inclusion membrane using ionic liquid plasticizer and Cyphos IL 104 carrier for Cr(VI) transport. J. Membr. Sci. 2011, 367, 85–90. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Recovery of gold from aqua regia digested electronic scrap using a PVDF-HFP based polymer inclusion membrane (PIM) containing Cyphos® IL 104. J. Membr. Sci. 2016, 514, 274–281. [Google Scholar] [CrossRef]
- Wang, D.; Cattrall, R.W.; Li, J.; Almeida, M.I.G.S.; Stevens, G.W.; Kolev, S.D. A poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP)-based polymer inclusion membrane (PIM) containing LIX84I for the extraction and transport of Cu(II) from its ammonium sulfate/ammonia solutions. J. Membr. Sci. 2017, 542, 272–279. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Selective extraction of vanadium(V) from sulfate solutions into a polymer inclusion membrane composed of poly(vinylidenefluoride-co-hexafluoro-propylene) and Cyphos® IL 101. J. Membr. Sci. 2018, 545, 57–65. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. Extraction of gold(III) from hydrochloric acid solutions with a PVC-based polymer inclusion membrane (PIM) containing Cyphos® IL 104. Membranes 2015, 5, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Nagul, E.A.; Fontàs, C.; McKelvie, I.D.; Cattrall, R.W.; Kolev, S.D. The use of a polymer inclusion membrane for separation and preconcentration of orthophosphate in flow analysis. Anal. Chim. Acta 2013, 803, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Pratiwi, A.I.; Sato, T.; Matsumoto, M.; Kondo, K. Permeation mechanism of succinic acid through polymer inclusion membranes with ionic liquid Aliquat 336. J. Chem. Eng. Jpn. 2014, 47, 314–318. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) in chemical analysis—A review. Anal. Chim. Acta 2017, 987, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pandey, A.K.; Tyagi, A.K.; Dey, G.K.; Ramagiri, S.V.; Bellare, J.R.; Goswami, A. In situ formation of stable gold nanoparticles in polymer inclusion membranes. J. Colloid Interface Sci. 2009, 337, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pandey, A.K.; Shukla, R.; Ramagiri, S.V.; Bellare, J.R. Plasticised polymer inclusion membrane as tunable host for stable gold nanoparticles. Int. J. Nanotechnol. 2010, 7, 953–966. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. The preparation of a gold nanoparticle monolayer on the surface of a polymer inclusion membrane using EDTA as the reducing agent. J. Membr. Sci. 2011, 379, 322–329. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. A method for the coating of a polymer inclusion membrane with a monolayer of silver nanoparticles. J. Membr. Sci. 2013, 428, 142–149. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. A method for coating a polymer inclusion membrane with a monolayer of palladium nanoparticles. React. Funct. Polym. 2015, 97, 30–36. [Google Scholar] [CrossRef]
- Pereira, N.; St John, A.; Cattrall, R.W.; Perera, J.M.; Kolev, S.D. Influence of the composition of polymer inclusion membranes on their homogeneity and flexibility. Desalination 2009, 236, 327–333. [Google Scholar] [CrossRef]
- Arous, O.; Kerdjoudj, H.; Seta, P. Comparison of carrier-facilitated silver(I) and copper(II) ions transport mechanisms in a supported liquid membrane and in a plasticized cellulose triacetate membrane. J. Membr. Sci. 2004, 241, 177–185. [Google Scholar] [CrossRef]
