Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production
Abstract
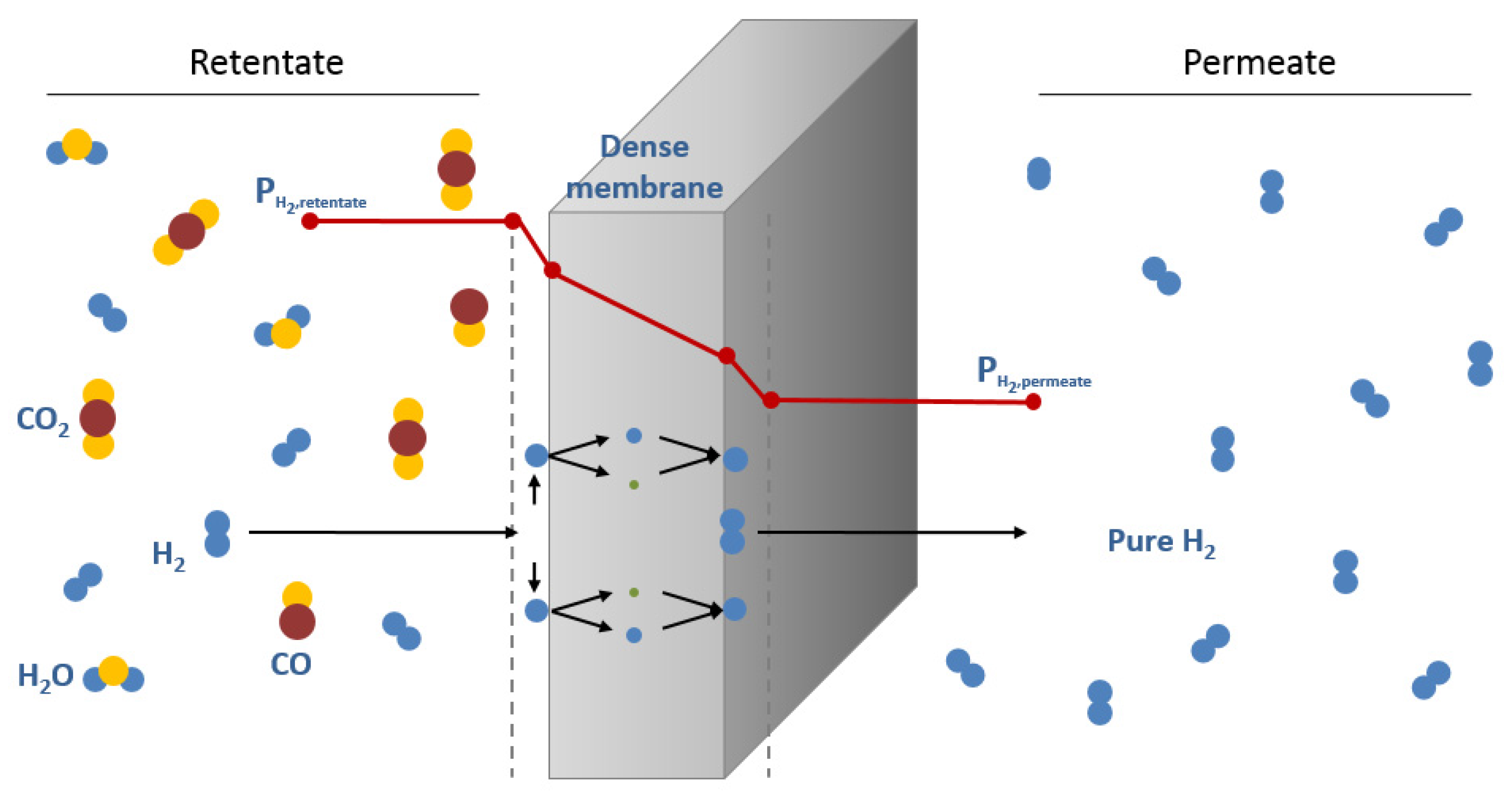
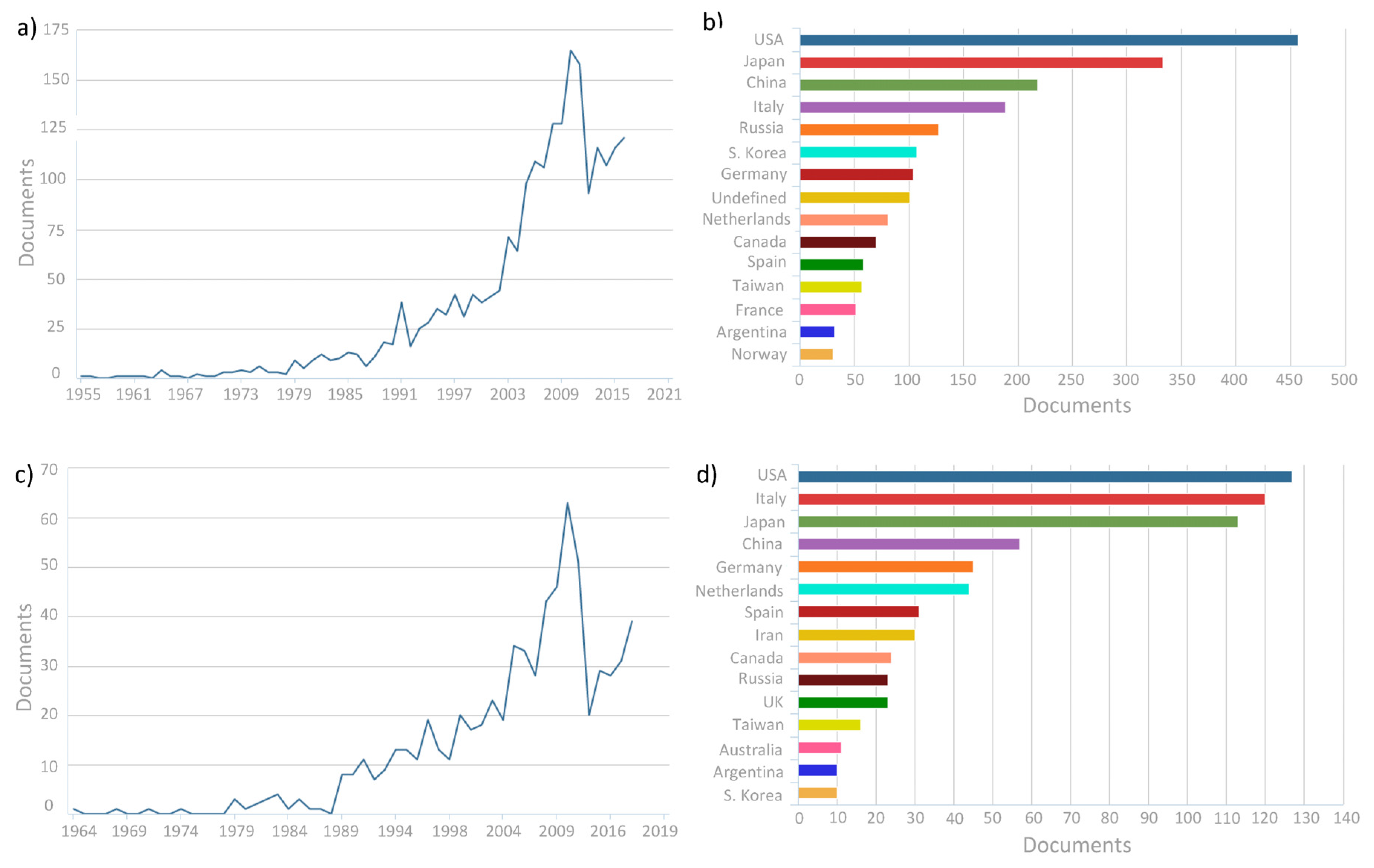
:1. Introduction
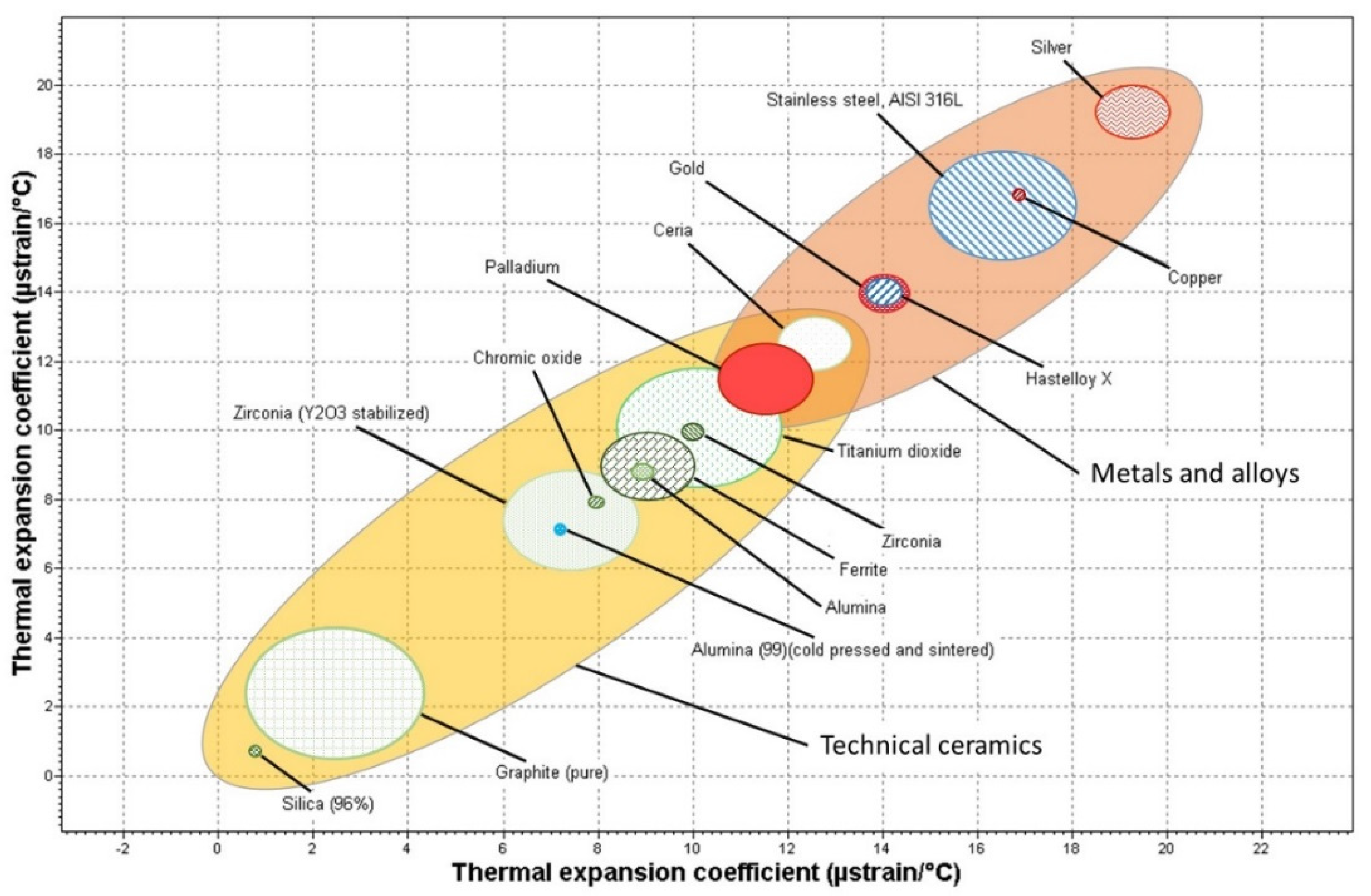
2. Membrane Supports
2.1. Chemical Treatment
2.2. Mechanical Treatment
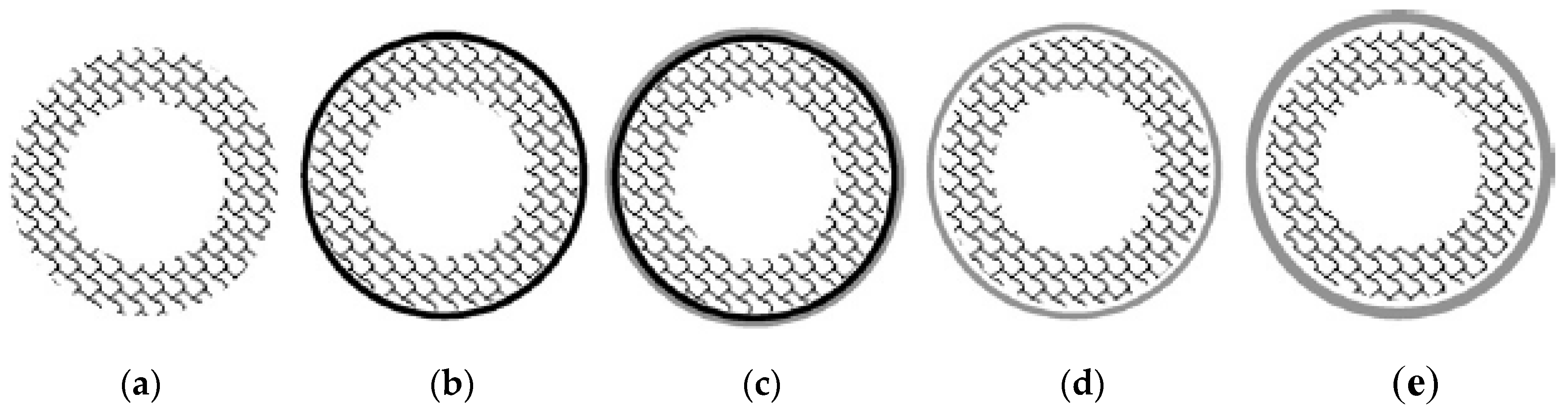
2.3. Incorporation of Intermediate Layers
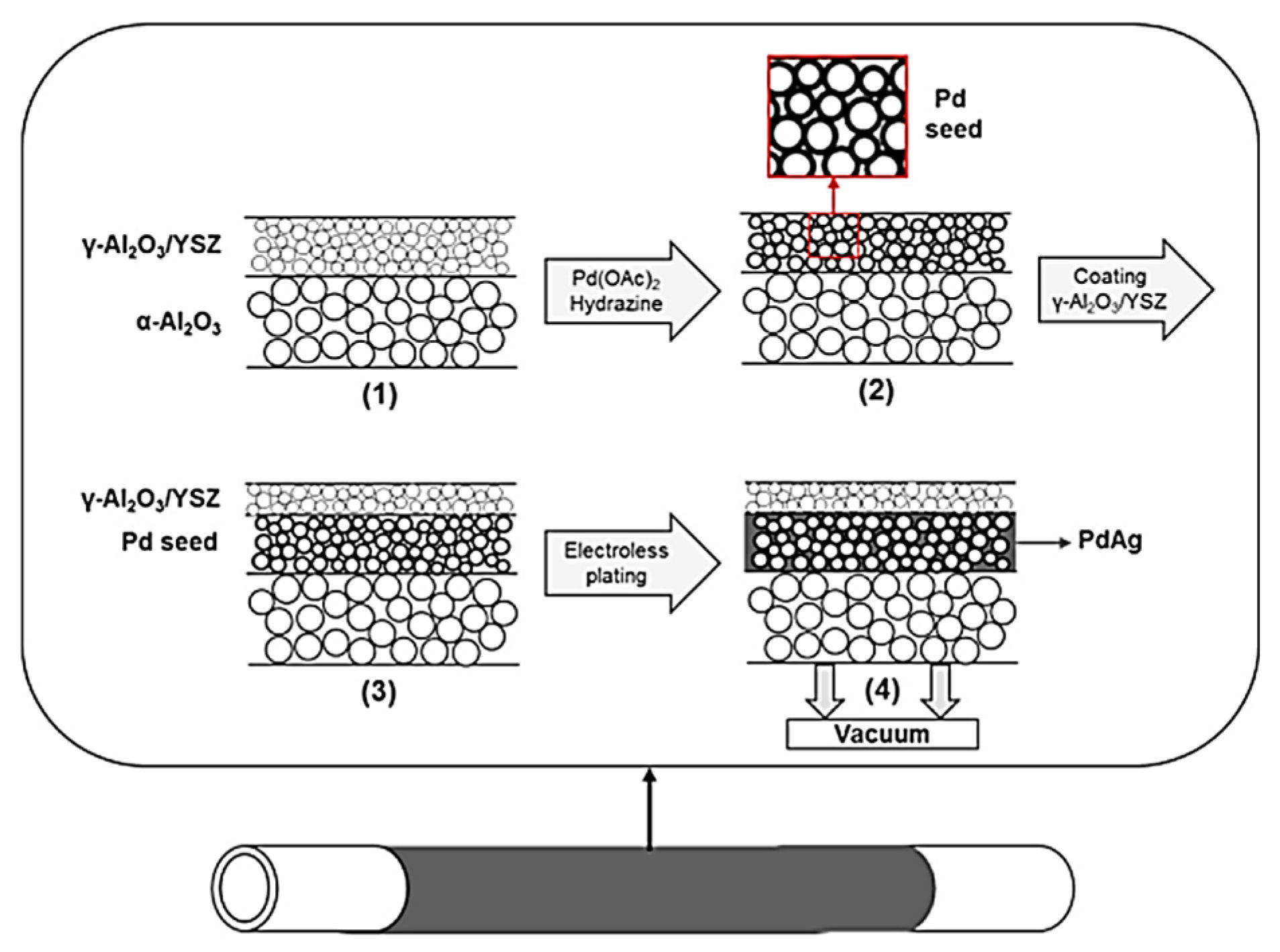
3. Palladium Incorporation by Electroless Plating
3.1. Electroless Plating Standard Method
3.2. Recent Developments in Electroless Plating
4. Pd-Alloy Membranes
4.1. Alloy Preparation
4.2. Binary Alloys
4.2.1. PdAg Membranes
4.2.2. PdCu Membranes
4.2.3. PdAu Membranes
4.2.4. Others Binary Alloys
4.3. Ternary Alloys
5. Concluding Remarks and Future Perspectives
Acknowledgments
Conflicts of Interest
References
- Grunewald, N.; Klasen, S.; Martínez-Zarzoso, I.; Muris, C. The Trade-off between Income Inequality and Carbon Dioxide Emissions. Ecol. Econ. 2017, 142, 249–256. [Google Scholar] [CrossRef]
- Henriques, I.; Sadorsky, P. Investor implications of divesting from fossil fuels. Glob. Financ. J. 2017. [Google Scholar] [CrossRef]
- Furlan, C.; Mortarino, C. Forecasting the impact of renewable energies in competition with non-renewable sources. Renew. Sustain. Energy Rev. 2018, 81, 1879–1886. [Google Scholar] [CrossRef]
- Hanley, E.S.; Deane, J.P.; Gallachóir, B.P.Ó. The role of hydrogen in low carbon energy futures—A review of existing perspectives. Renew. Sustain. Energy Rev. 2017. [Google Scholar] [CrossRef]
- Muradov, N. Low to near-zero CO2 production of hydrogen from fossil fuels: Status and perspectives. Int. J. Hydrog. Energy 2017, 42, 14058–14088. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. “Renewable” hydrogen: Prospects and challenges. Renew. Sustain. Energy Rev. 2011, 15, 3034–3040. [Google Scholar] [CrossRef]
- Vivas, F.J.; de las Heras, A.; Segura, F.; Andújar, J.M. A review of energy management strategies for renewable hybrid energy systems with hydrogen backup. Renew. Sustain. Energy Rev. 2018, 82, 126–155. [Google Scholar] [CrossRef]
- Valente, A.; Iribarren, D.; Dufour, J. Harmonised life-cycle global warming impact of renewable hydrogen. J. Clean. Prod. 2017, 149, 762–772. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 5–12. [Google Scholar] [CrossRef]
- Moniruddin, M.; Ilyassov, B.; Zhao, X.; Smith, E.; Serikov, T.; Ibrayev, N.; Asmatulu, R.; Nuraje, N. Recent progress on perovskite materials in photovoltaic and water splitting applications. Mater. Today Energy 2017. [Google Scholar] [CrossRef]
- Rau, F.; Herrmann, A.; Krause, H.; Fino, D.; Trimis, D. Production of hydrogen by autothermal reforming of biogas. Energy Procedia 2017, 120, 294–301. [Google Scholar] [CrossRef]
- Sengodan, S.; Lan, R.; Humphreys, J.; Du, D.; Xu, W.; Wang, H.; Tao, S. Advances in reforming and partial oxidation of hydrocarbons for hydrogen production and fuel cell applications. Renew. Sustain. Energy Rev. 2018, 82, 761–780. [Google Scholar] [CrossRef]
- Li, X.; Li, A.; Lim, C.J.; Grace, J.R. Hydrogen permeation through Pd-based composite membranes: Effects of porous substrate, diffusion barrier and sweep gas. J. Membr. Sci. 2016, 499, 143–155. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Audichon, T. Chapter 2—Hydrogen Production from Thermal Reforming BT—Hydrogen Electrochemical Production. In Hydrogen Energy Fuel Cells Primary; Academic Press: Cambridge, MA, USA, 2018; pp. 7–15. [Google Scholar]
- Hossain, M.A.; Jewaratnam, J.; Ganesan, P. Prospect of hydrogen production from oil palm biomass by thermochemical process—A review. Int. J. Hydrog. Energy 2016, 41, 16637–16655. [Google Scholar] [CrossRef]
- Devasahayam, S.; Strezov, V. Thermal Decomposition of Magnesium Carbonate with Biomass and Plastic Wastes for Simultaneous Production of Hydrogen and Carbon Avoidance. J. Clean. Prod. 2018, 174, 1089–1095. [Google Scholar] [CrossRef]
- Tosti, S.; Cavezza, C.; Fabbricino, M.; Pontoni, L.; Palma, V.; Ruocco, C. Production of hydrogen in a Pd-membrane reactor via catalytic reforming of olive mill wastewater. Chem. Eng. J. 2015, 275, 366–373. [Google Scholar] [CrossRef]
- Tian, T.; Li, Q.; He, R.; Tan, Z.; Zhang, Y. Effects of biochemical composition on hydrogen production by biomass gasification. Int. J. Hydrog. Energy 2017, 42, 19723–19732. [Google Scholar] [CrossRef]
- Zahedi, S.; Solera, R.; García-Morales, J.L.; Sales, D. Effect of the addition of glycerol on hydrogen production from industrial municipal solid waste. Fuel 2016, 180, 343–347. [Google Scholar] [CrossRef]
- Murugan, A.; Brown, A.S. Review of purity analysis methods for performing quality assurance of fuel cell hydrogen. Int. J. Hydrog. Energy 2015, 40, 4219–4233. [Google Scholar] [CrossRef]
- Pinacci, P.; Basile, A. 3–Palladium-based composite membranes for hydrogen separation in membrane reactors. In Handbook of Membrane Reactors; Elsevier: Amsterdam, The Netherlands, 2013; pp. 149–182. [Google Scholar]
- Di Marcoberardino, G.; Binotti, M.; Manzolini, G.; Viviente, J.L.; Arratibel, A.; Roses, L.; Gallucci, F. Achievements of European projects on membrane reactor for hydrogen production. J. Clean. Prod. 2017, 161, 1442–1450. [Google Scholar] [CrossRef]
- Yin, H.; Yip, A.C.K. A Review on the Production and Purification of Biomass-Derived Hydrogen Using Emerging Membrane Technologies. Catalysts 2017, 7, 297. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Boeltken, T.; Piermartini, P.; Selinsek, M.; Loewert, M.; Dallmann, F.; Kreuder, H.; Cholewa, M.; Wunsch, A.; Belimov, M.; et al. Micro and micro membrane reactors for advanced applications in chemical energy conversion. Curr. Opin. Chem. Eng. 2017, 17, 108–125. [Google Scholar] [CrossRef]
- Al-Mufachi, N.A.; Rees, N.V.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- Deveau, N.D.; Ma, Y.H.; Datta, R. Beyond Sieverts’ law: A comprehensive microkinetic model of hydrogen permeation in dense metal membranes. J. Membr. Sci. 2013, 437, 298–311. [Google Scholar] [CrossRef]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Rahimpour, M.R.; Samimi, F.; Babapoor, A.; Tohidian, T.; Mohebi, S. Palladium membranes applications in reaction systems for hydrogen separation and purification: A review. Chem. Eng. Process. Process Intensif. 2017, 121, 24–49. [Google Scholar] [CrossRef]
- Deville, H.; Troost, L. Sur la permeabilitè du fer a haute temperature. Comptes Rendus 1863, 57, 965–967. [Google Scholar]
- Deville, H. Note sur le passage des gaz au travers des corps solides homogènes. Comptes Rendus 1864, 59, 102. [Google Scholar]
- Thomas, G. On the absorption and dialytic separation of gases by colloid septa. Philos. Trans. R. Soc. A 1866, 156, 399–439. [Google Scholar]
- Dunbar, Z.W.; Lee, I.C. Effects of elevated temperatures and contaminated hydrogen gas mixtures on novel ultrathin palladium composite membranes. Int. J. Hydrog. Energy 2017, 42, 29310–29319. [Google Scholar] [CrossRef]
- Yun, S.; Oyama, S.T. Correlations in palladium membranes for hydrogen separation: A review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Helmi, A.; Gallucci, F.; van Sint Annaland, M. Resource scarcity in palladium membrane applications for carbon capture in integrated gasification combined cycle units. Int. J. Hydrog. Energy 2014, 39, 10498–10506. [Google Scholar] [CrossRef]
- Jayaraman, V.; Lin, Y.S. Synthesis and hydrogen permeation properties of ultrathin palladium-silver alloy membranes. J. Membr. Sci. 1995, 104, 251–262. [Google Scholar] [CrossRef]
