Abstract
The separator membrane is an essential component of lithium-ion batteries, separating the anode and cathode, and controlling the number and mobility of the lithium ions. Among the polymer matrices most commonly investigated for battery separators are poly(vinylidene fluoride) (PVDF) and its copolymers poly(vinylidene fluoride-co-trifluoroethylene) (PVDF-TrFE), poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), and poly(vinylidene fluoride-cochlorotrifluoroethylene) (PVDF-CTFE), due to their excellent properties such as high polarity and the possibility of controlling the porosity of the materials through binary and ternary polymer/solvent systems, among others. This review presents the recent advances on battery separators based on PVDF and its copolymers for lithium-ion batteries. It is divided into the following sections: single polymer and co-polymers, surface modification, composites, and polymer blends. Further, a critical comparison between those membranes and other separator membranes is presented, as well as the future trends on this area.
1. Introduction
In the field of mobile applications, the efficient storage of energy is one of the most critical issues, since there is a fundamental need to maximize the amount of energy stored. This issue can be accomplished by increasing the gravimetric and volumetric energy density of the batteries [1].
The electrochemical lithium ion battery is used to provide power to a large variety of mobile appliances, such as smartphones, tablets, and laptops, as well as an increasing number of sensors and actuators, which will have a fundamental role in the shaping of the Internet of Things and Industry 4.0 concepts, the main trend for current technological evolution [2]. Lithium ion batteries can also power electric and hybrid vehicles, and take part in the management of renewable energy production, being essential in a more sustainable energy paradigm. As some renewable resources, such as solar and wind, are intermittent over time, storing energy for their use during periods of lack of resources is a critical issue for lithium ion batteries [3,4].
Lithium ion batteries are very suitable for the aforementioned applications due to their advantages with respect to other battery types, as they are lighter, cheaper, have a higher energy density (250 Wh·kg−1, 650 Wh·L−1), lower charge lost, no memory effect, a prolonged service-life, and a higher number of charge/discharge cycles [5].
Furthermore, the global market of lithium ion batteries is currently growing, and it is expected that in 2022, the market value will reach $46.21 billion, with an annual growth rate of 10.8% [6].
The first commercial lithium ion battery, which was by Sony, entered the market in 1991, with the fundamental contribution of John Goodenough in the development of LiCoO2 as the active material for the cathode [7].
The main components of a battery are the anode, the cathode, and the separator, which are represented in Figure 1, together with the working principle of a lithium ion battery.
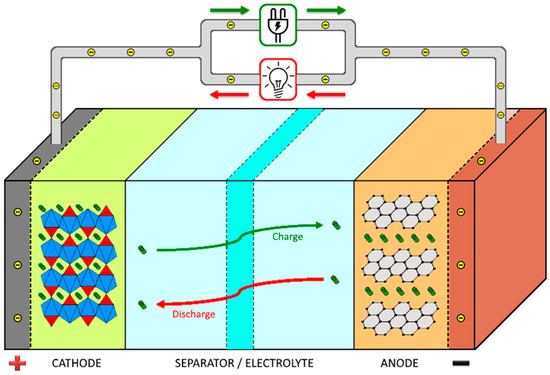
Figure 1.
Schematic representation of a lithium ion battery and its working operation.
During the discharge process of the battery, the cathode acts as an oxidizing element, receiving electrons from the external electric circuit and being reduced. The anode is the reducing element, releasing electrons to the external electrical circuit, being oxidized during the electrochemical reaction [8].
2. Battery Separator: Function, Characteristics, and Types
Separators play a key role in the operation of electrochemical devices. The main purpose of the separator membranes is to separate the cathode from the anode, avoiding the occurrence of short circuits, and controlling to the mobility of lithium ions between electrodes. The performance of a separator in a lithium ion battery is determined by some requirements such as porosity, chemical and thermal stability, electrical insulator, wettability, dimensional stability, and resistance to degradation by chemical reagents and electrolytes (Figure 2) [9]. Figure 2 shows the ideal values for the main requirements of a separator membrane.
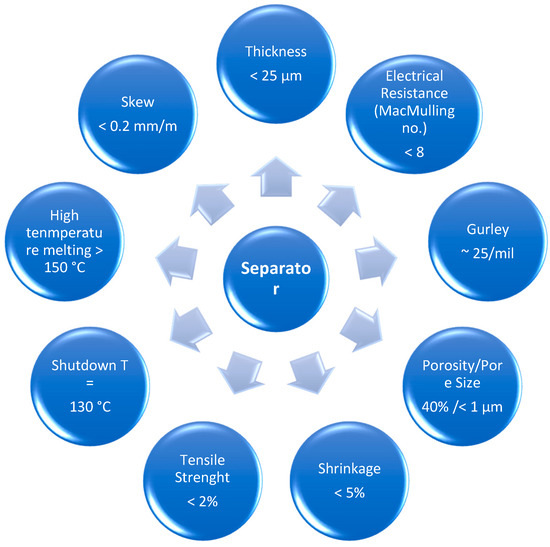
Figure 2.
Ideal values for the main requirements of a separator membrane.
There are different types of separators, but the most widely used consist of a polymer matrix embedded by the electrolyte solution, i.e., a liquid electrolyte where salts are dissolved in solvents, water, or organic molecules. The main types of separators are shown in Table 1 [10].

Table 1.
Types and characteristics of different separators adapted from [10].
The most commonly used materials as matrix for lithium ion battery separators are polymers, or polymer composites. Some of the most commonly used polymers are poly(propylene) (PP), poly(ethylene) (PE), poly(vinylidene fluoride) (PVDF) and its copolymers, poly(ethylene oxide) (PEO), and poly(acrylonitrile) (PAN) [11]. Some separators are developed by blending two different polymers to improve the characteristics of the membrane. In some cases, nanoparticles are added to the matrix as fillers to increase its mechanical stability or ionic conductivity. In composites separators, the most widely used fillers are oxide ceramics (ZrO2 [12,13], Al2O3 [14,15], SiO2 [16,17]), carbonaceous fillers (graphene [18], carbon black [19], carbon nanofiber [20]), and ionic liquids [21], among others.
The solvents must possess some requirements to ensure proper battery operation. The properties of a good solvent are high dielectric constant, low viscosity, high chemical stability, and in liquid form over a wide temperature range. For this application, solvents of ethylene carbonate (EC), propylene carbonate (PC), dimethyl carbonate (DMC), diethyl carbonate (DEC), and ethyl methyl carbonate (EMC) are the most commonly used [11].
3. Poly(vinylidene fluoride) and Its Copolymers
Considering the different polymer matrices used for battery separators, PVDF and its copolymers (poly(vinylidene fluoride-co-trifluoroethylene), poly(vinylidene fluoride-co-trifluoroethylene) (PVDF-TrFE), poly(vinylidene fluoride-co-hexafluoropropylene), poly(vinylidene fluoride-co-hexafluoropropylene) (PVDF-HFP), and poly(vinylidene fluoride-cochlorotrifluoroethylene) (PVDF-CTFE)) show exceptional properties and characteristics for the development of battery separators, highlighting high polarity, excellent thermal and mechanical properties, wettability by organic solvents, being chemically inert and stable in the cathodic environment, and possessing tailorable porosity through binary and ternary solvent/non-solvent systems [22,23]. The main properties of these polymers are presented in Table 2 [11].

Table 2.
Main properties of PVDF and its copolymers [26,27,28].
PVDF and its copolymers are partially fluorinated semi-crystalline polymers where the amorphous phase is located between the crystalline lamellae arranged in spherulites. It can crystallize in different crystalline phase, depending on the temperature and processing conditions [24,25]. In relation to the crystalline phases of PVDF and its copolymers, the most important phases are the β-phase, since it presents ferroelectric, piezoelectric, and pyroelectric properties, and the α-phase, which is the most stable thermodynamically, when material is obtained directly from the melt [24]. As illustrated in Table 2, PVDF and its polymers are characterized by excellent mechanical properties, good thermal stability up to 100 °C, and a high dielectric constant, which is essential for assisting the ionization of lithium salts.
PVDF copolymers have drawn increasing attention for battery separators, as the addition of other monomers to the VDF blocks increases the fluorine content and decreases the degree of crystallinity (Table 2), which is particularly relevant once the uptake of the electrode solution occurs in the amorphous region through a swelling process for accommodating the electrolyte and, as a result, increases the ionic conductivity [29]. The recent literature on PVDF and its battery separator copolymers is structured into four sections dedicated to single polymers, surface modification, composites, and polymer blends, respectively.
The main achievement for PVDF and co-polymers as battery separators was thoroughly reviewed in [11]. Since then, important contributions have been achieved, which are the subject of the present review.