- Xu, J.Y.; Wang, L.J.; Shen, W.; Paimin, R.; Wang, X.G. The influence of the interior structure of Aliquat 336/PVC membranes to their extraction behavior. Sep. Sci. Technol. 2004, 39, 3527–3539. [Google Scholar] [CrossRef]
- St John, A.M.; Best, S.P.; Wang, Y.D.; Tobin, M.J.; Puskar, L.; Siegele, R.; Cattrall, R.W.; Kolev, S.D. Micrometer-scale 2D mapping of the composition and homogeneity of polymer inclusion membranes. Aust. J. Chem. 2011, 64, 930–938. [Google Scholar] [CrossRef]
- St John, A.M.; Cattrall, R.W.; Kolev, S.D. Determination of the initial flux of polymer inclusion membranes. Sep. Purif. Technol. 2013, 116, 41–45. [Google Scholar] [CrossRef]
- Cho, Y.; Xu, C.L.; Cattrall, R.W.; Kolev, S.D. A polymer inclusion membrane for extracting thiocyanate from weakly alkaline solutions. J. Membr. Sci. 2011, 367, 85–90. [Google Scholar] [CrossRef]
- Cho, Y.; Cattrall, R.W.; Kolev, S.D. A novel polymer inclusion membrane based method for continuous clean-up of thiocyanate from gold mine tailings water. J. Hazard. Mater. 2017, 341, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Argiropoulos, G.; Cattrall, R.W.; Hamilton, I.C.; Kolev, S.D.; Paimin, R. The study of a membrane for extracting gold(III) from hydrochloric acid solutions. J. Membr. Sci. 1998, 138, 279–285. [Google Scholar] [CrossRef]
- Kolev, S.D.; Argiropoulos, G.; Cattrall, R.W.; Hamilton, I.C.; Paimin, R. Mathematical modelling of membrane extraction of gold(III) from hydrochloric acid solutions. J. Membr. Sci. 1997, 137, 261–269. [Google Scholar] [CrossRef]
- Bonggotgetsakul, Y.Y.N.; Ashokkumar, M.; Cattrall, R.W.; Kolev, S.D. The use of sonication to increase extraction rate in polymer inclusion membranes. An application to the extraction of gold(III). J. Membr. Sci. 2010, 365, 242–247. [Google Scholar] [CrossRef]


| Aliquat 336 Concentration (wt %) | Initial Flux for Au(III) Extraction (J0) (mol·m−2·s−1) | Critical Au(III) Extraction Time (h) | Critical AuNP Mass (mg) |
|---|---|---|---|
| 20 | 3.12 × 10−8 | 2.5 | 0.16 |
| 25 | 1.56 × 10−7 | 3.5 | 0.45 |
| 30 | 5.20 × 10−7 | 6.5 | 3.45 |
| 35 | 2.60 × 10−6 | 7.5 | 5.44 |
| DD/NPOE Concentration (wt %) | Initial Flux for Au(III) Extraction (J0) (mol·m−2·s−1) | Critical Au(III) Extraction Time (h) | Critical AuNP Mass (mg) |
|---|---|---|---|
| 0 | 3.12 × 10−8 | 2.5 | 0.16 |
| DD | |||
| 5 | 4.68 × 10−7 | 8 | 3.05 |
| 10 | 2.08 × 10−6 | 12 | 4.54 |
| 15 | 2.08 × 10−6 | 12 | 4.57 |
| NPOE | |||
| 5 | 2.60 × 10−7 | 4.5 | 0.88 |
| 10 | 3.64 × 10−7 | 4.5 | 2.02 |
| 15 | 5.20 × 10−7 | 4.5 | 2.84 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonggotgetsakul, Y.Y.N.; Cattrall, R.W.; Kolev, S.D. The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier. Membranes 2018, 8, 6. https://doi.org/10.3390/membranes8010006
Bonggotgetsakul YYN, Cattrall RW, Kolev SD. The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier. Membranes. 2018; 8(1):6. https://doi.org/10.3390/membranes8010006
Chicago/Turabian StyleBonggotgetsakul, Ya Ya N., Robert W. Cattrall, and Spas D. Kolev. 2018. "The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier" Membranes 8, no. 1: 6. https://doi.org/10.3390/membranes8010006
APA StyleBonggotgetsakul, Y. Y. N., Cattrall, R. W., & Kolev, S. D. (2018). The Effect of Surface Confined Gold Nanoparticles in Blocking the Extraction of Nitrate by PVC-Based Polymer Inclusion Membranes Containing Aliquat 336 as the Carrier. Membranes, 8(1), 6. https://doi.org/10.3390/membranes8010006