- Yun, S.; Ko, J.H.; Oyama, S.T. Ultrathin palladium membranes prepared by a novel electric field assisted activation. J. Membr. Sci. 2011, 369, 482–489. [Google Scholar] [CrossRef]
- Maneerung, T.; Hidajat, K.; Kawi, S. Ultra-thin (<1 µm) internally-coated Pd–Ag alloy hollow fiber membrane with superior thermal stability and durability for high temperature H2 separation. J. Membr. Sci. 2014, 452, 127–142. [Google Scholar] [CrossRef]
- Melendez, J.; Fernandez, E.; Gallucci, F.; van Annaland, M.; Arias, P.L.; Tanaka, D.A.P. Preparation and characterization of ceramic supported ultra-thin (~1 µm) Pd-Ag membranes. J. Membr. Sci. 2017, 528, 12–23. [Google Scholar] [CrossRef]
- Feitosa, J.L.C.S.; da Cruz, A.G.B.; Souza, A.C.; Duda, F.P. Stress effects on hydrogen permeation through tubular multilayer membranes: Modeling and simulation. Int. J. Hydrog. Energy 2015, 40, 17031–17037. [Google Scholar] [CrossRef]
- Dittmar, B.; Behrens, A.; Schödel, N.; Rüttinger, M.; Franco, T.; Straczewski, G.; Dittmeyer, R. Methane steam reforming operation and thermal stability of new porous metal supported tubular palladium composite membranes. Int. J. Hydrog. Energy 2013, 38, 8759–8771. [Google Scholar] [CrossRef]
- Melendez, J.; de Nooijer, N.; Coenen, K.; Fernandez, E.; Viviente, J.L.; van Sint Annaland, M.; Arias, P.L.; Tanaka, D.A.P.; Gallucci, F. Effect of Au addition on hydrogen permeation and the resistance to H2S on Pd-Ag alloy membranes. J. Membr. Sci. 2017, 542, 329–341. [Google Scholar] [CrossRef]
- El Hawa, H.W.A.; Lundin, S.-T.B.; Paglieri, S.N.; Harale, A.; Way, J.D. The influence of heat treatment on the thermal stability of Pd composite membranes. J. Membr. Sci. 2015, 494, 113–120. [Google Scholar] [CrossRef]
- Lundin, S.-T.B.; Patki, N.S.; Fuerst, T.F.; Wolden, C.A.; Way, J.D. Inhibition of hydrogen flux in palladium membranes by pressure–induced restructuring of the membrane surface. J. Membr. Sci. 2017, 535, 70–78. [Google Scholar] [CrossRef]
- Livshits, A.I. The hydrogen transport through the metal alloy membranes with a spatial variation of the alloy composition: Potential diffusion and enhanced permeation. Int. J. Hydrog. Energy 2017, 42, 13111–13119. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Medrano, J.A.; Coenen, K.; Arratibel, A.; Melendez, J.; de Nooijer, N.C.A.; Spallina, V.; Viviente, J.L.; Zuñiga, J.; et al. Palladium based membranes and membrane reactors for hydrogen production and purification: An overview of research activities at Tecnalia and TU/e. Int. J. Hydrog. Energy 2017, 42, 13763–13776. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, H.; Fan, X.; Zhou, L.; Chen, Q. Palladium composite membrane fabricated on rough porous alumina tube without intermediate layer for hydrogen separation. Int. J. Hydrog. Energy 2017, 42, 9958–9965. [Google Scholar] [CrossRef]
- Nayebossadri, S.; Fletcher, S.; Speight, J.D.; Book, D. Hydrogen permeation through porous stainless steel for palladium-based composite porous membranes. J. Membr. Sci. 2016, 515, 22–28. [Google Scholar] [CrossRef]
- Itoh, N.; Suga, E.; Sato, T. Composite palladium membrane prepared by introducing metallic glue and its high durability below the critical temperature. Sep. Purif. Technol. 2014, 121, 46–53. [Google Scholar] [CrossRef]
- Calles, J.A.; Sanz, R.; Alique, D.; Furones, L.; Marín, P.; Ordoñez, S. Influence of the selective layer morphology on the permeation properties for Pd-PSS composite membranes prepared by electroless pore-plating: Experimental and modeling study. Sep. Purif. Technol. 2018, 194, 10–18. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Preparation of “pore-fill” type Pd–YSZ–γ-Al2O3 composite membrane supported on α-Al2O3 tube for hydrogen separation. J. Membr. Sci. 2008, 320, 436–441. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L. New synthesis method of Pd membranes over tubular {PSS} supports via “pore-plating” for hydrogen separation processes. Int. J. Hydrog. Energy 2012, 37, 18476–18485. [Google Scholar] [CrossRef]
- Pacheco, D.A.; Llosa, M.A.; Niwa, S.; Wakui, Y.; Mizukami, F.; Namba, T.; Suzuki, T.M. Preparation of palladium and silver alloy membrane on a porous α-alumina tube via simultaneous electroless plating. J. Membr. Sci. 2005, 247, 21–27. [Google Scholar] [CrossRef]
- Zeng, G.; Goldbach, A.; Shi, L.; Xu, H. On alloying and low-temperature stability of thin, supported PdAg membranes. Int. J. Hydrog. Energy 2012, 37, 6012–6019. [Google Scholar] [CrossRef]
- Zhao, L.; Goldbach, A.; Bao, C.; Xu, H. Sulfur inhibition of PdCu membranes in the presence of external mass flow resistance. J. Membr. Sci. 2015, 496, 301–309. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Braun, F.; Cornaglia, L.M. Novel PdAgCu ternary alloy: Hydrogen permeation and surface properties. Appl. Surf. Sci. 2011, 257, 6626–6635. [Google Scholar] [CrossRef]
- Jia, H.; Wu, P.; Zeng, G.; Salas-Colera, E.; Serrano, A.; Castro, G.R.; Xu, H.; Sun, C.; Goldbach, A. High-temperature stability of Pd alloy membranes containing Cu and Au. J. Membr. Sci. 2017, 544, 151–160. [Google Scholar] [CrossRef]
- Tosti, S. Supported and laminated Pd-based metallic membranes. Int. J. Hydrog. Energy 2003, 28, 1445–1454. [Google Scholar] [CrossRef]
- Zhang, K.; Gade, S.K.; Hatlevik, Ø.; Way, J.D. A sorption rate hypothesis for the increase in H2 permeability of palladium-silver (Pd–Ag) membranes caused by air oxidation. Int. J. Hydrog. Energy 2012, 37, 583–593. [Google Scholar] [CrossRef]
- Tosti, S.; Bettinali, L.; Violante, V. Rolled thin Pd and Pd–Ag membranes for hydrogen separation and production. Int. J. Hydrog. Energy 2000, 25, 319–325. [Google Scholar] [CrossRef]
- Tosti, S.; Bettinali, L.; Castelli, S.; Sarto, F.; Scaglione, S.; Violante, V. Sputtered, electroless and rolled palladium-ceramic membranes. J. Membr. Sci. 2002, 196, 241–249. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Development of thin binary and ternary Pd-based alloy membranes for use in hydrogen production. J. Membr. Sci. 2011, 383, 124–134. [Google Scholar] [CrossRef]
- Calles, J.A.; Sanz, R.; Alique, D. Influence of the type of siliceous material used as intermediate layer in the preparation of hydrogen selective palladium composite membranes over a porous stainless steel support. Int. J. Hydrog. Energy 2012, 37, 6030–6042. [Google Scholar] [CrossRef]
- Peters, T.A.; Stange, M.; Bredesen, R. 2-Fabrication of palladium-based membranes by magnetron sputtering. In Palladium Membrane Technology Hydrogen Production, Carbon Capture and Other Application; Doukelis, A., Panopoulos, K., Koumanakos, A., Kakaras, E., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 25–41. [Google Scholar]
- Huang, L.; Chen, C.S.; He, Z.D.; Peng, D.K.; Meng, G.Y. Palladium membranes supported on porous ceramics prepared by chemical vapor deposition. Thin Solid Films 1997, 302, 98–101. [Google Scholar] [CrossRef]
- Jun, C.-S.; Lee, K.-H. Palladium and palladium alloy composite membranes prepared by metal-organic chemical vapor deposition method (cold-wall). J. Membr. Sci. 2000, 176, 121–130. [Google Scholar] [CrossRef]
- Lee, S.M.; Xu, N.; Kim, S.S.; Li, A.; Grace, J.R.; Lim, C.J.; Boyd, T.; Ryi, S.-K.; Susdorf, A.; Schaadt, A. Palladium/ruthenium composite membrane for hydrogen separation from the off-gas of solar cell production via chemical vapor deposition. J. Membr. Sci. 2017, 541, 1–8. [Google Scholar] [CrossRef]
- Chen, S.C.; Tu, G.C.; Hung, C.C.Y.; Huang, C.A.; Rei, M.H. Preparation of palladium membrane by electroplating on AISI 316L porous stainless steel supports and its use for methanol steam reformer. J. Membr. Sci. 2008, 314, 5–14. [Google Scholar] [CrossRef]