3.1. Single Polymer and Co-Polymers
As already mentioned, one of the main characteristics of PVDF and its co-polymers is their high dielectric permittivity, providing a large affinity with polar electrolytes when compared to other polymers [11]. The main characteristics of the developed PVDF and the copolymer membranes are shown in Table 3.

Table 3.
Separator membranes based on PVDF and co-polymers, indicating also the main properties, and the main goal/achievement of the investigation.
Table 3 shows that the electrospinning technique is widely used to produce functional membranes. Thus, electrospun separators have been developed for PVDF-PDA [31], PVDF-HFP [44], and PVDF-CTFE [47].
For the PVDF-CTFE membrane, the cell assembly considered for the battery performance tests is represented in Figure 3.
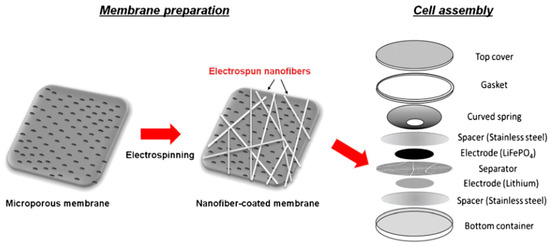
Figure 3.
Manufacturing of a testing cell based on PVDF-CTFE separators [47], with copyright permission from Springer Nature.
For PVDF-HFP electrospun membranes, it has been demonstrated that a single layer membrane shows good porosity and uptake value, but that the mechanical stability is negatively affected, with the viscosity of the solution playing an important role [44]. Also, a novel gel electrolyte was developed based on PVDF-HFP by the addition of disiloxane into the electrolyte solution [42], leading to a thermally stable separator that is not flammable, thus contributing to safer lithium ion batteries [45]. It this sense, ionic liquids have also been used in electrolyte solutions, improving both safety and the ionic conductivity of the membranes [43].
A multistep electrospinning technique for the production of PVDF membranes for electrical double-layer capacitors has been proposed, allowing for the manufacture of thinner and more densely packed separators [30].
Further, membranes have been developed based on PVDF for air-cathode in microbial fuel cells [38] and piezo-supercapacitors [39]. Dual asymmetric PVDF separators were produced by a thermally-induced phase separation method, in which the large and interconnected pores in the bulk structure ensures an improved electrolyte uptake and ionic conductivity, while the small pores in the surfaces prevent the loss of electrolyte and the growth of lithium dendrites. It is indicated that those separators ensure safer batteries with high discharge capacity and longer cycle life [36].
A further step towards the development of more environmentally friendly PVDF separator membranes was proposed by using DMPU as a solvent for PVDF, and IL [C2mim][NTf2] as an electrolyte. The use of the IL increased the ionic conductivity and discharge capacity of the membrane when compared with separators using conventional electrolytes [9].
Porous PVDF-HFP membranes were prepared with non-solvents using the phase inversion technique. When selecting different types of non-solvents such as water, methanol, ethanol, and propanol, and their contents in acetone, it was possible to control the size of the pores (Figure 4) [46].
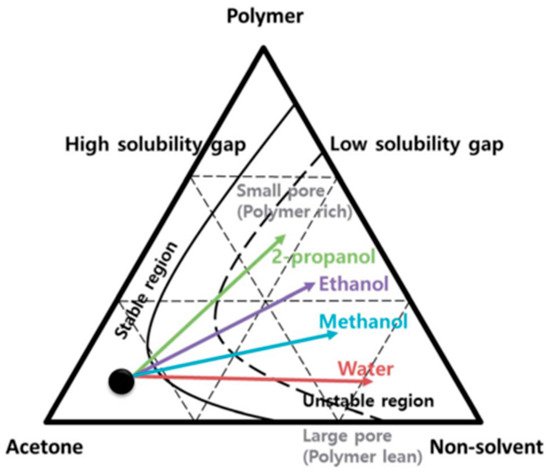
Figure 4.
Phase diagram of the ternary mixture—PVDF–HFP, acetone, and non-solvent—in order to control PVDF-HFP membrane morphology [46], with copyright permission from the Royal Society of Chemistry.
Finally, a correlation between the β-phase content of the separators, and the rate capability and cyclability of the batteries was demonstrated for different PVDF co-polymers, showing that the PVDF-TrFE membrane has the best battery performance for the highest β-phase content (100%) [41].
Thus, it is observed that for single (co)polymer membranes, the main focus is to tailor the morphology to obtain good uptake without mechanical deterioration, and to improve the interaction between the electrolyte solution and the separator membrane.
3.2. Surface Modification of the Separator Membranes
Typically, surface modification of the membranes is carried out to improve specific properties such as wettability, and thermal and mechanical stability. PVDF membranes have been prepared after different surface modifications, but also have been used to modify the properties of other polymer membranes, as presented in Table 4.

Table 4.
Surface modifications on PVDF and co-polymers, indicating also the main properties, goal and achievement.
The most commonly used surface modification is the use of PVDF and its copolymers for the coating of other polymers such as polyethylene porous separators. Thus, the coating of PE with a Al2O3 ceramic layer and a PVDF electrospun nanofiber layer leads to enhanced electrolyte uptake, improved capacity discharge, and cycle life [55]. Similarly, a PDA coating on PVDF improves hydrophilicity, enhancing electrolyte uptake and ionic conductivity of the separator [31].
A typical surface modification technique, such as plasma treatment, allows significant improvement of the electrolyte uptake of PVDF electrospun membranes [48].
A hot-pressing technique was proposed to develop PET/PVDF separators, with improved mechanical behavior properties [52].
The preparation of a PVDF/PMMA/PVDF separator showed great potential for its use in lithium-sulfur batteries, showing high initial discharge capacity and cycle stability, also reducing cell polarization and suppressing the shuttle effect, which is described as the transport of soluble polysulfides between both electrodes and the associated charge [54].
A composite membrane with a PVDF/HEC/PVDF sandwich structure was developed, leading to higher electrolyte uptake, ionic conductivity, and cycling performance. It is also greener and safer because of the fire-retardant behavior of its components [58].
For PVDF-HFP membranes, several coatings have been applied, such as ZrO2 nanoparticles [64], PP polymer [59], PMMA polymer [60], PDA layer [12], and SiO2-modified PET [61], leading mainly to improved electrolyte uptake.
Surface modifications are also achieved by modifying the drying temperature of PVDF-HFP/PET separators prepared by dip-coating, with a drying temperature of 80 °C improving cycle and rate performances with respect to batteries with a conventional PP separator [58].
The dip-coating of a PE separator with γ-Al2O3/PVDF-HFP/TTT, proved to increase electrolyte uptake and ionic conductivity when compared with conventional membranes, as shown in Figure 5 where its microstructure and cycling performance are presented. The discharge performance was also enhanced as well as the thermal resistance [13].
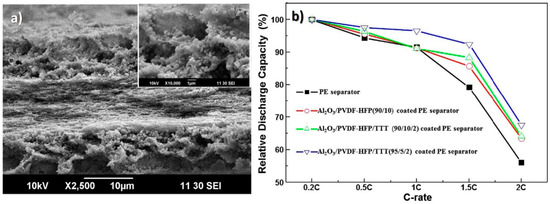
Figure 5.
(a) Cross-section scanning electron microscopy (SEM) images of the γ-Al2O3/PVDF-HFP/TTT(95/5/2)- coated PE separator and (b) relative discharge capacities as a function of the C-rate [13], with copyright permission from Elsevier.
Basically, surface modifications are essential for improve the electrolyte wettability of the separators, and are realized in several polymer membranes of single and multiple layers with many polymers (PP, PET, PMMA, etc.) and filler nanoparticles.
3.3. Composite Membranes
Polymer composites are used to improve battery performance by incorporating suitable fillers, such as oxides ceramic, zeolites, and carbon nanotubes, among others, with the objective of increasing ionic conductivity, mechanical strength, and thermal stability. The main properties of composite separator membranes based on PVDF and its copolymers are presented in Table 5.

Table 5.
Polymer composites based on PVDF and co-polymers with main properties, goal, and achievement.
Several fillers such as n-butanol [90], SiO2 [103], ZnO [86] MgAl2O4 [105], and MMT [107] particles were used with PVDF and its copolymer composites in order to improve thermal and mechanical stability as well as the ionic conductivity value.
Mechanical improvement of separators has been achieved by developing sandwich-type composite separators, by a successive electrospinning method and based on PMIA [79].
The addition of DNA-CTMA in a PVDF matrix allows the development of flexible membranes, with interesting mechanical properties, highlighting its favorable stretch properties, allowing foldable separators with elevated elasticity [71].
The addiction of cellulose nanoparticles in the separator structure proved to significantly increase the mechanical strength of the membrane. It also improves the wettability and induces the β-phase formation in PVDF. However, the presence of NCC reduces the ionic conductivity of the membrane [73].