- Chen, C.-H.; Huang, Y.-R.; Liu, C.-W.; Wang, K.-W. Preparation and modification of PdAg membranes by electroless and electroplating process for hydrogen separation. Thin Solid Films 2016, 618, 189–194. [Google Scholar] [CrossRef]
- Yoshii, K.; Oshino, Y.; Tachikawa, N.; Toshima, K.; Katayama, Y. Electrodeposition of palladium from palladium(II) acetylacetonate in an amide-type ionic liquid. Electrochem. Commun. 2015, 52, 21–24. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L.; Ordóñez, S.; Marín, P.; Corengia, P.; Fernandez, E. Preparation, testing and modelling of a hydrogen selective Pd/YSZ/SS composite membrane. Int. J. Hydrog. Energy 2011, 36, 15783–15793. [Google Scholar] [CrossRef]
- Alique, D.; Imperatore, M.; Sanz, R.; Calles, J.A.; Baschetti, M.G. Hydrogen permeation in composite Pd-membranes prepared by conventional electroless plating and electroless pore-plating alternatives over ceramic and metallic supports. Int. J. Hydrog. Energy 2016, 41, 19430–19438. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Lee, S.-W.; Oh, D.-K.; Seo, B.-S.; Park, J.-W.; Park, J.-S.; Lee, D.-W.; Kim, S.S. Electroless plating of Pd after shielding the bottom of planar porous stainless steel for a highly stable hydrogen selective membrane. J. Membr. Sci. 2014, 467, 93–99. [Google Scholar] [CrossRef]
- Sari, R.; Yaakob, Z.; Ismail, M.; Daud, W.R.W.; Hakim, L. Palladium-alumina composite membrane for hydrogen separator fabricated by combined sol-gel and electroless plating technique. Ceram. Int. 2013, 39, 3211–3219. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Bosko, M.L.; Cornaglia, L.M. Electroless Plating of Pd Binary and Ternary Alloys and Surface Characteristics for Application in Hydrogen Separation A2—Hashmi, MSJ BT. In Comprehensive Materials Finishing; Elsevier: Oxford, UK, 2017; pp. 1–24. [Google Scholar]
- Zhang, B. Chapter 1—History–From the Discovery of Electroless Plating to the Present BT. In Amorphous and Nano Alloys Electroless Depositions; Elsevier: Oxford, UK, 2016; pp. 3–48. [Google Scholar]
- Tosti, S.; Fabbricino, M.; Pontoni, L.; Palma, V.; Ruocco, C. Catalytic reforming of olive mill wastewater and methane in a Pd-membrane reactor. Int. J. Hydrog. Energy 2016, 41, 5465–5474. [Google Scholar] [CrossRef]
- Tosti, S.; Adrover, A.; Basile, A.; Camilli, V.; Chiappetta, G.; Violante, V. Characterization of thin wall Pd–Ag rolled membranes. Int. J. Hydrog. Energy 2003, 28, 105–112. [Google Scholar] [CrossRef]
- Calles, J.A.; Sanz, R.; Alique, D.; Furones, L. Thermal stability and effect of typical water gas shift reactant composition on H2 permeability through a Pd-YSZ-PSS composite membrane. Int. J. Hydrog. Energy 2014, 39, 1398–1409. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L. H2 production via water gas shift in a composite Pd membrane reactor prepared by the pore-plating method. Int. J. Hydrog. Energy 2014, 39, 4739–4748. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Alique, D.; Furones, L.; Ordóñez, S.; Marín, P. Hydrogen production in a Pore-Plated Pd-membrane reactor: Experimental analysis and model validation for the Water Gas Shift reaction. Int. J. Hydrog. Energy 2015, 40, 3472–3484. [Google Scholar] [CrossRef]
- Masuda, H.; Nishio, K.; Baba, N. Preparation of microporous metal membrane using two-step replication of interconnected structure of porous glass. J. Mater. Sci. Lett. 1994, 13, 338–340. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Yeung, K.L. Palladium-silver composite membranes by electroless plating technique. J. Membr. Sci. 1999, 158, 127–141. [Google Scholar] [CrossRef]
- Augustine, A.S.; Mardilovich, I.P.; Kazantzis, N.K.; Ma, Y.H. Durability of PSS-supported Pd-membranes under mixed gas and water-gas shift conditions. J. Membr. Sci. 2012, 415–416, 213–220. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D.; McCormick, R.L.; Paglieri, S.N. Preparation and characterization of Pd-Cu composite membranes for hydrogen separation. Chem. Eng. J. 2003, 93, 11–22. [Google Scholar] [CrossRef]
- Lewis, A.E.; Kershner, D.C.; Paglieri, S.N.; Slepicka, M.J.; Way, J.D. Pd-Pt/YSZ composite membranes for hydrogen separation from synthetic water-gas shift streams. J. Membr. Sci. 2013, 437, 257–264. [Google Scholar] [CrossRef]
- Kong, S.Y.; Kim, D.H.; Henkensmeier, D.; Kim, H.J.; Ham, H.C.; Han, J.; Yoon, S.P.; Yoon, C.W.; Choi, S.H. Ultrathin layered Pd/PBI–HFA composite membranes for hydrogen separation. Sep. Purif. Technol. 2017, 179, 486–493. [Google Scholar] [CrossRef]
- Kim, D.H.; Kong, S.Y.; Lee, G.; Yoon, C.W.; Ham, H.C.; Han, J.; Song, K.H.; Henkensmeier, D.; Choi, S.H. Effect of PBI-HFA surface treatments on Pd/PBI-HFA composite gas separation membranes. Int. J. Hydrog. Energy 2017, 42, 22915–22924. [Google Scholar] [CrossRef]
- Kumar, R.; Kamakshi; Kumar, M.; Awasthi, K. Selective deposition of Pd nanoparticles in porous PET membrane for hydrogen separation. Int. J. Hydrog. Energy 2017, 42, 15203–15210. [Google Scholar] [CrossRef]
- Alique, D. Processing and Characterization of Coating and Thin Film Materials. In Advanced Ceramic and Metallic Coating and Thin Film Materials Energy and Environmental Applications; Zhang, J., Jung, Y., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Plazaola, A.A.; Tanaka, D.P.; Van Sint Annaland, M.; Gallucci, F. Recent Advances in Pd-Based Membranes for Membrane Reactors. Molecules 2017, 22, 51. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Caravella, A.; Xu, H.Y. Recent progress in Pd-based composite membranes. J. Mater. Chem. A 2016, 4, 14069–14094. [Google Scholar] [CrossRef]
- Mardilovich, I.P.; Engwall, E.; Ma, Y.H. Dependence of hydrogen flux on the pore size and plating surface topology of asymmetric Pd-porous stainless steel membranes. Desalination 2002, 144, 85–89. [Google Scholar] [CrossRef]
- Tarditi, A.; Gerboni, C.; Cornaglia, L. PdAu membranes supported on top of vacuum-assisted ZrO2-modified porous stainless steel substrates. J. Membr. Sci. 2013, 428, 1–10. [Google Scholar] [CrossRef]
- Chen, C.; Ma, Y.H. The effect of H2S on the performance of Pd and Pd/Au composite membrane. J. Membr. Sci. 2010, 362, 535–544. [Google Scholar] [CrossRef]
- Fernandez, E.; Helmi, A.; Coenen, K.; Melendez, J.; Luis, J.; Alfredo, D.; Tanaka, P.; van Sint, M.; Gallucci, F. Development of thin Pd-Ag supported membranes for fluidized bed membrane reactors including WGS related gases. Int. J. Hydrog. Energy 2014, 40, 3506–3519. [Google Scholar] [CrossRef]
- Shackelford, J.F.; William, A. Materials Science Engineering Hand Book, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Ryi, S.K.; Xu, N.; Li, A.; Lim, C.J.; Grace, J.R. Electroless Pd membrane deposition on alumina modified porous Hastelloy substrate with EDTA-free bath. Int. J. Hydrog. Energy 2010, 35, 2328–2335. [Google Scholar] [CrossRef]
- Fernandez, E.; Medrano, J.A.; Melendez, J.; Parco, M.; Viviente, J.L.; van Sint Annaland, M.; Gallucci, F.; Tanaka, D.A.P. Preparation and characterization of metallic supported thin Pd-Ag membranes for hydrogen separation. Chem. Eng. J. 2016, 305, 182–190. [Google Scholar] [CrossRef]
- Ryi, S.K.; Ahn, H.S.; Park, J.S.; Kim, D.W. Pd-Cu alloy membrane deposited on CeO2 modified porous nickel support for hydrogen separation. Int. J. Hydrog. Energy 2014, 39, 4698–4703. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, S.; Fan, Y.; Xu, N.; He, Y. Preparation of dense Pd composite membranes on porous Ti–Al alloy supports by electroless plating. J. Membr. Sci. 2012, 387–388, 24–29. [Google Scholar] [CrossRef]
- Kim, S.S.; Xu, N.; Li, A.; Grace, J.R.; Lim, C.J.; Ryi, S.K. Development of a new porous metal support based on nickel and its application for Pd based composite membranes. Int. J. Hydrog. Energy 2015, 40, 3520–3527. [Google Scholar] [CrossRef]
- Kulprathipanja, A.; Alptekin, G.O.; Falconer, J.L.; Way, J.D. Pd and Pd-Cu membranes: Inhibition of H2 permeation by H2S. J. Membr. Sci. 2005, 254, 49–62. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanaka, D.A.P.; Tanco, M.A.L.; Wakui, Y.; Ikeda, T.; Mizukami, F.; Suzuki, T.M. Preparation and Hydrogen Permeation Properties of Thin Pd-Au Alloy Membranes Supported on Porous α-Alumina Tube. Mater. Trans. 2008, 49, 449–452. [Google Scholar] [CrossRef]
- Wang, W.P.; Thomas, S.; Zhang, X.L.; Pan, X.L.; Yang, W.S.; Xiong, G.X. H2/N2 gaseous mixture separation in dense Pd/α-Al2O3 hollow fiber membranes: Experimental and simulation studies. Sep. Purif. Technol. 2006, 52, 177–185. [Google Scholar] [CrossRef]
- Nair, B.K.R.; Choi, J.; Harold, M.P. Electroless plating and permeation features of Pd and Pd/Ag hollow fiber composite membranes. J. Membr. Sci. 2007, 288, 67–84. [Google Scholar] [CrossRef]
- Incelli, M.; Santucci, A.; Tosti, S.; Sansovini, M.; Carlini, M. Heavy water decontamination tests through a Pd-Ag membrane reactor: Water Gas Shift and Isotopic Swamping performances. Fusion Eng. Des. 2016, 124, 692–695. [Google Scholar] [CrossRef]
- Yu, C.; Xu, H. An efficient palladium membrane reactor to increase the yield of styrene in ethylbenzene dehydrogenation. Sep. Purif. Technol. 2011, 78, 249–252. [Google Scholar] [CrossRef]
- Brunetti, A.; Caravella, A.; Fernandez, E.; Tanaka, D.A.P.; Gallucci, F.; Drioli, E.; Curcio, E.; Viviente, J.L.; Barbieri, G. Syngas upgrading in a membrane reactor with thin Pd-alloy supported membrane. Int. J. Hydrog. Energy 2015, 40, 10883–10893. [Google Scholar] [CrossRef]
- Rahman, M.A.; García-García, F.R.; Li, K. Development of a catalytic hollow fibre membrane microreactor as a microreformer unit for automotive application. J. Membr. Sci. 2012, 390–391, 68–75. [Google Scholar] [CrossRef]
- Mardilovich, P.P.; She, Y.; Ma, Y.H.; Rei, M.-H. Defect-free palladium membranes on porous stainless-steel support. AIChE J. 1998, 44, 310–322. [Google Scholar] [CrossRef]
- Li, A.; Grace, J.R.; Lim, C.J. Preparation of thin Pd-based composite membrane on planar metallic substrate: Part II. Preparation of membranes by electroless plating and characterization. J. Membr. Sci. 2007, 306, 159–165. [Google Scholar] [CrossRef]
- Jayaraman, V.; Lin, Y.S.; Pakala, M.; Lin, R.Y. Fabrication of ultrathin metallic membranes on ceramic supports by sputter deposition. J. Membr. Sci. 1995, 99, 89–100. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Park, J.-S.; Kim, S.-H.; Kim, D.-W.; Cho, K.-I. Formation of a defect-free Pd–Cu–Ni ternary alloy membrane on a polished porous nickel support (PNS). J. Membr. Sci. 2008, 318, 346–354. [Google Scholar] [CrossRef]
- Pinacci, P.; Drago, F. Influence of the support on permeation of palladium composite membranes in presence of sweep gas. Catal. Today 2012, 193, 186–193. [Google Scholar] [CrossRef]
- Ryi, S.-K.; Park, J.-S.; Hwang, K.-R.; Lee, C.-B.; Lee, S.-W. Repair of Pd-based composite membrane by polishing treatment. Int. J. Hydrog. Energy 2011, 36, 13776–13780. [Google Scholar] [CrossRef]
- Jemaa, N.; Shu, J.; Kaliaguine, S.; Grandjean, B.P.A. Thin Palladium Film Formation on Shot Peening Modified Porous Stainless Steel Substrates. Ind. Eng. Chem. Res. 1996, 35, 973–977. [Google Scholar] [CrossRef]
- Huang, Y.; Dittmeyer, R. Preparation of thin palladium membranes on a porous support with rough surface. J. Membr. Sci. 2007, 302, 160–170. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Fan, Y.; Xu, N. Palladium-based composite membranes: Principle, preparation and characterization. Prog. Chem. 2006, 18, 230–238. [Google Scholar]
- Collins, J.P.; Way, J.D. Catalytic decomposition of ammonia in a membrane reactor. J. Membr. Sci. 1994, 96, 259–274. [Google Scholar] [CrossRef]
- Huang, Y.; Shu, S.; Lu, Z.; Fan, Y. Characterization of the adhesion of thin palladium membranes supported on tubular porous ceramics. Thin Solid Films 2007, 515, 5233–5240. [Google Scholar] [CrossRef]
- Coronas, J.; Santamaría, J. Catalytic reactors based on porous ceramic membranes. Catal. Today 1999, 51, 377–389. [Google Scholar] [CrossRef]
- Tong, J.; Matsumura, Y.; Suda, H.; Haraya, K. Thin and dense Pd/CeO2/MPSS composite membrane for hydrogen separation and steam reforming of methane. Sep. Purif. Technol. 2005, 46, 1–10. [Google Scholar] [CrossRef]
- Qiao, A.; Zhang, K.; Tian, Y.; Xie, L.; Luo, H.; Lin, Y.S.; Li, Y. Hydrogen separation through palladium-copper membranes on porous stainless steel with sol-gel derived ceria as diffusion barrier. Fuel 2010, 89, 1274–1279. [Google Scholar] [CrossRef]
- Wang, D.; Tong, J.; Xu, H.; Matsumura, Y. Preparation of palladium membrane over porous stainless steel tube modified with zirconium oxide. Catal. Today 2004, 93–95, 689–693. [Google Scholar] [CrossRef]
- Gao, H.; Lin, J.Y.S.; Li, Y.; Zhang, B. Electroless plating synthesis, characterization and permeation properties of Pd–Cu membranes supported on ZrO2 modified porous stainless steel. J. Membr. Sci. 2005, 265, 142–152. [Google Scholar] [CrossRef]
- Lee, C.-B.; Lee, S.-W.; Park, J.-S.; Ryi, S.-K.; Lee, D.-W.; Hwang, K.-R.; Kim, S.-H. Ceramics used as intermetallic diffusion barriers in Pd-based composite membranes sputtered on porous nickel supports. J. Alloys Compd. 2013, 578, 425–430. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, H.; Rui, Z.; Liu, P.; Li, Y.; Lin, Y.S. High-Temperature Stability of Palladium Membranes on Porous Metal Supports with Different Intermediate Layers. Ind. Eng. Chem. Res. 2009, 48, 1880–1886. [Google Scholar] [CrossRef]
- Yepes, D.; Cornaglia, L.M.; Irusta, S.; Lombardo, E.A. Different oxides used as diffusion barriers in composite hydrogen permeable membranes. J. Membr. Sci. 2006, 274, 92–101. [Google Scholar] [CrossRef]
- Li, A.; Grace, J.R.; Lim, C.J. Preparation of thin Pd-based composite membrane on planar metallic substrate: Part I: Pre-treatment of porous stainless steel substrate. J. Membr. Sci. 2007, 298, 175–181. [Google Scholar] [CrossRef]
- Broglia, M.; Pinacci, P.; Radaelli, M.; Bottino, A.; Capannelli, G.; Comite, A.; Vanacore, G.; Zani, M. Synthesis and characterization of Pd membranes on alumina-modified porous stainless steel supports. Desalination 2009, 245, 508–515. [Google Scholar] [CrossRef]
- Chi, Y.-H.; Yen, P.-S.; Jeng, M.-S.; Ko, S.-T.; Lee, T.-C. Preparation of thin Pd membrane on porous stainless steel tubes modified by a two-step method. Int. J. Hydrog. Energy 2010, 35, 6303–6310. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, K.-H. Hydrogen separation by Pd alloy composite membranes: Introduction of diffusion barrier. J. Membr. Sci. 2001, 192, 177–185. [Google Scholar] [CrossRef]
- Van Gestel, T.; Hauler, F.; Bram, M.; Meulenberg, W.A.; Buchkremer, H.P.; van Gestel, T.; Hauler, F.; Bram, M.; Meulenberg, W.A.; Buchkremer, H.P. Synthesis and characterization of hydrogen-selective sol–gel SiO2 membranes supported on ceramic and stainless steel supports. Sep. Purif. Technol. 2014, 121, 20–29. [Google Scholar] [CrossRef]
- Kanezashi, M.; Fuchigami, D.; Yoshioka, T.; Tsuru, T. Control of Pd dispersion in sol–gel-derived amorphous silica membranes for hydrogen separation at high temperatures. J. Membr. Sci. 2013, 439, 78–86. [Google Scholar] [CrossRef]