The use of SiO2 nanoparticles in a PVDF electrospun separator can raise the mechanical strength of the membrane, thus leading to a more tough and durable battery [106].
Improved security operation for lithium ion batteries, due to suitable flammability resistance, has been addressed by developing PVDF/LiPVAOB composites membranes [33].
The direct application of a ceramic suspension of PVDF/Al2O3 in the electrode, resulting in a separator-cathode assembly, enhances the adhesion between these structures, and improves electrochemical cell performance [66].
PVP/PVDF membranes incorporated with carbon black nanoparticles were produced for supercapacitor applications. The separators showed improvements in mechanical properties and dielectric constant values [19].
GPEs based on boron-containing cross-linker proved to have high thermal resistance, maintaining their dimensional stability up to 150 °C, due to their stable PVDF matrix. Also, ionic conductivity and electrochemical stability were improved when compared to commercial separators [68].
Studies on the influence of solvents in nanoclay/PVDF separators showed that using DMAc as a solvent improves the porosity and electrolyte uptake of the membrane when compared with most used solvents such as NMP or DMF. Furthermore, the addition of PVP to the separator structure contributed to increase the pore size and to reduce the degree of crystallinity [72].
The addition of a metal-organic framework to a polymer structure proved to increase the conductivity of the produced membrane without needing an electrolyte. The membrane also showed high durability and good mechanical properties [76].
The dipping of PVDF nanofiber membranes into Al2O3 proved to improve the thermal stability of the produced separator and its ionic conductivity. It also shows a low discharge capacity decay, even at high discharge rates [111].
A double-layer separator was prepared with PVDF and reduced graphene oxide, for lithium-sulfur batteries. It is shown that the two layers combined their properties to enhance the thermal stability of the membrane and the cycling performance of the cells [82].
The use of inorganic fibers as substrate for separators lead to improved thermal and mechanical stability when compared to commercial membranes. It was also proven that it enhanced the electrochemical performance of lithium ion cells [115].
CNF/PVDF composite membranes showed greater performance when applied in Li-S batteries, with enhanced cycling stability. The produced batteries retained a capacity of 768.6 mAhg−1 after 200 cycles at a 0.5 C rate [20]. The development of PVDF-C separators by the phase-inversion method for Li-S batteries also leads to outstanding electrochemical performance results, associated with the presence of the conductive carbon network in the polymer matrix [69].
In the search for more environmental friendly materials, a separator with PVDF, cellulose acetate and Al(HO)3 particles was developed by non-solvent induced phase separation (NIPS), the microstructure being presented in Figure 6a. This membrane exhibited high porosity, electrolyte uptake, and ionic conductivity, as well as good cycling capacity, even at high C-rates, as demonstrated in Figure 6b [70].
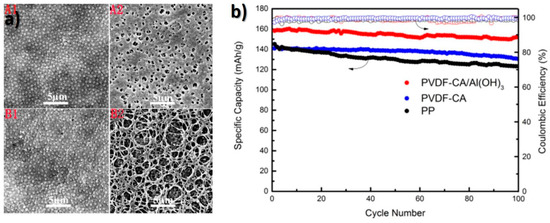
Figure 6.
(a) SEM images of separators microstructure and (b) cycle performance of cells assembled [70], with copyright permission from Elsevier.
PVDF was also used in the study of the potential of zeolitic imidazolate framework-4 in separators. The prepared membranes showed high thermal stability, porosity, ionic conductivity, and cycling performance when compared with conventional separators [120].
The incorporation of Meldrum’s acid groups in the PVDF structure proved to increase the ionic conductivity of the membrane, as well as the cycling performance, in particular at high C-rates [74].
PVDF/PFSA electrospun nanofibers allow the development of membrane with high mechanical stability and ionic conductivity with high discharge capacity and cycling stability [81].
A GPE membrane was developed by blending PVDF with PEO and ZrO2. This membrane showed high electrolyte uptake, and excellent rate performance and discharge capacity for application in lithium-sulfur batteries [88].
Electrospun membranes with Octaphenyl-POSS nanoparticles showed a significant improvement in porosity and electrolyte uptake. For a ratio of 2:100 (w:w), the separator proved to have high mechanical stability, ionic conductivity, and thermal stability [77].
A nonaflate anion-based IL and lithium salt was introduced on a GPE, allowing the development of a membrane with high thermal stability and electrochemical properties. When used alongside with a LiCoO2 cathode, this separator also showed good discharge capacity and capacity of retention [96].
The addition of MgAl2O4 as filler in electrospun fibrous PVDF-HFP separators contributes to improving electrochemical performance, with high discharge capacity and excellent cycle life results [104].
The integration of m-SBA15 as filler in a polymer matrix, on the other hand, is advantageous as it decreases the degree of crystallinity of PVDF-HFP, increasing electrolyte uptake and enhancing the ionic conductivity [109,110].
The enhancement of the electrochemical performance has been extensively addressed by composites membranes with TiO2 nanoparticles [119], and clay nanosheets [95], the latter improving the interfacial areal connection between the polymer structure and clay, facilitating ion transport.
The NaA zeolite is considered to be a very interesting material for incorporation as filler in lithium ion battery separators. It allows the formation of voids in the composite separator structure, which are filled with electrolyte, substantially increasing the ionic conductivity [108].
The safety operation of lithium ion batteries can be upgraded by the addition of metal hydroxides such as Al(OH)3 and Mg(OH)2, in PVDF-HFP composite separators. These metal hydroxides endow a fire-retardant behavior to the cells, due to their natural thermal stability [91].
Kuo et al. synthesized an oligomeric ionic liquid from a phenolic epoxy resin. By blending this ionic liquid with PVDF-HFP, a high performance, non-flammable gel polymer membrane was obtained. This membrane exhibits high ionic conductivity, although with a low liquid electrolyte uptake (<50%) [111].
The addiction of ZrO2 filler increases the porosity, ionic conductivity, and thermal resistance of the PVDF membranes. The presence of polar constituents and high connected interstitial voids facilitate electrolyte absorption, increasing the ionic conductivity and the performance of the membranes [113]. When a layer of ZrO2 was added between two layers of PVDF-HFP, the obtained separator presented even better electrochemical properties [114].
Graphene oxide nanosheets incorporated during the phase inversion of PVDF-HFP improve electrochemical battery performances of the produced separators, as well as thermal stability and the mechanical properties of the membrane [97].
HMSS/PVDF-HFP composite separators with improved porosity were developed; the presence of SiO2 spheres created a well-developed microporous structure, leading to higher wettability and ionic conductivity [98].
The incorporation of a superfine LLTO in a PVDF-HFP separator enhanced the ionic conductivity of the membrane. It was also been shown that a cell with a this type of separator presents improved discharge capacity and rate performance [101].
Bohemite composite separators were produced, exhibiting cycling performances comparable to the conventional ones. These membranes are also safer because of the limitation to Li dendrite formation, preventing the occurrence of short circuits [67].
A comparative study of Al2O3 and NaAlO2 particles in a gel polymer electrolyte proved that NaAlO2 membranes present higher ionic conductivity than Al2O3, as well as improved mechanical properties [14].
ZrO2 membranes with PVDF-HFP as a binder were produced by solvent casting methods. These separators present high porosity and thermal stability, but show lower mechanical strength than commercially available membranes [116].
A GPE produced by thermal crosslinking of PEGDA and PEGMEA proved to be compatible with lithium ion batteries, with a high coulombic efficiency of 94% after 100 cycles [78].
Liu et al. produced a GPE with PVDF-HFP and graphene via NIPS. The addition of a small concentration of graphene (0.002 wt. %) proved to significantly improve the properties of the membrane by increasing porosity, electrolyte uptake, ionic conductivity, and cycling performance, when compared to commercial separators [18].
Regardless of the fillers type used, Table 5 shows that most of the work is devoted to increasing ionic conductivity and electrochemical performance compared to the pure matrix. In particular, inert oxide ceramics (Al2O3, TiO2, SiO2, ZrO2) reduce the degree of crystallinity, and enhance mechanical properties and ionic conductivity value. Carbon materials (CNF, Graphene, rGO) improve safety and interfacial stability between electrodes and separator membranes, and lithium fillers such as Li1,3Al0,3Ti1,7(PO4)3, LiTSFI, and LLTO increase ionic conductivity value of the separators.
In addition, there are other fillers types such as zeolites and clays that are being intensely used for the development of separators, allowing the improvement of electrochemical behavior.
3.4. Polymer Blend Separator Membranes
Finally, another type of separator membrane are polymer blends where two different polymers with complementary properties are used; for example one showing excellent mechanical properties and the other with a hydrophilic character. The main properties of polymer blends based on PVDF and its copolymer are presented in Table 6.