- Zheng, L.; Li, H.; Xu, T.; Bao, F.; Xu, H. Defect size analysis approach combined with silicate gel/ceramic particles for defect repair of Pd composite membranes. Int. J. Hydrog. Energy 2016, 41, 18522–18532. [Google Scholar] [CrossRef]
- Bosko, M.L.; Ojeda, F.; Lombardo, E.A.; Cornaglia, L.M. NaA zeolite as an effective diffusion barrier in composite Pd/PSS membranes. J. Membr. Sci. 2009, 331, 57–65. [Google Scholar] [CrossRef]
- Mobarake, M.D.; Jafari, P.; Irani, M. Preparation of Pd-based membranes on Pd/TiO2 modified NaX/PSS substrate for hydrogen separation: Design and optimization. Microporous Mesoporous Mater. 2016, 226, 369–377. [Google Scholar] [CrossRef]
- Yu, J.; Qi, C.; Zhang, J.; Bao, C.; Xu, H. Synthesis of a zeolite membrane as a protective layer on a metallic Pd composite membrane for hydrogen purification. J. Mater. Chem. A 2015, 3, 5000–5006. [Google Scholar] [CrossRef]
- Sato, K.; Natsui, M.; Hasegawa, Y. Preparation of Double Layer Membrane Combined with Palladium Metal and FAU Zeolite for Catalytic Membrane Reactor. Mater. Trans. 2015, 56, 473–478. [Google Scholar] [CrossRef]
- Wang, X.; Tan, X.; Meng, B.; Zhang, X.; Liang, Q.; Pan, H.; Liu, S. TS-1 zeolite as an effective diffusion barrier for highly stable Pd membrane supported on macroporous α-Al2O3 tube. RSC Adv. 2013, 3, 4821–4834. [Google Scholar] [CrossRef]
- Abate, S.; Díaz, U.; Prieto, A.; Gentiluomo, S.; Palomino, M.; Perathoner, S.; Corma, A.; Centi, G. Influence of Zeolite Protective Overlayer on the Performances of Pd Thin Film Membrane on Tubular Asymmetric Alumina Supports. Ind. Eng. Chem. Res. 2016, 55, 4948–4959. [Google Scholar] [CrossRef]
- Ma, Y.H.; Mardilovich, P.P.; She, Y. Hydrogen Gas-Extraction Module and Method of Fabrication. U.S. Patent 6152987 A, 28 November 2000. [Google Scholar]
- Guazzone, F.; Engwall, E.E.; Ma, Y.H. Effects of surface activity, defects and mass transfer on hydrogen permeance and n-value in composite palladium-porous stainless steel membranes. Catal. Today 2006, 118, 24–31. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Soria, M.A.; Rodríguez-Ramos, I.; Guerrero-Ruiz, A. Modifications of porous stainless steel previous to the synthesis of Pd membranes. Stud. Surf. Sci. Catal. 2010, 175, 779–783. [Google Scholar] [CrossRef]
- Nam, S.-E.; Lee, K.-H. Preparation and Characterization of Palladium Alloy Composite Membranes with a Diffusion Barrier for Hydrogen Separation. Ind. Eng. Chem. Res. 2005, 44, 100–105. [Google Scholar] [CrossRef]
- Ayturk, M.E.; Mardilovich, I.P.; Engwall, E.E.; Ma, Y.H. Synthesis of composite Pd-porous stainless steel (PSS) membranes with a Pd/Ag intermetallic diffusion barrier. J. Membr. Sci. 2006, 285, 385–394. [Google Scholar] [CrossRef]
- Lee, J.-H.; Han, J.-Y.; Kim, K.-M.; Ryi, S.-K.; Kim, D.-W. Development of homogeneous Pd–Ag alloy membrane formed on porous stainless steel by multi-layered films and Ag-upfilling heat treatment. J. Membr. Sci. 2015, 492, 242–248. [Google Scholar] [CrossRef]
- Pujari, M.; Agarwal, A.; Uppaluri, R.; Verma, A. Role of electroless nickel diffusion barrier on the combinatorial plating characteristics of dense Pd/Ni/PSS composite membranes. Appl. Surf. Sci. 2014, 305, 658–664. [Google Scholar] [CrossRef]
- Tong, J.; Suda, H.; Haraya, K.; Matsumura, Y. A novel method for the preparation of thin dense Pd membrane on macroporous stainless steel tube filter. J. Memb. Sci. 2005, 260, 10–18. [Google Scholar] [CrossRef]
- Tong, J.; Su, L.; Haraya, K.; Suda, H. Thin Pd membrane on α-Al2O3 hollow fiber substrate without any interlayer by electroless plating combined with embedding Pd catalyst in polymer template. J. Membr. Sci. 2008, 310, 93–101. [Google Scholar] [CrossRef]
- Hu, X.; Chen, W.; Huang, Y. Fabrication of Pd/ceramic membranes for hydrogen separation based on low-cost macroporous ceramics with pencil coating. Int. J. Hydrog. Energy 2010, 35, 7803–7808. [Google Scholar] [CrossRef]
- Zhao, H.-B.; Pflanz, K.; Gu, J.-H.; Li, A.-W.; Stroh, N.; Brunner, H.; Xiong, G.-X. Preparation of palladium composite membranes by modified electroless plating procedure. J. Membr. Sci. 1998, 142, 147–157. [Google Scholar] [CrossRef]
- Bottino, A.; Broglia, M.; Capannelli, G.; Comite, A.; Pinacci, P.; Scrignari, M.; Azzurri, F. Sol-gel synthesis of thin alumina layers on porous stainless steel supports for high temperature palladium membranes. Int. J. Hydrog. Energy 2014, 39, 4717–4724. [Google Scholar] [CrossRef]
- Brenner, A.; Riddell, G.E. Nickel plating on steel by chemical reduction. J. Res. Natl. Bur. Stand. 1946, 37, 31–34. [Google Scholar] [CrossRef]
- Zornoza, B.; Casado, C.; Navajas, A. Chapter 11—Advances in Hydrogen Separation and Purification with Membrane Technology. In Renewable Hydrogen Technologies; Gandía, L.M., Arzamendi, G., Diéguez, P.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 245–268. [Google Scholar]
- De Falco, M.; Iaquaniello, G.; Palo, E.; Cucchiella, B.; Palma, V.; Ciambelli, P. 11—Palladium-based membranes for hydrogen separation: Preparation, economic analysis and coupling with a water gas shift reactor. In Handbook of Membrane Reactors; Basile, A., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 456–486. [Google Scholar]
- Den Exter, M.J. 3—The use of electroless plating as a deposition technology in the fabrication of palladium-based membranes. In Palladium Membrane Technology Hydrogen Production, Carbon Capture and Other Application; Doukelis, A., Panopoulos, K., Koumanakos, A., Kakaras, E., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 43–67. [Google Scholar]
- Basile, A.; Tong, J.; Millet, P. 2—Inorganic membrane reactors for hydrogen production: An overview with particular emphasis on dense metallic membrane materials. In Handbook of Membrane Reactors; Basile, A., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 42–148. [Google Scholar]
- Cheng, Y.S.; Yeung, K.L. Effects of electroless plating chemistry on the synthesis of palladium membranes. J. Membr. Sci. 2001, 182, 195–203. [Google Scholar] [CrossRef]
- Dogan, M.; Kilicarslan, S. Effects of process parameters on the synthesis of palladium membranes. Nucl. Instrum. Methods Phys. Res. Sect. B 2008, 266, 3458–3466. [Google Scholar] [CrossRef]
- Yeung, K.L.; Christiansen, S.C.; Varma, A. Palladium composite membranes by electroless plating technique: Relationships between plating kinetics, film microstructure and membrane performance. J. Membr. Sci. 1999, 159, 107–122. [Google Scholar] [CrossRef]
- Djokić, S.S. Fundamentals of Electroless Deposition BT. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Mallory, G.O.; Hajdu, J.B. Electroless Plating: Fundamentals and Applications; American Electroplaters and Surface Finishers Society: Orlando, FL, USA, 1990. [Google Scholar]
- Shu, J.; Grandjean, B.P.A.; Ghali, E.; Kaliaguine, S. Simultaneous deposition of Pd and Ag on porous stainless steel by electroless plating. J. Membr. Sci. 1993, 77, 181–195. [Google Scholar] [CrossRef]
- Rothenberger, K.S.; Cugini, A.V.; Howard, B.H.; Killmeyer, R.P.; Ciocco, M.V.; Morreale, B.D.; Enick, R.M.; Bustamante, F.; Mardilovich, I.P.; Ma, Y.H. High pressure hydrogen permeance of porous stainless steel coated with a thin palladium film via electroless plating. J. Membr. Sci. 2004, 244, 55–68. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Way, J.D. Innovations in palladium membrane research. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Wei, L.; Yu, J.; Hu, X.; Wang, R.; Huang, Y. Effects of Sn residue on the high temperature stability of the H2-permeable palladium membranes prepared by electroless plating on Al2O3 substrate after SnCl2–PdCl2 process: A case study. Chin. J. Chem. Eng. 2016, 24, 1154–1160. [Google Scholar] [CrossRef]