Table 6.
Polymer blends based on PVDF and co-polymers with main properties, goal, and achievement.
PVDF composite separators with methyl cellulose as host of gel polymer electrolyte allows the development of low cost and environmentally friendlier separators with excellent mechanical, thermal, and electrochemical performances [123].
A trilayer porous membrane of PVDF-HFP with PVC as the middle layer was developed. It was shown that a good porosity and uptake value can be achieved, though the mechanical stability was negatively affected [44].
Cells produced with PVDF-NCC separators presented good battery performance at high C-rates, which is very critical for meeting the minimum and maximum power-assist requirements for integration in hybrid electric vehicles [124,125].
A mechanically strengthened electrospun composite PVDF-HFP/PEG/PEGDMA separator was developed. PEG and PEGDMA allow the improvement of the mechanical strength of the composite membrane, which is confirmed by the existence of physical bonded structures [143].
P(MMA-co-PEGMA) and PDMS-g-(PPO-PEO) copolymers within PVDF allow the reduction of the crystallinity of the PVDF matrix, and gently improve the electrolyte uptake, thus leading to an enhanced ionic conductivity [129,136].
PLTB can be successfully used in a PVDF-HFP composite separator. In comparison with a typical PP separator, it is more safe and efficient, due to its thermal and electrochemical stability. This separator is very promising in terms of security operation, because of flame retardant characteristics [144].
An eco-friendly technique to recover cellulose acetate from wasted cigarette filters (Figure 7) was developed, and the material can be integrated in a PVDF/CA membrane for lithium ion batteries, which presents a good performance [140].
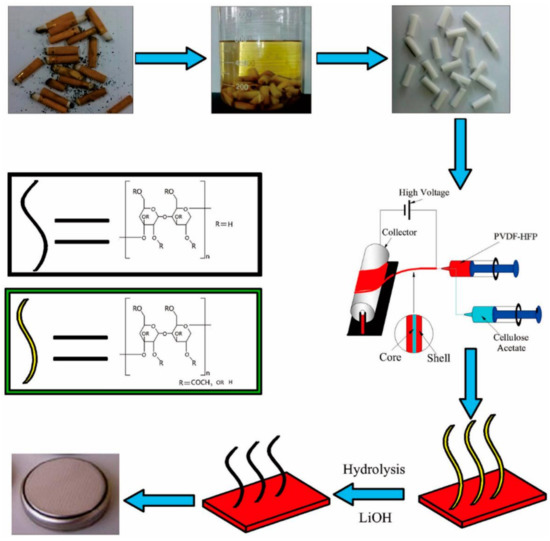
Figure 7.
Preparation of PVDF-HFP/CA nanofiber separators for lithium ion batteries [140], with copyright permission from the American Chemical Society.
PVDF separators were manufactured by a phase inversion technique, with two different cross-linking agents (TAIC and MEP) and with the application of gamma radiation. The produced membranes are characterized by good mechanical behavior and low electrical resistance [34].
Electrospun PVDF membranes blended with PMMA/SiO2 showed good porosity and elevated electrolyte uptake [137]. Blending with PI further enhanced their thermal and mechanical properties, ensuring a better battery performance than commercial PE separators [134].
PVDF/PEO blend membranes show an increase of the ionic conductivity and electrolyte uptake when compared with PVDF membranes. The improved wettability and porosity in x-PEGDA-coated PEI/PVDF membranes has been also reported [147].
PVDF-HFP/HDPE membranes were prepared by non-solvent induced phase separation. This separator presents good cycling performance in lithium ion batteries and a high ionic conductivity [141]. Further studies showed an increased discharge capacity of these membranes, by decreasing the size of the HDPE fillers [121].
PVDF/PAN blend separators were produced by TIPS [126] and electrospinning [127], with improved thermal and mechanical properties. The best PVDF/PAN ratio was 90:10. Despite the lower ionic conductivity when compared with conventional separators, these membranes showed higher cycle and C-rate performance [126].
PVDF/PAN electrospun membrane have excellent dimensional stability even at high temperatures, high electrolyte uptake and ionic conductivity, and superior discharge capacity [127].
The blending of PVDF and PEO in an electrospun membrane proved to increase significantly the electrolyte uptake of the separator, while decreasing the shutdown temperature [132]
Cross-linked PBA/PVDF GPE were prepared by soaking semi-interpenetrating polymer networks with liquid electrolyte. For a PBA/PVDF ratio of 1:0.5, the best results of electrolyte uptake, ionic conductivity, and cycling stability were obtained [128].
A PVDF/PET hybrid separator was produced via a mechanical pressing process. The obtained membrane presented high wettability and electrolyte uptake, while maintaining good thermal stability [133].
The introduction of PANI in a PVDF separator by the breath figure method proved to increase the electrolyte uptake and ionic conductivity of the membrane. The best results were obtained for 30% of PANI, with a uniform pore structure and excellent thermal stability [142].
The use of PVDF-HFP/PVSK membranes in lithium-sulfur batteries has been reported. It has been proved that even small amounts of PVSK (5 wt. %) increase the discharge capacity of the cell and reduce the capacity decay [146].
An increase of the use of natural polymers and biopolymers is observed for the preparation of PVDF and copolymer blends, considering the environmental issues. It is demonstrated in Table 6 that they allow to improve mechanical properties and wettability, and consequently the battery performance. In addition, the use of conductive polymers such as PANI in polymer blends has acquired special attention in recent years, considering that the electrical properties are improved without mechanical deterioration. Typically, the most commonly used PVDF and PVDF-HFP blends are developed with PAN and PEO polymers, allowing the improve thermal and mechanical stability, as well as wettability and ionic conductivity value, respectively.
4. Conclusions and Future Trends
In this review, the latest advances in PVDF-based battery separators for lithium-ion battery applications are presented.
Considering the excellent properties of PVDF and its copolymers as a separation membrane and the importance of the role of the battery separator in battery applications, this review was divided into four different sections—single polymers, surface modification, polymer composites, and blends, where, for each category, the improvement of the main properties of the separators’ degree of porosity, uptake value, mechanical and thermal properties, ionic conductivity, and cycling performance, as well as safety and environmental impacts,- for the different developed materials was presented.
In the single polymer category, PVDF and PVDF-HFP stand out as the most commonly applied polymers produced by various processing techniques, with TIPS and electrospinning methods being the most commonly used to tailor microstructure (degree of porosity and pore size) to improve battery performance.
The number of research papers on surface modifications of the membranes has increased in recent years, as the surface of the polymer membrane strongly affects the uptake process. Surface modification is accomplished by coating hydrophilic polymers or plasma treatment to increase the interaction between the polymer membrane and the electrolytic solution.
Generally, the addition of fillers increases battery performance through the improvement of ionic conductivity in polymer composites, but has not yet demonstrated the best filler for PVDF and its copolymer membranes. The most commonly used fillers are inert oxide ceramics, carbon materials, and lithium fillers. The most improved properties are mechanical properties, interfacial stability, between electrodes and separator membranes and ionic conductivity value, respectively.
In relation to the polymer blends, the appearance of new blends based on natural and conductive polymers within PVDF for battery separators has been observed.
The blends of PVDF and its copolymers widely used are with PAN and PEO polymers, allowing the improvement of mechanical properties and wettability and electric properties, respectively.
The future trends for single polymer separators are to obtain single polymers with a porosity above 50% but a smaller pore size below 500 nm to prevent dendrite growth. Further, it is expected an increase in the use of ionic liquids as electrolytic solution. In relation to surface modifications, the use of poly (ionic liquids) and natural polymers as a surface modification coating of PVDF polymer membranes will be interesting, considering environmental issues.
With respect to polymer composites, future perspectives are related to improving the interaction between polymer matrix and fillers, in order to optimize filler content without decreasing electrical properties or hindering mechanical stability. Also, the use of more than one filler with complementary properties may be the way for improving cycling performance.
The progress with respect to polymer blends is related to the scalability of the fabrication process, increasing the interaction and compatibilization of the two polymers.
In summary, PVDF-based battery separators allow the tailoring of all the properties/characteristics required for a new generation of separator membranes for lithium-ion batteries with high power and excellent cycling performance.
List of Symbols and Abbreviations
| (C2H5)3CH3NBF4 | Triethylmethylammonium tetrafluoroborate |
| [C2mim][NTf2] | 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| Al(OH)3 | Aluminum hydroxide |
| Al2O3 | Aluminum oxide |
| AlO(OH) | Bohemite |
| AN | Acetonitrile |
| BC | Boron-containing cross-linker |
| CA | Cellulose acetate |
| CMC | carboxymethyl cellulose |
| CNF | Carbon nanofiber |
| DEC | Diethyl carbonate |
| DEM | Diethoxymethane |
| DMAc | Dimethyl acetamide |
| DMC | Dimethyl carbonate |
| DME | 1,2-dimethoxyethane |
| DMF | Dimethyl formamide |
| DMSO | Dimethyl sulfoxide |
| DNA-CTMA | Deoxyribonucleic acid-cetyltrimethylammonium |
| DOL | 1,3-dioxolane |
| EC | Ethylene carbonate |
| EMC | Ethyl methyl carbonate |
| EMImNfO-LiNfO | 1-ethyl-3- methylimidazolium nonafluoro-1-butanesulfonate/lithium nonafluoro-1-butanesulfonate |
| EMITf | 1-ethyl 3-methyl imidazolium trifluoromethane sulfonate |
| EMITFSI | 1-ethyl-3-methyl-imidazolium bis(trifluoromethanesulfonyl)imide |
| EP | Ethyl propionate |
| Et4N-BF4 | Tetraethylammonium tetrafluoroboratein |
| GF | Glass fiber |
| GO | Graphene oxides |
| GPE | Gel polymer electrolyte |
| H2SO4 | Sulfuric acid |
| HDPE | High density polyethylene |
| HEC | Hydroxyethyl cellulose |
| HMSS | Hollow mesoporous silica spheres |
| HTPB-g-MPEG | Hydroxyl-terminated polybutadiene-grafted-methoxyl polyethylene glycol |
| KOH | Potassium hydroxide |
| LiClO4 | Lithium percholorate |
| LiCoO2 | Lithium cobalt oxide |
| LiFAP | Lithium Tris(pentafluoroethane)-trifluorophosphate |
| LiNfO/BMImNfO | Lithium nonafluorobutanesulfonate/1-butyl-3-me-thylimidazolium nonafluorobutanesulfonate |
| LiNO3 | Lithium nitrate |
| LiPF6 | Lithium hexafluorophosphate |
| LiPVAOB | Lithium polyvinyl alcohol oxalate borate |
| Li-S | Lithium-sulfur |
| LiTFSI | lithium bis(trifluoromethanesulfonyl)imide |
| LLTO | Li0.33La0.557TiO3 |
| MA | Meldrum’s acid |
| MC | Methyl cellulose |
| MEP | Ethylene oxide-propylene oxide |
| Mg(OH)2 | Magnesium hydroxide |
| MgAl2O4 | Magnesium aluminate |
| MMT | Montmorillonite |
| MOF-808 | Zirconium (IV) metal-organic framework |
| m-SBA 15 | Mesoporous silica |
| NaA | NaA zeolite |
| NaClO4 | Sodium perchlorate |
| NaTf | Sodium trifluoromethane sulfonate |
| NCC | Nanocrystalline cellulose |
| NIPS | Non-solvent induced phase separation |
| NMP | N-methyl-2-pyrrolidone |
| OIL | Oligomeric ionic liquid (bromide bis(tri-fluoromethane)sulfonimide) |
| P(MMA-co-PEGMA) | Poly(methyl methacrylate-co-poly(ethylene glycol) methacrylate) |
| PAN | Polyacrylonitrile |
| PANI | Polyaniline |
| PBA | Poly(butyl acrylate) |
| PC | Propylene carbonate |
| PDA | Polydopamine |
| PDMS-g-(PPO-PEO) | Poly(dimethylsiloxane)-graft-poly(propylene oxide)-block-poly(ethylene oxide) |
| PE | Polyethylene |
| PEG | Polyethylene glycol |
| PEGDA | Poly(ethylene glicol)diacrylate |
| PEGDMA | Polyethylene glycol dimethacrylate |
| PEGMEA | Poly(ethylene glycol) methyl ether acrylate |
| PEI | Polyetherimide |
| PEO | Polyethilene oxide |
| PET | Polyethylene terephthalate |
| PFSA | Perflourosulfonic acid |
| PI | Polyimide |
| PLTB | Polimeric lithium tartaric acid borate |
| PMIA | Poly(m-phenylene isophthalamide) |
| PMMA | Polymethyl methacrylate |
| POSS | Polyhedral oligomeric silsesquioxane |
| PP | Polypropylene |
| P-PAEK | Phenolphthaleyne-poly(aryl ether ketone) |
| PSx-PEO3 | Polysiloxane-comb-propyl(triethylene oxide) |
| PSU | Poly(sulfone) |
| PTFE | Poly(tetrafluoroethylene) |
| PVA | Polyvinyl alcohol |
| PVC | Poly(vinyl chloride) |
| PVDF | Poly(vinylidene fluoride) |
| PVDF-co-CTFE | Polyvinylidene fluoride-co-chlorotrifluoroethylene |
| PVDF-co-HFP | Poly(vinylidene fluoride-co-hexafluoropropylene) |
| PVDF-HFP | Poly(vinylidene fluoride-co-hexafluoropropene)Poly(vinylidene fluoride-hexafluoropropylene) |
| PVDF-PE | Polyvinylidene difluoride-coated polyethylene |
| PVDF-TrFE | Poly(vinylidene fluoride-trifluoroethylene) |
| PVP | Polyvinylpyrrolidone |
| PVSK | Polyvinylsulfate potassium salt |
| rGO | Reduced graphene oxide |
| SCPC | Self-charging power cell |
| SiO2 | Silicon dioxide |
| SN | Succinonitrile |
| SnO2 | Tin oxide |
| TAIC | Triallyl isocyanurate |
| TEABF4 | Tetraethyl ammonium tetrafluoroborate |
| TiO2 | Titanium dioxide |
| TIPS | Thermal-induced phase separation |
| TTT | 1,3,5-trially-1,3,5-triazine-2,4,6(1 H,3 H,5 H)-trione |
| VC | Vinylene carbonate |
| x-PEGDA | x-polyethylene glycol diacrylate |
| ZnO | Zinc oxide |
| ZrO2 | Zirconium dioxide |
Funding
Portuguese Foundation for Science and Technology (FCT): UID/FIS/04650/2013, PTDC/CTM-ENE/5387/2014, UID/CTM/50025/2013, project NO. 28157/02/SAICT/2017 and grants SFRH/BPD/112547/2015 (C.M.C.), including FEDER funds through the COMPETE 2020 programme and National Funds through FCT. Financial support from the Basque Government Industry Department under the ELKARTEK and HAZITEK programs is also acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Megahed, S.; Ebner, W. Lithium-ion battery for electronic applications. J. Power Sources 1995, 54, 155–162. [Google Scholar] [CrossRef]
- Oliveira, J.; Correia, V.; Castro, H.; Martins, P.; Lanceros-Mendez, S. Polymer-based smart materials by printing technologies: Improving application and integration. Add. Manuf. 2018, 21, 269–283. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Park, K.S. The li-ion rechargeable battery: A perspective. JACS 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Lanceros-Méndez, S.; Costa, C.M. Printed Batteries: Materials, Technologies and Applications; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Manthiram, A. An outlook on lithium ion battery technology. ACS Cent. Sci. 2017, 3, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Research, A.M. Lithium-Ion Battery Market—Global Opportunity Analysis and Industry Forecast; Allied Market Research: London, UK, 2016; pp. 2015–2022. [Google Scholar]
- Yoshio, M.; Brodd, R.J.; Kozawa, A. Lithium-Ion Batteries Science and Technologies; Springer: Berlin, Germany, 2009. [Google Scholar]
- Wu, Y.P. Lithium-Ion Batteries Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Costa, C.M.; Rodrigues, H.M.; Gören, A.; Machado, A.V.; Silva, M.M.; Lanceros-Méndez, S. Preparation of poly(vinylidene fluoride) lithium-ion battery separators and their compatibilization with ionic liquid—A green solvent approach. Chem. Select 2017, 2, 5394–5402. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.J. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.M.; Silva, M.M.; Lanceros-Méndez, S. Battery separators based on vinylidene fluoride (vdf) polymers and copolymers for lithium ion battery applications. RSC Adv. 2013, 3, 11404–11417. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Huang, S.; Li, C.; Shen, X.; Zhang, P.; Wu, D.; Sun, D.; Zhao, J. A simple method to prepare a polydopamine modified core-shell structure composite separator for application in high-safety lithium-ion batteries. J. Membr. Sci. 2016, 518, 168–177. [Google Scholar] [CrossRef]
- Nho, Y.C.; Sohn, J.Y.; Shin, J.; Park, J.S.; Lim, Y.M.; Kang, P.H. Preparation of nanocomposite γ-al2o3/polyethylene separator crosslinked by electron beam irradiation for lithium secondary battery. Radiat. Phys. Chem. 2017, 132, 65–70. [Google Scholar] [CrossRef]