- Chi, Y.-H.; Uan, J.-Y.; Lin, M.-C.; Lin, Y.-L.; Huang, J.-H. Preparation of a novel Pd/layered double hydroxide composite membrane for hydrogen filtration and characterization by thermal cycling. Int. J. Hydrog. Energy 2013, 38, 13734–13741. [Google Scholar] [CrossRef]
- Guo, Y.; Jin, Y.; Wu, H.; Zhou, L.; Chen, Q.; Zhang, X.; Li, X. Preparation of palladium membrane on Pd/silicalite-1 zeolite particles modified macroporous alumina substrate for hydrogen separation. Int. J. Hydrog. Energy 2014, 39, 21044–21052. [Google Scholar] [CrossRef]
- Seshimo, M.; Ozawa, M.; Sone, M.; Sakurai, M.; Kameyama, H. Fabrication of a novel Pd/γ-alumina graded membrane by electroless plating on nanoporous γ-alumina. J. Membr. Sci. 2008, 324, 181–187. [Google Scholar] [CrossRef]
- Touyeras, F.; Hihn, J.Y.; Delalande, S.; Viennet, R.; Doche, M.L. Ultrasound influence on the activation step before electroless coating. Ultrason. Sonochem. 2003, 10, 363–368. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Foo, K.Y.; Way, J.D.; Collins, J.P.; Harper-Nixon, D.L. A New Preparation Technique for Pd/Alumina Membranes with Enhanced High-Temperature Stability. Ind. Eng. Chem. Res. 1999, 38, 1925–1936. [Google Scholar] [CrossRef]
- Zhu, B.; Tang, C.H.; Xu, H.Y.; Su, D.S.; Zhang, J.; Li, H. Surface activation inspires high performance of ultra-thin Pd membrane for hydrogen separation. J. Membr. Sci. 2017, 526, 138–146. [Google Scholar] [CrossRef]
- Uemiya, S.; Sato, N.; Ando, H.; Kikuchi, E. The water gas shift reaction assisted by a palladium membrane reactor. Ind. Eng. Chem. Res. 1991, 30, 585–589. [Google Scholar] [CrossRef]
- Shi, Z.; Wu, S.; Szpunar, J.A.; Roshd, M. An observation of palladium membrane formation on a porous stainless steel substrate by electroless deposition. J. Membr. Sci. 2006, 280, 705–711. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, G.; Yang, W. A modified electroless plating technique for thin dense palladium composite membranes with enhanced stability. J. Membr. Sci. 2008, 314, 226–237. [Google Scholar] [CrossRef]
- Yeung, K.L.; Sebastian, J.M.; Varma, A. Novel preparation of Pd/Vycor composite membranes. Catal. Today 1995, 25, 231–236. [Google Scholar] [CrossRef]
- Souleimanova, R.S.; Mukasyan, A.S.; Varma, A. Effects of osmosis on microstructure of Pd-composite membranes synthesized by electroless plating technique. J. Membr. Sci. 2000, 166, 249–257. [Google Scholar] [CrossRef]
- Li, A.; Liang, W.; Hughes, R. Characterisation and permeation of palladium/stainless steel composite membranes. J. Membr. Sci. 1998, 149, 259–268. [Google Scholar] [CrossRef]
- Tanaka, D.A.P.; Tanco, M.A.L.; Nagase, T.; Okazaki, J.; Wakui, Y.; Mizukami, F.; Suzuki, T.M. Fabrication of hydrogen-permeable composite membranes packed with palladium nanoparticles. Adv. Mater. 2006, 18, 630–632. [Google Scholar] [CrossRef]
- Thoen, P.M.; Roa, F.; Way, J.D. High flux palladium–copper composite membranes for hydrogen separations. Desalination 2006, 193, 224–229. [Google Scholar] [CrossRef]
- Gade, S.K.; Thoen, P.M.; Way, J.D. Unsupported palladium alloy foil membranes fabricated by electroless plating. J. Membr. Sci. 2008, 316, 112–118. [Google Scholar] [CrossRef]
- Chi, Y.-H.; Lin, J.-J.; Lin, Y.-L.; Yang, C.-C.; Huang, J.-H. Influence of the rotation rate of porous stainless steel tubes on electroless palladium deposition. J. Membr. Sci. 2015, 475, 259–265. [Google Scholar] [CrossRef]
- Zeng, G.; Goldbach, A.; Xu, H. Defect sealing in Pd membranes via point plating. J. Membr. Sci. 2009, 328, 6–10. [Google Scholar] [CrossRef]
- Sanz, R.; Calles, J.A.; Ordóñez, S.; Marín, P.; Alique, D.; Furones, L. Modelling and simulation of permeation behaviour on Pd/PSS composite membranes prepared by “pore-plating” method. J. Membr. Sci. 2013, 446, 410–421. [Google Scholar] [CrossRef]
- Hatlevik, Ø.; Gade, S.K.; Keeling, M.K.; Thoen, P.M.; Davidson, A.P.; Way, J.D. Palladium and palladium alloy membranes for hydrogen separation and production: History, fabrication strategies and current performance. Sep. Purif. Technol. 2010, 73, 59–64. [Google Scholar] [CrossRef]
- Abu El Hawa, H.W.; Paglieri, S.N.; Morris, C.C.; Harale, A.; Way, J.D. Identification of thermally stable Pd-alloy composite membranes for high temperature applications. J. Membr. Sci. 2014, 466, 151–160. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Shu, J.; Grandjean, B.P.A.; van Neste, A. Catalytic Palladium-Based Membrane Reactors: A Review. Can. J. Chem. Eng. 1991. [Google Scholar] [CrossRef]
- Lin, W.H.; Chang, H.F. Characterizations of Pd-Ag membrane prepared by sequential electroless deposition. Surf. Coat. Technol. 2005, 194, 157–166. [Google Scholar] [CrossRef]
- Santucci, A.; Borgognoni, F.; Vadrucci, M.; Tosti, S. Testing of dense Pd-Ag tubes: Effect of pressure and membrane thickness on the hydrogen permeability. J. Membr. Sci. 2013, 444, 378–383. [Google Scholar] [CrossRef]
- Medrano, J.A.; Fernandez, E.; Melendez, J.; Parco, M.; Tanaka, D.A.P.; van Sint Annaland, M.; Gallucci, F. Pd-based metallic supported membranes: High-temperature stability and fluidized bed reactor testing. Int. J. Hydrog. Energy 2016, 41, 8706–8718. [Google Scholar] [CrossRef]
- Abu El Hawa, H.W.; Paglieri, S.N.; Morris, C.C.; Harale, A.; Way, J.D. Application of a Pd-Ru composite membrane to hydrogen production in a high temperature membrane reactor. Sep. Purif. Technol. 2015, 147, 388–397. [Google Scholar] [CrossRef]
- Abu El Hawa, H.W.; Lundin, S.-T.B.; Patki, N.S.; Way, J.D. Steam methane reforming in a PdAu membrane reactor: Long-term assessment. Int. J. Hydrog. Energy 2016, 41, 10193–10201. [Google Scholar] [CrossRef]
- Patki, N.S.; Lundin, S.T.; Way, J.D. Rapid annealing of sequentially plated Pd-Au composite membranes using high pressure hydrogen. J. Membr. Sci. 2016, 513, 197–205. [Google Scholar] [CrossRef]
- Morreale, B.D.; Ciocco, M.V.; Howard, B.H.; Killmeyer, R.P.; Cugini, A.V.; Enick, R.M. Effect of hydrogen-sulfide on the hydrogen permeance of palladium–copper alloys at elevated temperatures. J. Membr. Sci. 2004, 241, 219–224. [Google Scholar] [CrossRef]
- Kurokawa, H.; Yakabe, H.; Yasuda, I.; Peters, T.; Bredesen, R. Inhibition effect of CO on hydrogen permeability of Pd-Ag membrane applied in a microchannel module conFiguration. Int. J. Hydrog. Energy 2014, 39, 17201–17209. [Google Scholar] [CrossRef]
- Peters, T.A.; Liron, O.; Tschentscher, R.; Sheintuch, M.; Bredesen, R. Investigation of Pd-based membranes in propane dehydrogenation (PDH) processes. Chem. Eng. J. 2016, 305, 191–200. [Google Scholar] [CrossRef]
- Peters, T.A.; Kaleta, T.; Stange, M.; Bredesen, R. Hydrogen transport through a selection of thin Pd-alloy membranes: Membrane stability, H2S inhibition and flux recovery in hydrogen and simulated WGS mixtures. Catal. Today 2012, 193, 8–19. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Imhoff, C.; Braun, F.; Miller, J.B.; Gellman, A.J.; Cornaglia, L. PdCuAu ternary alloy membranes: Hydrogen permeation properties in the presence of H2S. J. Membr. Sci. 2015, 479, 246–255. [Google Scholar] [CrossRef]
- Braun, F.; Tarditi, A.M.; Miller, J.B.; Cornaglia, L.M. Pd-based binary and ternary alloy membranes: Morphological and perm-selective characterization in the presence of H2S. J. Membr. Sci. 2014, 450, 299–307. [Google Scholar] [CrossRef]