- Hashmi, S.A.; Bhat, M.Y.; Singh, M.K.; Sundaram, N.T.K.; Raghupathy, B.P.C.; Tanaka, H. Ionic liquid-based sodium ion-conducting composite gel polymer electrolytes: Effect of active and passive fillers. J. Solid State Electrochem. 2016, 20, 2817–2826. [Google Scholar] [CrossRef]
- Wu, D.; Deng, L.; Sun, Y.; Teh, K.S.; Shi, C.; Tan, Q.; Zhao, J.; Sun, D.; Lin, L. A high-safety pvdf/Al2O3 composite separator for Li-ion batteries via tip-induced electrospinning and dip-coating. RSC Adv. 2017, 7, 24410–24416. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, S.; Sun, D.; Jin, Y. Preparation and evaluation of a separator with an asymmetric structure for lithium-ion batteries. RSC Adv. 2016, 6, 105461–105468. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Gao, H.; Jin, X.; Xie, X. Composite melt-blown nonwoven fabrics with large pore size as li-ion battery separator. Int. J. Hydrog. Energy 2016, 41, 324–330. [Google Scholar] [CrossRef]
- Liu, J.; Wu, X.; He, J.; Li, J.; Lai, Y. Preparation and performance of a novel gel polymer electrolyte based on poly(vinylidene fluoride)/graphene separator for lithium ion battery. Electrochim. Acta 2017, 235, 500–507. [Google Scholar] [CrossRef]
- Jabbarnia, A.; Khan, W.S.; Ghazinezami, A.; Asmatulu, R. Investigating the thermal, mechanical, and electrochemical properties of pvdf/pvp nanofibrous membranes for supercapacitor applications. J. Appl. Polym. Sci. 2016, 133, 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Yang, Y.; Yue, X.; Hao, X.; Sun, W.; Rooney, D.; Sun, K. Flexible carbon nanofiber/polyvinylidene fluoride composite membranes as interlayers in high-performance lithium[sbnd]sulfur batteries. J. Power Sources 2016, 329, 305–313. [Google Scholar] [CrossRef]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Kim, J.F.; Jung, J.T.; Wang, H.H.; Lee, S.Y.; Moore, T.; Sanguineti, A.; Drioli, E.; Lee, Y.M. Microporous pvdf membranes via thermally induced phase separation (tips) and stretching methods. J. Membr. Sci. 2016, 509, 94–104. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Martins, P.; Lopes, A.C.; Lanceros-Mendez, S. Electroactive phases of poly(vinylidene fluoride): Determination, processing and applications. Prog. Polym. Sci. 2014, 39, 683–706. [Google Scholar] [CrossRef]
- Nalwa, H.S. Ferroelectric Polymers: Chemistry: Physics, and Applications; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Sousa, R.E.; Ferreira, J.C.C.; Costa, C.M.; Machado, A.V.; Silva, M.M.; Lanceros-Mendez, S. Tailoring poly(vinylidene fluoride-co-chlorotrifluoroethylene) microstructure and physicochemical properties by exploring its binary phase diagram with dimethylformamide. J. Polym. Sci. Part B Polym. Phys. 2015, 53, 761–773. [Google Scholar] [CrossRef]
- Sousa, R.E.; Nunes-Pereira, J.; Ferreira, J.C.C.; Costa, C.M.; Machado, A.V.; Silva, M.M.; Lanceros-Mendez, S. Microstructural variations of poly(vinylidene fluoride co-hexafluoropropylene) and their influence on the thermal, dielectric and piezoelectric properties. Polym. Test. 2014, 40, 245–255. [Google Scholar] [CrossRef]
- Costa, C.M.; Rodrigues, L.C.; Sencadas, V.; Silva, M.M.; Rocha, J.G.; Lanceros-Méndez, S. Effect of degree of porosity on the properties of poly(vinylidene fluoride–trifluorethylene) for li-ion battery separators. J. Membr. Sci. 2012, 407–408, 193–201. [Google Scholar] [CrossRef]
- Idris, N.H.; Rahman, M.M.; Wang, J.-Z.; Liu, H.-K. Microporous gel polymer electrolytes for lithium rechargeable battery application. J. Power Sources 2012, 201, 294–300. [Google Scholar] [CrossRef]
- Tõnurist, K.; Vaas, I.; Thomberg, T.; Jänes, A.; Kurig, H.; Romann, T.; Lust, E. Application of multistep electrospinning method for preparation of electrical double-layer capacitor half-cells. Electrochim. Acta 2014, 119, 72–77. [Google Scholar] [CrossRef]
- Cao, C.; Tan, L.; Liu, W.; Ma, J.; Li, L. Polydopamine coated electrospun poly(vinyldiene fluoride) nanofibrous membrane as separator for lithium-ion batteries. J. Power Sources 2014, 248, 224–229. [Google Scholar] [CrossRef]
- Saito, Y.; Morimura, W.; Kuratani, R.; Nishikawa, S. Ion transport in separator membranes of lithium secondary batteries. J. Phys. Chem. C 2015, 119, 4702–4708. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, S.; Shi, Y.; Yang, Y.; Hou, Y.; Wu, Y. A composite gel polymer electrolyte with high performance based on poly(vinylidene fluoride) and polyborate for lithium ion batteries. Adv. Energy Mater. 2014, 4, 1300647. [Google Scholar] [CrossRef]
- Karabelli, D.; Leprêtre, J.C.; Dumas, L.; Rouif, S.; Portinha, D.; Fleury, E.; Sanchez, J.Y. Crosslinking of poly(vinylene fluoride) separators by gamma-irradiation for electrochemical high power charge applications. Electrochim. Acta 2015, 169, 32–36. [Google Scholar] [CrossRef]
- Musil, M.; Pléha, D. Nonwoven separators fabrication and analysis methods. ECS Trans. 2015, 70, 127–133. [Google Scholar] [CrossRef]
- Liang, H.Q.; Wan, L.S.; Xu, Z.K. Poly(vinylidene fluoride) separators with dual-asymmetric structure for high-performance lithium ion batteries. Chin. J. Polym. Sci. (Engl. Ed.) 2016, 34, 1423–1435. [Google Scholar] [CrossRef]
- He, H.; Fu, Y.; Zhao, T.; Gao, X.; Xing, L.; Zhang, Y.; Xue, X. All-solid-state flexible self-charging power cell basing on piezo-electrolyte for harvesting/storing body-motion energy and powering wearable electronics. Nano Energy 2017, 39, 590–600. [Google Scholar] [CrossRef]
- Song, J.; Liu, L.; Yang, Q.; Liu, J.; Yu, T.; Yang, F.; Crittenden, J. Pvdf layer as a separator on the solution-side of air-cathodes: The electricity generation, fouling and regeneration. RSC Adv. 2015, 5, 52361–52368. [Google Scholar] [CrossRef]
- Song, R.; Jin, H.; Li, X.; Fei, L.; Zhao, Y.; Huang, H.; Lai-Wa Chan, H.; Wang, Y.; Chai, Y. A rectification-free piezo-supercapacitor with a polyvinylidene fluoride separator and functionalized carbon cloth electrodes. J. Mater. Chem. A 2015, 3, 14963–14970. [Google Scholar] [CrossRef]
- Janakiraman, S.; Surendran, A.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electroactive poly(vinylidene fluoride) fluoride separator for sodium ion battery with high coulombic efficiency. Solid State Ionics 2016, 292, 130–135. [Google Scholar] [CrossRef]
- Kundu, M.; Costa, C.M.; Dias, J.; Maceiras, A.; Vilas, J.L.; Lanceros-Méndez, S. On the relevance of the polar β-phase of poly(vinylidene fluoride) for high performance lithium-ion battery separators. J. Phys. Chem. C 2017, 121, 26216–26225. [Google Scholar] [CrossRef]
- Jeschke, S.; Mutke, M.; Jiang, Z.; Alt, B.; Wiemhofer, H.D. Study of carbamate-modified disiloxane in porous pvdf-hfp membranes: New electrolytes/separators for lithium-ion batteries. Chemphyschem 2014, 15, 1761–1771. [Google Scholar] [CrossRef] [PubMed]
- Karuppasamy, K.; Reddy, P.A.; Srinivas, G.; Tewari, A.; Sharma, R.; Shajan, X.S.; Gupta, D. Electrochemical and cycling performances of novel nonafluorobutanesulfonate (nonaflate) ionic liquid based ternary gel polymer electrolyte membranes for rechargeable lithium ion batteries. J. Membr. Sci. 2016, 514, 350–357. [Google Scholar] [CrossRef]