- Bosko, M.L.; Miller, J.B.; Lombardo, E.A.; Gellman, A.J.; Cornaglia, L.M. Surface characterization of Pd-Ag composite membranes after annealing at various temperatures. J. Membr. Sci. 2011, 369, 267–276. [Google Scholar] [CrossRef]
- Gao, H.; Lin, Y.S.; Li, Y.; Zhang, B. Chemical Stability and Its Improvement of Palladium-Based Metallic Membranes. Ind. Eng. Chem. Res. 2004, 43, 6920–6930. [Google Scholar] [CrossRef]
- Okazaki, J.; Ikeda, T.; Tanaka, D.A.P.; Sato, K.; Suzuki, T.M.; Mizukami, F. An investigation of thermal stability of thin palladium-silver alloy membranes for high temperature hydrogen separation. J. Membr. Sci. 2011, 366, 212–219. [Google Scholar] [CrossRef]
- Zhang, K.; Way, J.D. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Gade, S.K.; Payzant, E.A.; Park, H.J.; Thoen, P.M.; Way, J.D. The effects of fabrication and annealing on the structure and hydrogen permeation of Pd-Au binary alloy membranes. J. Membr. Sci. 2009, 340, 227–233. [Google Scholar] [CrossRef]
- Bond, R.A.; Evans, J.; Harris, I.R.; Ross, D.K. Hydrogen isotope separation using palladium alloy membranes. In Proceedings of the 12th Symposium on Fusion Technology, Juelich, Germany, 13–17 September 1982; Elsevier: Amsterdam, The Netherlands, 1983; pp. 537–542. [Google Scholar]
- Evans, J.; Harris, I.R.; Ross, D.K. A proposed method of hydrogen isotope separation using palladium alloy membranes. J. Less Common Met. 1983, 89, 407–414. [Google Scholar] [CrossRef]
- Pérez, P.; Cornaglia, C.A.; Mendes, A.; Madeira, L.M.; Tosti, S. Surface effects and CO/CO2 influence in the H2 permeation through a Pd–Ag membrane: A comprehensive model. Int. J. Hydrog. Energy 2015, 40, 6566–6572. [Google Scholar] [CrossRef]
- Augustine, A.S.; Ma, Y.H.; Kazantzis, N.K. High pressure palladium membrane reactor for the high temperature water-gas shift reaction. Int. J. Hydrog. Energy 2011, 36, 5350–5360. [Google Scholar] [CrossRef]
- Bhandari, R.; Ma, Y.H. Pd-Ag membrane synthesis: The electroless and electro-plating conditions and their effect on the deposits morphology. J. Membr. Sci. 2009, 334, 50–63. [Google Scholar] [CrossRef]
- Bosko, M.L.; Yepes, D.; Irusta, S.; Eloy, P.; Ruiz, P.; Lombardo, E.A.; Cornaglia, L.M. Characterization of Pd-Ag membranes after exposure to hydrogen flux at high temperatures. J. Membr. Sci. 2007, 306, 56–65. [Google Scholar] [CrossRef]
- Bosko, M.L.; Múnera, J.F.; Lombardo, E.A.; Cornaglia, L.M. Dry reforming of methane in membrane reactors using Pd and Pd-Ag composite membranes on a NaA zeolite modified porous stainless steel support. J. Membr. Sci. 2010, 364, 17–26. [Google Scholar] [CrossRef]
- Bosko, M.L.; Lombardo, E.A.; Cornaglia, L.M. The effect of electroless plating time on the morphology, alloy formation and H2 transport properties of Pd-Ag composite membranes. Int. J. Hydrog. Energy 2011, 36, 4068–4078. [Google Scholar] [CrossRef]
- Huerta, E.O. Corrosión y degradación de materiales, n.d. Comput. Fraud Secur. 2013, 2000, 3. [Google Scholar]
- Roa, F.; Block, M.J.; Way, J.D. The influence of alloy composition on the H2 flux of composite Pd-Cu membranes. Desalination 2002, 147, 411–416. [Google Scholar] [CrossRef]
- Roa, F.; Way, J.D. The effect of air exposure on palladium-copper composite membranes. Appl. Surf. Sci. 2005, 240, 85–104. [Google Scholar] [CrossRef]
- Lu, H.; Zhu, L.; Wang, W.; Yang, W.; Tong, J. Pd and Pd-Ni alloy composite membranes fabricated by electroless plating method on capillary α-Al2O3 substrates. Int. J. Hydrog. Energy 2015, 40, 3548–3556. [Google Scholar] [CrossRef]
- Reboredo, J.C.; Ugolini, A. Quantile causality between gold commodity and gold stock prices. Resour. Policy 2017, 53, 56–63. [Google Scholar] [CrossRef]
- Zhu, H.; Peng, C.; You, W. Quantile behaviour of cointegration between silver and gold prices. Financ. Res. Lett. 2016, 19, 119–125. [Google Scholar] [CrossRef]
- Liu, C.; Hu, Z.; Li, Y.; Liu, S. Forecasting copper prices by decision tree learning. Resour. Policy 2017, 52, 427–434. [Google Scholar] [CrossRef]
- Balcilar, M.; Hammoudeh, S.; Asaba, N.-A.F. A regime-dependent assessment of the information transmission dynamics between oil prices, precious metal prices and exchange rates. Int. Rev. Econ. Financ. 2015, 40, 72–89. [Google Scholar] [CrossRef]
- Sumrunronnasak, S.; Tantayanon, S.; Kiatgamolchai, S. Influence of layer compositions and annealing conditions on complete formation of ternary PdAgCu alloys prepared by sequential electroless and electroplating methods. Mater. Chem. Phys. 2017, 185, 98–103. [Google Scholar] [CrossRef]
- Tarditi, A.M.; Cornaglia, L.M. Novel PdAgCu ternary alloy as promising materials for hydrogen separation membranes: Synthesis and characterization. Surf. Sci. 2011, 605, 62–71. [Google Scholar] [CrossRef]
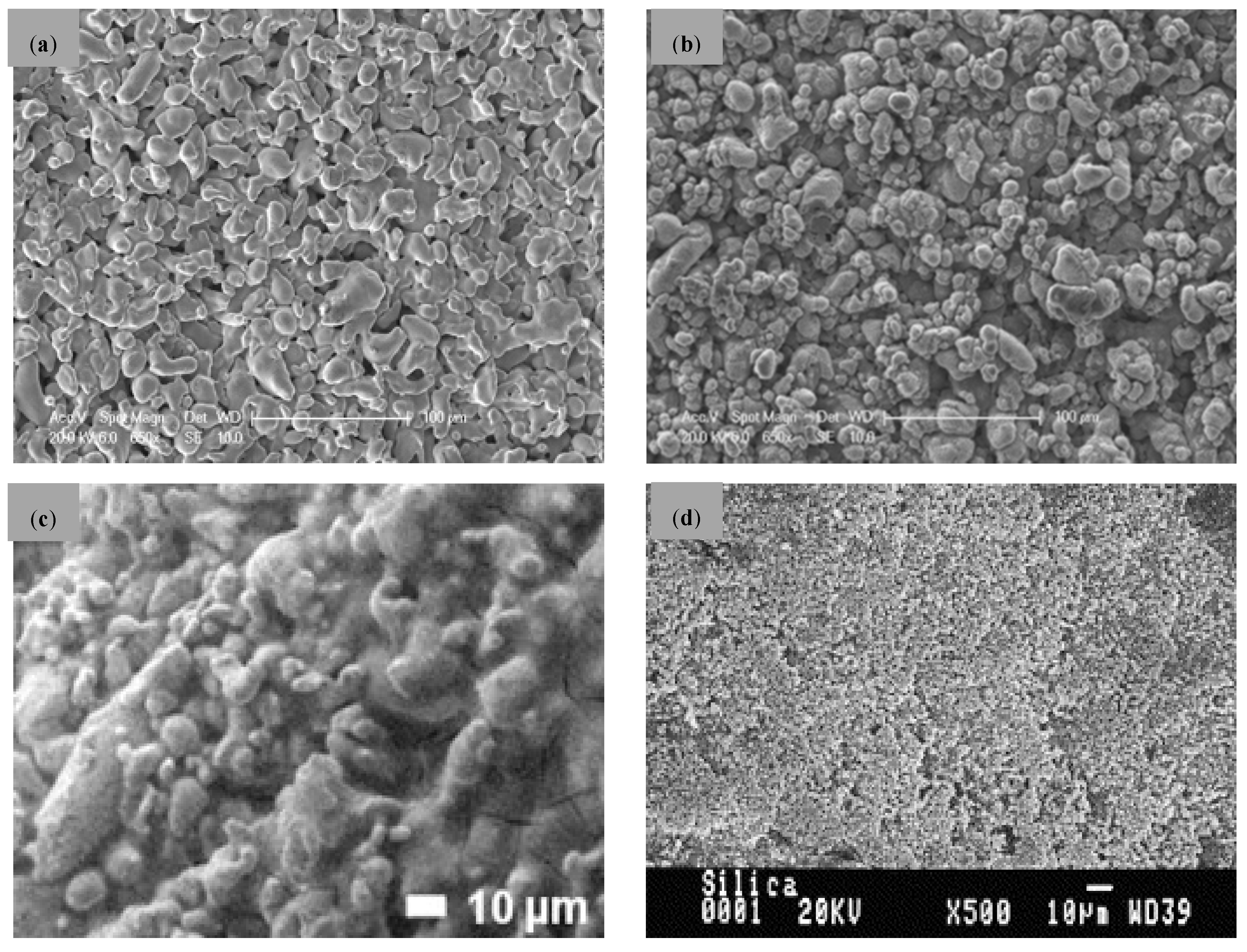
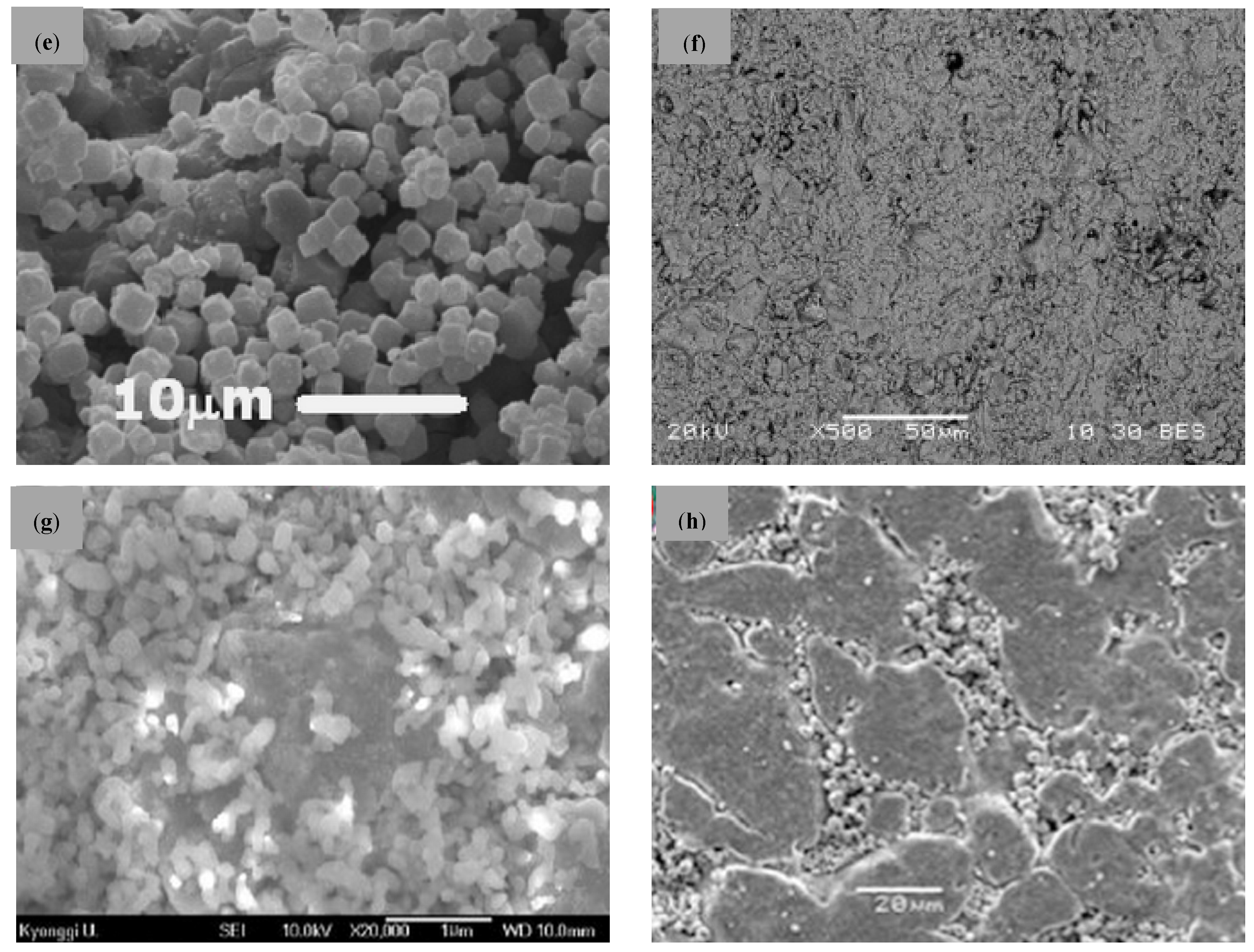

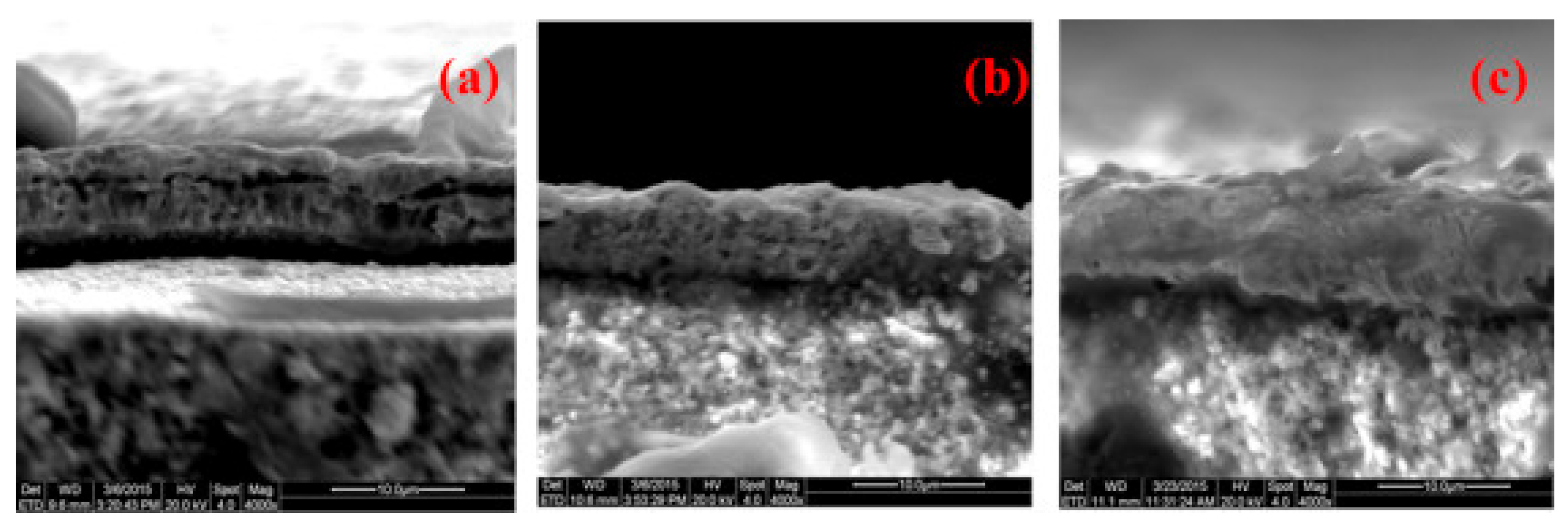
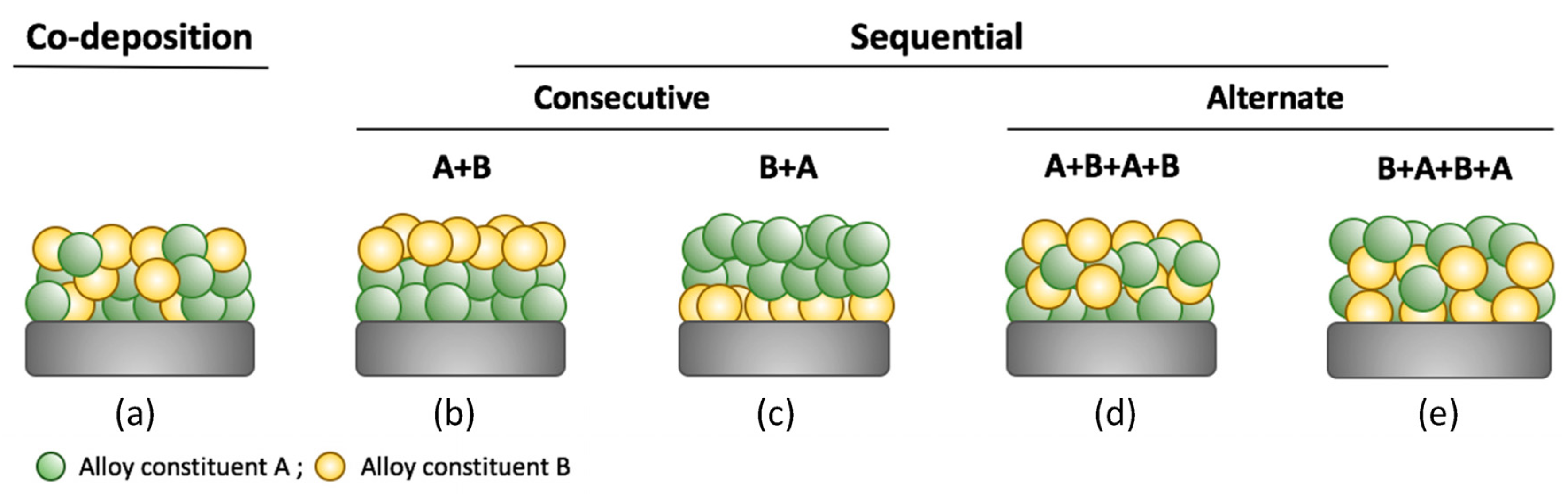
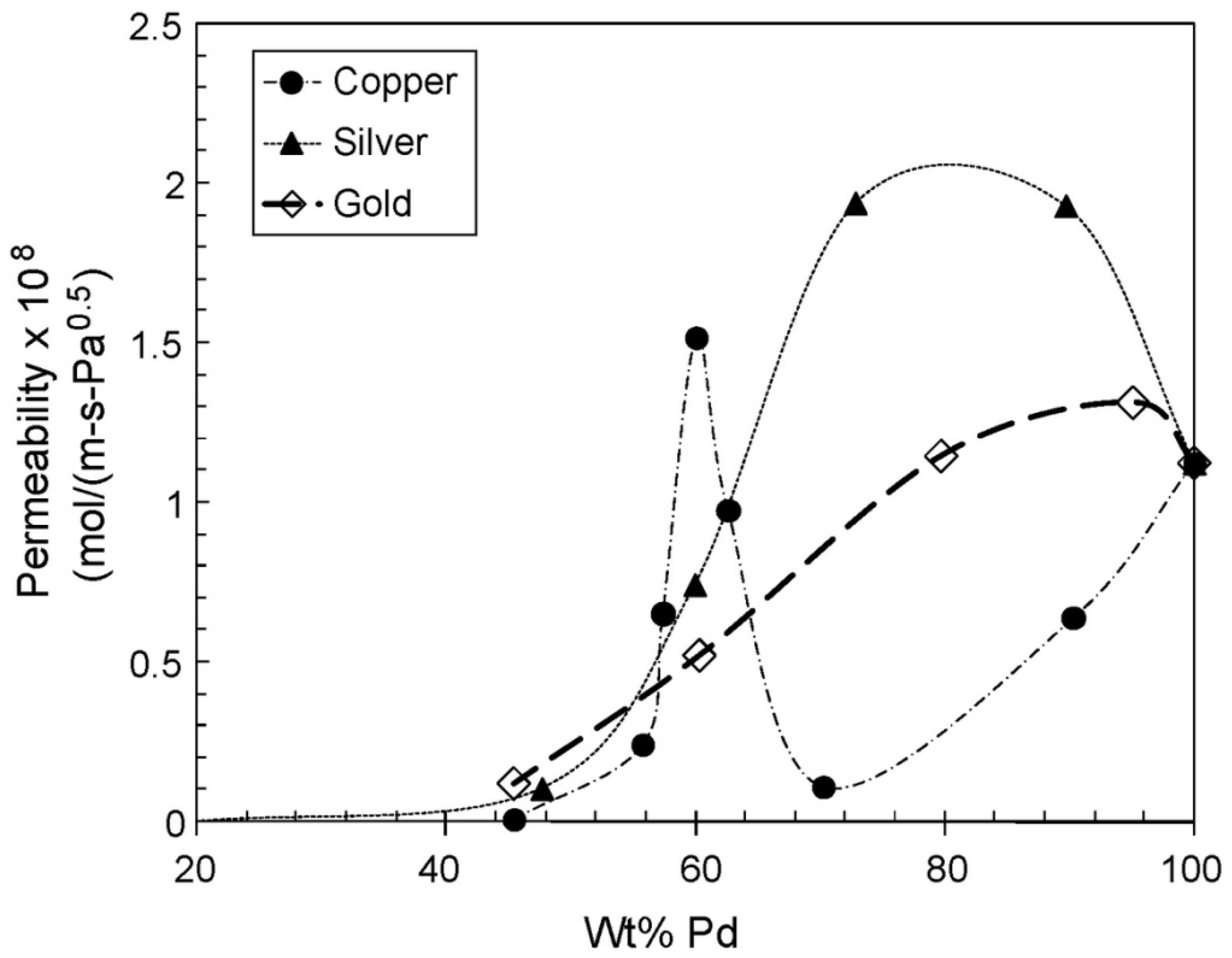
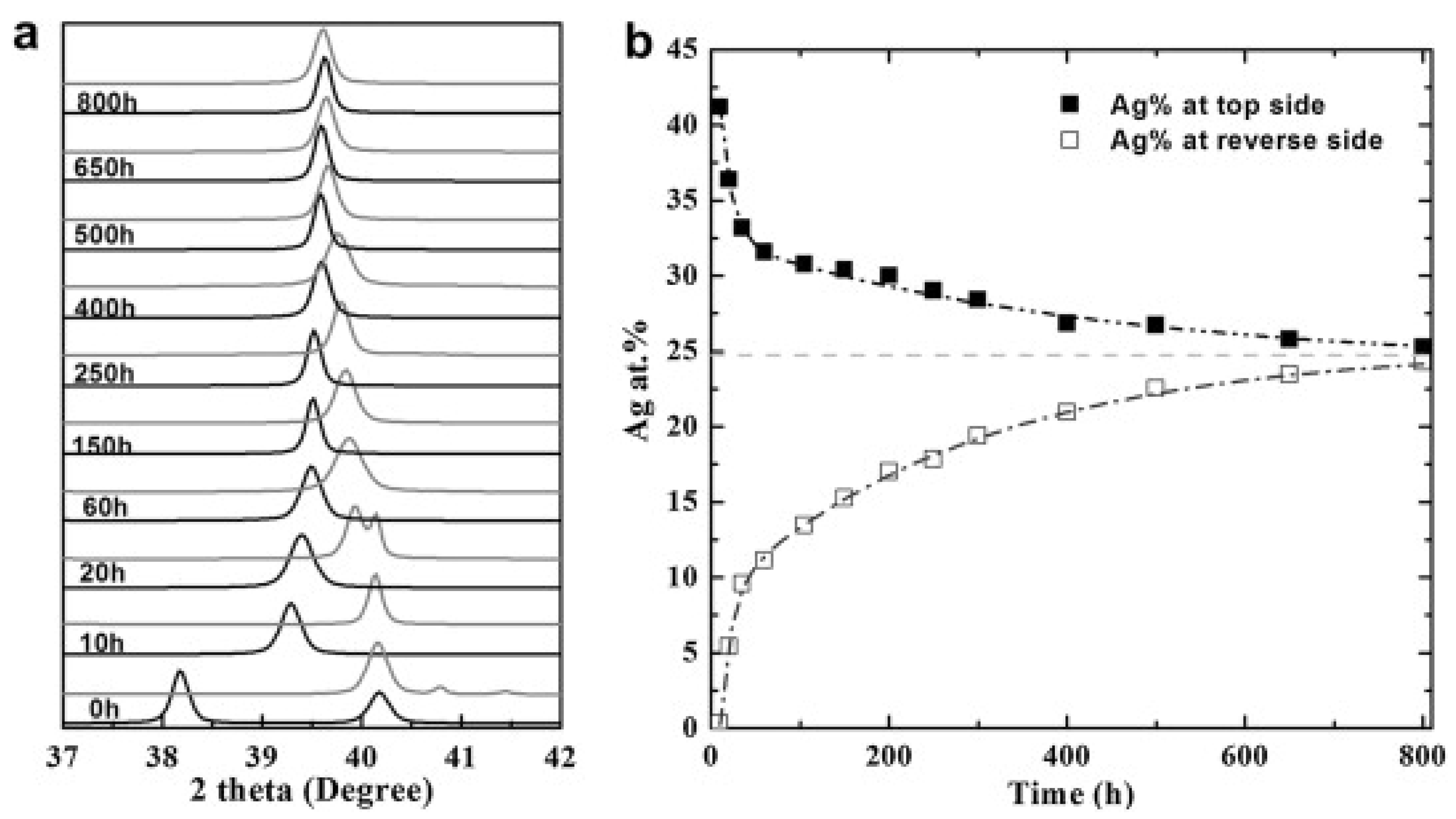
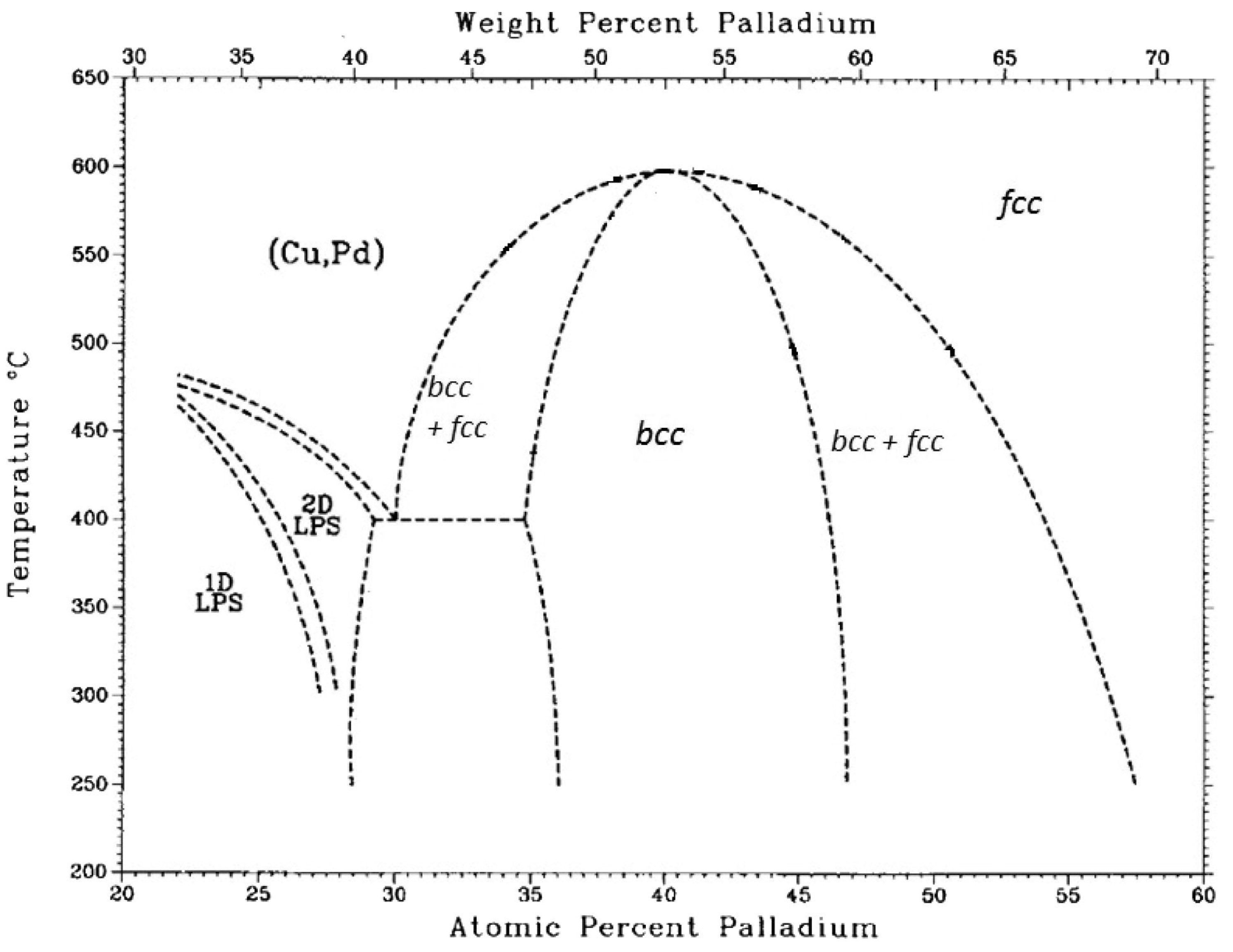
| Company | Material | Geometry | Thickness (mm) | Porosity (%) | Pore Size (nm) |
|---|---|---|---|---|---|
| Mott | Stainless steel: 304 L, 316 L, 310, 347, 430 | Disc, sheet, cup, tube | 1–3 | 0.1–100 × 103 (media grade) | |
| Hastelloy: C-22, C-276, X, N, B, B2 | |||||
| Inconel: 600, 625, 690 | |||||
| GKN | Stainless steel: 304 L, 316 L, 904 L, 310 | Disc, tube | 1.5–3 | 0.1–200 × 103 (media grade) | |
| Hastelloy: C-22, C-276, X | |||||
| Inconel: 600, 625 | |||||
| Monel: 400 | |||||
| Bronze | |||||
| Titanium | |||||
| Pall | Stainless steel: 304 L, 316 L, 310 SC | Cup, tube | - (a) | >0.1 × 103 (a) (media grade) | |
| Hastelloy: X | |||||
| Inconel: 600 | |||||
| Monel: 400 | |||||
| SiC/Al2O3 | |||||
| Mullite | |||||
| Inopor | α-Al2O3 | Tube, multichannel tube | - | 40–55 | 70–800 |
| TiO2 | 40–55 | 100–800 | |||
| 30–55 | 5–30 | ||||
| 30–40 | 1 | ||||
| ZrO2 | 40–55 | 110 | |||
| 30–55 | 3 | ||||
| γ-Al2O3 | 30–55 | 5–10 | |||
| SiO2 | 30–40 | 1 | |||
| Tami | TiO2/ZrO2 | Tube, multichannel tube | 2 | 4.5 × 103 (b) |
| Support | Modification Alternative | Particular Details | Selective Layer | Tselective Layer (m) | Permeation Conditions | Permeation Capacity | H2 Separation Factor | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | P (kPa) | ||||||||
| PSS | Chemical treatment | HCl, 5 min. | Pd | 20.0 | 350 | 100 | 3.11 × 10−4 (a) | 5000 | [110] |
| PSS | Chemical treatment | HCl-HNO3 mixture | Pd | 5.0 | 450–550 | 100 | 3.24 × 10−1–4.34×10−1 (c) | n.a. | [111] |
| Ni | Chemical treatment | HCl | Pd | 0.3 | 450 | 100 | 1.44 × 10−1 (c) | 1600 | [113] |
| Al2O3 | Mechanical treatment | Sandpapers: #320, #500 and #800 | Pd | 0.5 | n.a. | n.a. | n.a. | n.a. | [112] |
| Ni | Mechanical treatment | Sandpapers: #1200 | PdCuNi | 12.0 | 350–500 | 138–276 | 1.30 × 10−7–3.80 × 10−7 (b) | ∞ | [113] |
| PSS | Mechanical treatment | Ion shot penning | Pd | 6.0 | 400 | 100 | 5.80 × 10−2 (c) | n.a. | [116] |
| PSS | Permanent Intermediate layer | CeO2 particles | Pd | 13.0 | 550 | 200 | 2.75 × 10−1 (c) | ∞ | [122] |
| PSS | Permanent Intermediate layer | CeO2, sol-gel | PdCu | 8.0 | 450 | 100 | 74.00 (a) | 2369 | [123] |
| PSS | Permanent Intermediate layer | ZrO2, sol-gel | 10.0 | 500 | 100 | 8.30 × 10−2 (c) | n.a. | [124] | |
| PSS | Permanent Intermediate layer | ZrO2, sol-gel | PdCu | 10.0 | 480 | 100 | 1.10 × 10−7 (b) | ∞ | [125] |