- Angulakshmi, N.; Stephan, A.M. Electrospun trilayer polymeric membranes as separator for lithium–ion batteries. Electrochim. Acta 2014, 127, 167–172. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Huang, X.; Chen, S.; Wang, G. Honeycomb-like porous gel polymer electrolyte membrane for lithium ion batteries with enhanced safety. Sci. Rep. 2014, 4, 6007. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Choi, Y.; Chung, K.Y.; Park, J.H. Controlled pore evolution during phase inversion from the combinatorial non-solvent approach: Application to battery separators. J. Mater. Chem. A 2016, 4, 9496–9501. [Google Scholar] [CrossRef]
- Lee, H.; Alcoutlabi, M.; Toprakci, O.; Xu, G.; Watson, J.V.; Zhang, X. Preparation and characterization of electrospun nanofiber-coated membrane separators for lithium-ion batteries. J. Solid State Electrochem. 2014, 18, 2451–2458. [Google Scholar] [CrossRef]
- Laurita, R.; Zaccaria, M.; Gherardi, M.; Fabiani, D.; Merlettini, A.; Pollicino, A.; Focarete, M.L.; Colombo, V. Plasma processing of electrospun li-ion battery separators to improve electrolyte uptake. Plasma Process. Polym. 2016, 13, 124–133. [Google Scholar] [CrossRef]
- Fang, L.-F.; Shi, J.; Li, H.; Zhu, B.K.; Zhu, L.P. Construction of porous pvdf coating layer and electrochemical performances of the corresponding modified polyethylene separators for lithium ion batteries. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Kim, C.-S.; Jeong, K.M.; Kim, K.; Yi, C.-W. Effects of capacity ratios between anode and cathode on electrochemical properties for lithium polymer batteries. Electrochim. Acta 2015, 155, 431–436. [Google Scholar] [CrossRef]
- Xu, R.; Huang, X.; Lin, X.; Cao, J.; Yang, J.; Lei, C. The functional aqueous slurry coated separator using polyvinylidene fluoride powder particles for lithium-ion batteries. J. Electroanal. Chem. 2017, 786, 77–85. [Google Scholar] [CrossRef]
- Wu, D.; Huang, S.; Xu, Z.; Xiao, Z.; Shi, C.; Zhao, J.; Zhu, R.; Sun, D.; Lin, L. Polyethylene terephthalate/poly(vinylidene fluoride) composite separator for li-ion battery. J. Phys. D Appl. Phys. 2015, 48, 245304. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Li, M.X.; Chang, Z.; Wang, Y.F.; Gao, J.; Zhu, Y.S.; Wu, Y.P.; Huang, W. A sandwich pvdf/hec/pvdf gel polymer electrolyte for lithium ion battery. Electrochim. Acta 2017, 245, 752–759. [Google Scholar] [CrossRef]
- Yang, W.; Yang, W.; Feng, J.; Ma, Z.; Shao, G. High capacity and cycle stability rechargeable lithium-sulfur batteries by sandwiched gel polymer electrolyte. Electrochim. Acta 2016, 210, 71–78. [Google Scholar] [CrossRef]
- An, M.-Y.; Kim, H.-T.; Chang, D.-R. Multilayered separator based on porous polyethylene layer, Al2O3 layer, and electro-spun pvdf nanofiber layer for lithium batteries. J. Solid State Electrochem. 2014, 18, 1807–1814. [Google Scholar] [CrossRef]
- Wu, D.; Shi, C.; Huang, S.; Qiu, X.; Wang, H.; Zhan, Z.; Zhang, P.; Zhao, J.; Sun, D.; Lin, L. Electrospun nanofibers for sandwiched polyimide/poly (vinylidene fluoride)/polyimide separators with the thermal shutdown function. Electrochim. Acta 2015, 176, 727–734. [Google Scholar] [CrossRef]
- Kim, J.I.; Choi, Y.; Chung, K.Y.; Park, J.H. A structurable gel-polymer electrolyte for sodium ion batteries. Adv. Funct. Mater. 2017, 27, 1–7. [Google Scholar] [CrossRef]
- Li, W.; Li, X.; Xie, X.; Yuan, A.; Xia, B. Effect of drying temperature on a thin pvdf-hfp/pet composite nonwoven separator for lithium-ion batteries. Ionics 2017, 23, 929–935. [Google Scholar] [CrossRef]
- Liu, H.; Dai, Z.; Xu, J.; Guo, B.; He, X. Effect of silica nanoparticles/poly(vinylidene fluoride-hexafluoropropylene) coated layers on the performance of polypropylene separator for lithium-ion batteries. J. Energy Chem. 2014, 23, 582–586. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, S.; Xie, X.; Kretschmer, K.; Huang, X.; Sun, B.; Wang, G. Porous poly(vinylidene fluoride-co-hexafluoropropylene) polymer membrane with sandwich-like architecture for highly safe lithium ion batteries. J. Membr. Sci. 2014, 472, 133–140. [Google Scholar] [CrossRef]
- Wu, Y.S.; Yang, C.C.; Luo, S.P.; Chen, Y.L.; Wei, C.N.; Lue, S.J. Pvdf-hfp/pet/pvdf-hfp composite membrane for lithium-ion power batteries. Int. J. Hydrog. Energy 2017, 42, 6862–6875. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, H.; Lee, T.; Ryou, M.-H.; Lee, Y.M. Synergistic thermal stabilization of ceramic/co-polyimide coated polypropylene separators for lithium-ion batteries. J. Power Sources 2015, 294, 537–544. [Google Scholar] [CrossRef]
- Alcoutlabi, M.; Lee, H.; Zhang, X. Nanofiber-based membrane separators for lithium-ion batteries. MRS Proc. 2015, 1718. [Google Scholar] [CrossRef]
- Kim, K.J.; Kwon, H.K.; Park, M.S.; Yim, T.; Yu, J.S.; Kim, Y.J. Ceramic composite separators coated with moisturized zro2 nanoparticles for improving the electrochemical performance and thermal stability of lithium ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 9337–9343. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, C.; Shi, C.; Yang, C.; Deng, L.; Zhang, W.; Peng, L.; Dai, J.; Wu, D.; Zhang, P.; et al. Core-shell structured ceramic nonwoven separators by atomic layer deposition for safe lithium-ion batteries. Appl. Surf. Sci. 2018, 441, 165–173. [Google Scholar] [CrossRef]
- Xiao, W.; Zhao, L.; Gong, Y.; Wang, S.; Liu, J.; Yan, C. Preparation of high performance lithium-ion batteries with a separator–cathode assembly. RSC Adv. 2015, 5, 34184–34190. [Google Scholar] [CrossRef]
- Holtmann, J.; Schäfer, M.; Niemöller, A.; Winter, M.; Lex-Balducci, A.; Obeidi, S. Boehmite-based ceramic separator for lithium-ion batteries. J. Appl. Electrochem. 2016, 46, 69–76. [Google Scholar] [CrossRef]
- Shim, J.; Lee, J.S.; Lee, J.H.; Kim, H.J.; Lee, J.-C. Gel polymer electrolytes containing anion-trapping boron moieties for lithium-ion battery applications. ACS Appl. Mater. Interfaces 2016, 8, 27740–27752. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Ma, J.; Li, B.; Zuo, Y.; Xia, D. Enhanced cycle performance of lithium-sulfur batteries using a separator modified with a pvdf-c layer. ACS Appl. Mater. Interfaces 2014, 6, 20276–20281. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Liu, J.; He, C.; Li, J.; Wu, X. Composite of polyvinylidene fluoride–cellulose acetate with Al(OH)3 as a separator for high-performance lithium ion battery. J. Membr. Sci. 2017, 541, 661–667. [Google Scholar] [CrossRef]
- Kobayashi, N.; Ouchen, F.; Rau, I.; Kumar, J.; Ouchen, F.; Smarra, D.A.; Subramanyam, G.; Grote, J.G. DNA based electrolyte/separator for lithium battery application. In Nanobiosystems: Processing, Characterization, and Applications VIII; Society of Photo-optical Instrumentation Engineers: Bellingham, WA, USA, 2015; Volume 9557, p. 95570A. [Google Scholar]
- Rahmawati, S.A.; Sulistyaningsih; Putro, A.Z.A.; Widyanto, N.F.; Jumari, A.; Purwanto, A.; Dyartanti, E.R. Preparation and characterization of nanocomposite polymer electrolytes poly(vinylidone fluoride)/nanoclay. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2016; Volume 1710, p. 030053. [Google Scholar]
- Bolloli, M.; Antonelli, C.; Molméret, Y.; Alloin, F.; Iojoiu, C.; Sanchez, J.Y. Nanocomposite poly(vynilidene fluoride)/nanocrystalline cellulose porous membranes as separators for lithium-ion batteries. Electrochim. Acta 2016, 214, 38–48. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Liu, Y.L. Crosslinked electrospun poly(vinylidene difluoride) fiber mat as a matrix of gel polymer electrolyte for fast-charging lithium-ion battery. Electrochim. Acta 2017, 258, 1329–1335. [Google Scholar] [CrossRef]