| PSS | Permanent Intermediate layer | ZrO2, sol-gel, vacuum assisted method | PdAu | 10.0 | 400 | 100 | 1.10 × 10−3 (a) | >10,000 | [93] |
| PSS | Permanent Intermediate layer | YSZ particles | Pd | 27.7 | 350–450 | 30–400 | 4.50 × 10−4 (a) | ∞ | [70] |
| PSS | Permanent Intermediate layer | YSZ particles | Pd | 13.8 | 350–450 | 0–250 | 4.10 × 10−5–4.10 × 10−4 (a) | ∞ | [78] |
| Hast X | Permanent Intermediate layer | YSZ–Al2O3/YSZ | PdAg | 4.0–5.0 | 400–600 | 100 | 100.00 × 10−8 (b) | >200,000 | [98] |
| PSS | Permanent Intermediate layer | γ-Al2O3, dip-coating | Pd | 11.0 | n.a. | n.a. | n.a. | n.a. | [130] |
| PSS | Permanent Intermediate layer | Graded Al2O3 particles | Pd | <5.0 | 500 | n.a. | 2.94 × 10−3 (a) | 1124 | [131] |
| PSS | Permanent Intermediate layer | SiO2 particles | PdCu | 2.0 | 450 | n.a. | 8.37 × 10−7 (d) | 70,000 | [132] |
| PSS | Permanent Intermediate layer | Silicalite-1, sol-gel and dip-coating | Pd | 5.0 | 350–450 | 50–250 | 1.42 × 10−4 (a) | ∞ | [62] |
| PSS | Permanent Intermediate layer | Zeolite NaA | Pd | 19.0 | 450 | 50 | 1.10 × 10−3 (a) | 608 | [136] |
| PSS | Permanent Intermediate layer | Zeolite FAU-type | Pd | 1.0 | 200 | 100 | 1.20 × 10−4 (a) | n.a. | [139] |
| Al2O3 | Permanent Intermediate layer | Zeolite TS-1 | Pd | 2.0 | 350–450 | 50–500 | 1.48 × 10−1 (c) | 148 | [141] |
| PSS | Permanent Intermediate layer | Fe2O3-Cr2O3, oxidation in air (T = 600 °C) | Pd | 33.0 | 300 | n.a. | 2.66 × 10−4 (a) | n.a. | [143] |
| PSS | Permanent Intermediate layer | Fe2O3-Cr2O3, oxidation in air (T = 600 °C) | Pd | 19.0 | n.a. | n.a. | n.a. | n.a. | [144] |
| PSS | Permanent Intermediate layer | Tungsten particles | PdCu | 5.0–20.0 | n.a. | n.a. | n.a. | n.a. | [47] |
| PSS | Temporary intermediate layer | Aluminum hydroxide gel/polymer | Pd | 5.0 | 600 | 200 | 3.50 × 10−3 (a) | ∞ | [150] |
| Al2O3 | Permanent Intermediate layer | Graphite-Clay (from 2B pencil) | Pd | 5.0 | 450 | 100 | 3.10 × 10−1 (c) | 3700 | [151] |
| Al2O3 | Permanent Intermediate layer | Pd(II)-modified bohamite sol | Pd | 1.0 | 450 | n.a. | 2.23 × 10−2–1.07 (c) | 20–130 | [152] |
| Al2O3 | Permanent Intermediate layer | YSZ particles | Pd | 5.0 | 150–500 | 150–400 | 0.10–0.60 (c) | n.a. | [50] |
| ELP Improvement | Particular Details | Support | Support Modification | Tselective Layer (m) | Permeation Conditions | Permeation Capacity | H2 Separation Factor | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| T (°C) | P (kPa) | ||||||||
| Deposition around pores | Vacuum asisted-deposition | Al2O3 | - | 6.0 | 500 | n.a. | 8.78 × 10−4 (a) | 3000 | [176] |
| Deposition around pores | Vacuum asisted-deposition | Al2O3 | Pd(II)-modified bohamite sol | 1.0 | 450 | n.a. | 2.23 × 10−2–1.07 (b) | 20–130 | [152] |
| Deposition around pores | Osmotic effect with aqueous sucrose solution | Vycor glass | - | 1.6 | n.a. | n.a. | n.a. | n.a. | [177] |
| Deposition around pores | Osmotic effect with aqueous sucrose solution | Vycor glass | - | 2.5 | n.a. | n.a. | n.a. | n.a. | [178] |
| Protecting selective layer | Pore- filled, vacuum asissted-deposition between two ZrO2 layers | Al2O3 | YSZ particles | 5.0 | 150–500 | 150–400 | 0.10–0.60 (b) | n.a. | [50] |
| Reduction of carbon deposits | Free-EDTA baths | Al2O3 | ZrO2 | 1.3 | 365 | 138 | 394.61 (a) | n.a. | [181] |
| Reduction of carbon deposits | Free-EDTA baths | PSS | Al2O3 | 5.0 | 400 | 100 | 3.05·× 10−3 (a) | 500 | [182] |
| Increase film homogeneity | Support rotation | Al2O3 | ZrO2 | 5.0 | 350–450 | 100–400 | 3.00·× 10−3 (a) | >400 | [183] |
| Membrane repairing | Osmotic effect to close defects without thickness increase | PSS | - | 10.0 | 425–475 | 68–136 | 2.00·10−4 (b) | 400–1600 | [179] |
| Membrane repairing | Point plating to close defects without thickness increase | α-Al2O3 | γ-Al2O3 | n.a. | 500 | 100 | 7.20 × 10−1–8.50 × 10−1 (b) | n.a. | [184] |
| Reducing rejected membranes | ELP-PP. Pd-source and reducing agent from opposite sides of support | PSS | Fe2O3-Cr2O3 | 11.0–20.0 | 350–450 | 100–250 | 1.00 × 10−4–6.00 × 10−4 (a) | ∞ | [51] |
| Pd microstructure | Heat treatment at T > 640 °C | PSS | YSZ | 4.9 | 600 | 82 | 2.40 × 10−3 (a) | 200–2000 | [42] |
| Alloy Type | Alloy Composition | ELP Metal Incorporation | Support | Support Modification | Tselective Layer (m) | Annealing | Permeation Conditions | Permeation Capacity | H2 Separation Factor | Sulfur Tolerance | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T (°C) | P (kPa) | |||||||||||
| Binary | Pd75Ag25 | Sequential | Inconel | - | 10.0 | 500 °C, 24 h | 250–500 | 100 | - | 60–436 | - | [211] |
| Binary | Pd75Ag25 | Sequential | PSS | α-Al2O3/γ-Al2O4 | 20.0–26.0 | 500 °C | 450 | 100 | 3.10 × 10−4 (a) | 954 | - | [202] |
| Binary | Pd77Ag23 | Sequential | α-Al2O3/γ-Al2O3 | - | 2.3–2.5 | 500 °C, 800 h in H2 | 500 | 100 | 1.61–1.57 × 10−2 (a) | 3770–5600 | - | [53] |
| Binary | Pd77Ag23 | Co-deposition | Al2O3 | - | 3.2 | 500 °C, 2 h in N2 | 400 | 100 | 3.10 × 10−6 (a) | 8000–10,000 | - | [95] |
| Binary | PdAg | Co-deposition | Hast X | YSZ–Al2O3 | 4.0–5.0 | n.a. | 4–600 | 100 | 100.00 × 10−8 (b) | >200,000 | - | [192] |
| Binary | Pd81Cu19 | Sequential | Al2O3 | - | 5.0 | 500 °C, 48 h in N2 | 400 | 100 | 1.20 × 10−3 (a) | 1194 | Yes (35 ppm) | [54] |
| Binary | Pd60Cu40 | Sequential | α-Al2O3/γ-Al2O3; α-Al2O3/ZrO2 | - | 11.0 | H2 atmosphere | 450 | 345 | 0.80 (b) | 1150 | Yes | [84] |
| Binary | Pd62Cu38 | Sequential | PSS | CeO2 | 8.0 | 480 °C, 6 h in H2 | 450 | 100 | 74.00 (a) | 2369 | Yes | [123] |
| Binary | Pd90Au10 | Sequential, galvanic displacement | PSS | Oxidation in air (700 °C, 12 h) | <15.0 | 500 °C, 48 h in H2 | 3–500 | 100 | 9.35 × 10−4 (a) | ∞ | Yes (54.8 ppm) | [94] |
| Binary | Pd91Au9 | Sequential, galvanic displacement | PSS | ZrO2 | 10.0 | 500 °C in H2 | 400 | 100 | 1.10 × 10−3 (a) | >10,000 | Yes (54.8 ppm) | [93] |
| Binary | PdxNiy | Sequential | α-Al2O3 | - | 7.0 | n.a. | 500 | 20–120 | 2.74 × 10−3 (a) | 640 | - | [218] |
| Binary | Pd98Ru2 | Co-deposition | PSS | YSZ | 6.0 | n.a. | 550 | n.a. | 2.10 × 10−3 (a) | 1860 | - | [187] |
| Binary | P75Pt25 | Co-deposition | PSS | YSZ | 6.0 | n.a. | 550 | n.a. | 1.39 × 10−4 (a) | 1590 | - | [187] |
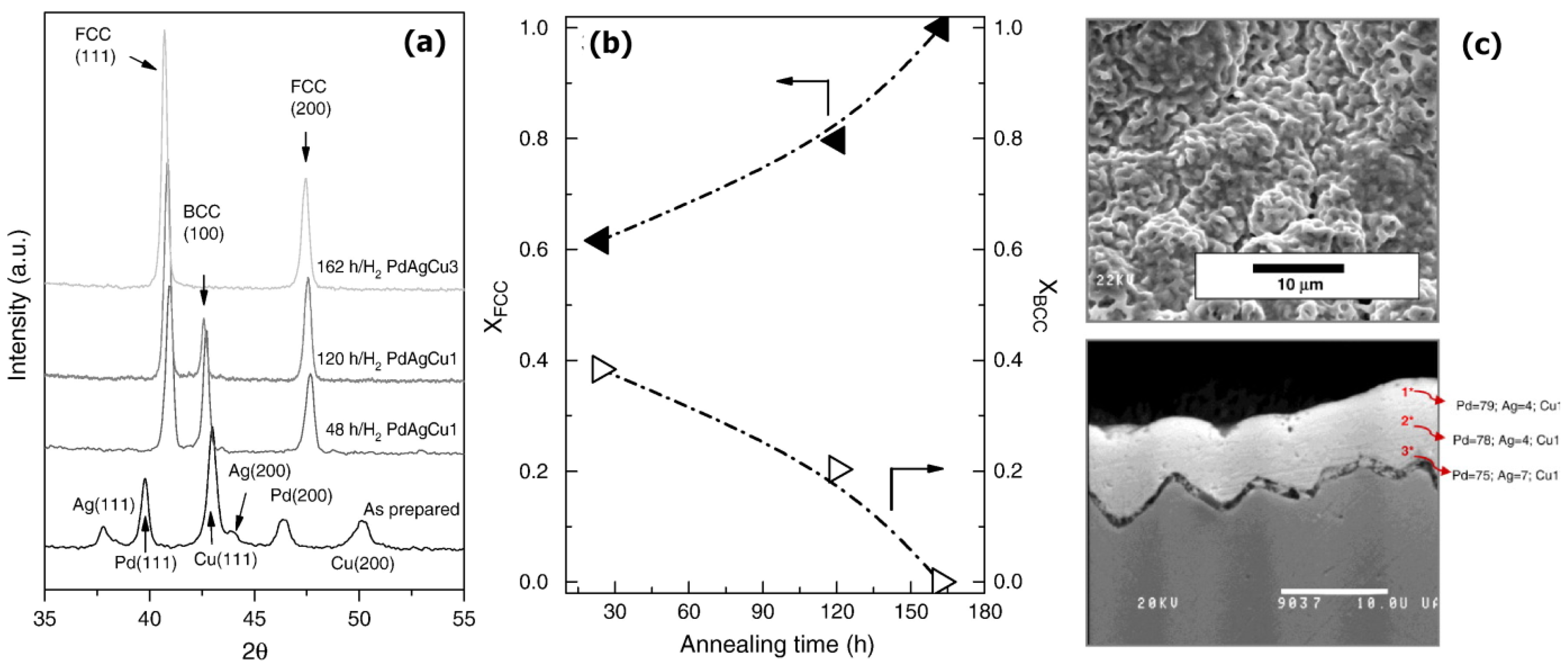
| Ternary | PdxAgyCuz | Sequential | PSS | Oxidation in air (500 °C, 12 h) | 24.0–27.0 | 500 °C, 162 h | 3–450 | 10–100 | 1.70–2.10 × 10−4 (a) | 300–10,000 | n.a. | [224] |
| Ternary | Pd91.7Ag4.8Au3.5 | Co-deposition/Sequential | α-Al2O3/γ-Al2O3 | - | 2.7 | 550 °C, 8 h | 600 | n.a. | 4.71 × 10−3 (a) | n.a. | Yes (9 ppm) | [41] |
| Ternary | Pd91.5Ag4.7Au3.8 | Co-deposition/Sequential | α-Al2O3/γ-Al2O4 | - | 2.7 | 550 °C, 8 h | 600 | n.a. | 2.32 × 10−3 (a) | 4115–793 | Yes (9 ppm) | [41] |
| Ternary | Pd69Au17Cu14 | Sequential | PSS | ZrO2 | 14.0 | 500 °C in H2 | 400 | 50 | 6.20 × 10−4 (a) | n.a. | Yes (100 ppm) | [200] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alique, D.; Martinez-Diaz, D.; Sanz, R.; Calles, J.A. Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production. Membranes 2018, 8, 5. https://doi.org/10.3390/membranes8010005
Alique D, Martinez-Diaz D, Sanz R, Calles JA. Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production. Membranes. 2018; 8(1):5. https://doi.org/10.3390/membranes8010005
Chicago/Turabian StyleAlique, David, David Martinez-Diaz, Raul Sanz, and Jose A. Calles. 2018. "Review of Supported Pd-Based Membranes Preparation by Electroless Plating for Ultra-Pure Hydrogen Production" Membranes 8, no. 1: 5. https://doi.org/10.3390/membranes8010005