- Fang, C.; Yang, S.; Zhao, X.; Du, P.; Xiong, J. Electrospun montmorillonite modified poly(vinylidene fluoride) nanocomposite separators for lithium-ion batteries. Mater. Res. Bull. 2016, 79, 1–7. [Google Scholar] [CrossRef]
- Luo, H.B.; Wang, M.; Liu, S.X.; Xue, C.; Tian, Z.F.; Zou, Y.; Ren, X.M. Proton conductance of a superior water-stable metal-organic framework and its composite membrane with poly(vinylidene fluoride). Inorg. Chem. 2017, 56, 4169–4175. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.L.; Jiao, X.N. Preparation and characterization of polyvinylidene fluoride/octaphenyl-polyhedral oligomeric silsesquioxane hybrid lithium-ion battery separators by electrospinning. Solid State Ionics 2017, 310, 134–142. [Google Scholar] [CrossRef]
- Li, H.; Chao, C.Y.; Han, P.L.; Yan, X.R.; Zhang, H.H. Preparation and properties of gel-filled pvdf separators for lithium ion cells. J. Appl. Polym. Sci. 2017, 134, 6–11. [Google Scholar] [CrossRef]
- Zhai, Y.; Wang, N.; Mao, X.; Si, Y.; Yu, J.; Al-Deyab, S.S.; El-Newehy, M.; Ding, B. Sandwich-structured pvdf/pmia/pvdf nanofibrous separators with robust mechanical strength and thermal stability for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14511–14518. [Google Scholar] [CrossRef]
- Xie, M.; Yin, M.; Nie, G.; Wang, J.; Wang, C.; Chao, D.; Liu, X. Poly(aryl ether ketone) composite membrane as a high-performance lithium-ion batteries separator. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2714–2721. [Google Scholar] [CrossRef]
- Meng-Nan, H.; Jiang, Z.-Q.; Li, F.-B.; Yang, H.; Xu, Z.-L. Preparation and characterization of pfsa-pvdf blend nanofiber membrane and its preliminary application investigation. New J. Chem. 2017, 41, 7544–7552. [Google Scholar]
- Zhu, P.; Zhu, J.; Zang, J.; Chen, C.; Lu, Y.; Jiang, M.; Yan, C.; Dirican, M.; Kalai Selvan, R.; Zhang, X. A novel bi-functional double-layer rgo–pvdf/pvdf composite nanofiber membrane separator with enhanced thermal stability and effective polysulfide inhibition for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2017, 5, 15096–15104. [Google Scholar] [CrossRef]
- Yanilmaz, M.; Lu, Y.; Dirican, M.; Fu, K.; Zhang, X. Nanoparticle-on-nanofiber hybrid membrane separators for lithium-ion batteries via combining electrospraying and electrospinning techniques. J. Membr. Sci. 2014, 456, 57–65. [Google Scholar] [CrossRef]
- Zhang, F.; Ma, X.; Cao, C.; Li, J.; Zhu, Y. Poly(vinylidene fluoride)/SiO2 composite membranes prepared by electrospinning and their excellent properties for nonwoven separators for lithium-ion batteries. J. Power Sources 2014, 251, 423–431. [Google Scholar] [CrossRef]
- Zhang, S.; Cao, J.; Shang, Y.; Wang, L.; He, X.; Li, J.; Zhao, P.; Wang, Y. Nanocomposite polymer membrane derived from nano tio2-pmma and glass fiber nonwoven: High thermal endurance and cycle stability in lithium ion battery applications. J. Mater. Chem. A 2015, 3, 17697–17703. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Xie, Y.; Wen, X.; Wang, S.; Kim, S.J.; Song, H.-K.; Wang, Z.L. Highly porous piezoelectric pvdf membrane as effective lithium ion transfer channels for enhanced self-charging power cell. Nano Energy 2015, 14, 77–86. [Google Scholar] [CrossRef]
- Xing, L.; Nie, Y.; Xue, X.; Zhang, Y. Pvdf mesoporous nanostructures as the piezo-separator for a self-charging power cell. Nano Energy 2014, 10, 44–52. [Google Scholar] [CrossRef]
- Gao, S.; Wang, K.; Wang, R.; Jiang, M.; Han, J.; Gu, T.; Cheng, S.; Jiang, K. Poly(vinylidene fluoride)-based hybrid gel polymer electrolytes for additive-free lithium sulfur batteries. J. Mater. Chem. A 2017, 5, 17889–17895. [Google Scholar] [CrossRef]
- Shamshad, A.; Chao, T.; Muhammad, W.; Weiqiang, L.; Zhaohuan, W.; Songhao, W.; Bismark, B.; Jingna, L.; Junaid, A.; Jie, X.; et al. Highly efficient pvdf-hfp/colloidal alumina composite separator for high-temperature lithium-ion batteries. Adv. Mater. Interfaces 2018, 5, 1701147. [Google Scholar]
- Yu, L.; Wang, D.; Zhao, Z.; Han, J.; Zhang, K.; Cui, X.; Xu, Z. Pore-forming technology development of polymer separators for power lithium-ion battery. In Proceedings of Sae-China Congress 2014: Selected Papers; Springer: Berlin, Germany, 2014; pp. 89–95. [Google Scholar]
- Yeon, D.; Lee, Y.; Ryou, M.-H.; Lee, Y.M. New flame-retardant composite separators based on metal hydroxides for lithium-ion batteries. Electrochim. Acta 2015, 157, 282–289. [Google Scholar] [CrossRef]
- Deng, Y.; Song, X.; Ma, Z.; Zhang, X.; Shu, D.; Nan, J. Al2O3/pvdf-hfp-cmc/pe separator prepared using aqueous slurry and post-hot-pressing method for polymer lithium-ion batteries with enhanced safety. Electrochim. Acta 2016, 212, 416–425. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, Y.; Yang, Z.; Gao, T.; Kirsch, D.; Liu, B.; Song, J.; Yang, B.; Hu, L. 3d printed separator for the thermal management of high-performance li metal anodes. Energy Storage Mater. 2018, 12, 197–203. [Google Scholar] [CrossRef]
- Asghar, M.R.; Zhang, Y.; Wu, A.; Yan, X.; Shen, S.; Ke, C.; Zhang, J. Preparation of microporous cellulose/poly(vinylidene fluoride-hexafluoropropylene) membrane for lithium ion batteries by phase inversion method. J. Power Sources 2018, 379, 197–205. [Google Scholar] [CrossRef]
- Kim, M.; Kim, J.K.; Park, J.H. Clay nanosheets in skeletons of controlled phase inversion separators for thermally stable li-ion batteries. Adv. Funct. Mater. 2015, 25, 3399–3404. [Google Scholar] [CrossRef]
- Karuppasamy, K.; Reddy, P.A.; Srinivas, G.; Sharma, R.; Tewari, A.; Kumar, G.H.; Gupta, D. An efficient way to achieve high ionic conductivity and electrochemical stability of safer nonaflate anion-based ionic liquid gel polymer electrolytes (ilgpes) for rechargeable lithium ion batteries. J. Solid State Electrochem. 2017, 21, 1145–1155. [Google Scholar] [CrossRef]
- Choi, Y.; Zhang, K.; Chung, K.Y.; Wang, D.H.; Park, J.H. Pvdf-hfp/exfoliated graphene oxide nanosheet hybrid separators for thermally stable li-ion batteries. RSC Adv. 2016, 6, 80706–80711. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.; Wang, H.; Gong, Y.; Zhao, L.; Liu, J.; Yan, C. Hollow mesoporous silica sphere-embedded composite separator for high-performance lithium-ion battery. J. Solid State Electrochem. 2016, 20, 2847–2855. [Google Scholar] [CrossRef]
- Freitag, A.; Langklotz, U.; Rost, A.; Stamm, M.; Ionov, L. Ionically conductive polymer/ceramic separator for lithium-sulfur batteries. Energy Storage Mater. 2017, 9, 105–111. [Google Scholar] [CrossRef]
- Suleman, M.; Kumar, Y.; Hashmi, S.A. High-rate supercapacitive performance of go/r-go electrodes interfaced with plastic-crystal-based flexible gel polymer electrolyte. Electrochim. Acta 2015, 182, 995–1007. [Google Scholar] [CrossRef]
- Huang, F.; Liu, W.; Li, P.; Ning, J.; Wei, Q. Electrochemical properties of llto/fluoropolymer-shell cellulose-core fibrous membrane for separator of high performance lithium-ion battery. Materials 2016, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Ma, Y.; Liu, J.; Liu, X. Improved performance of pvdf-hfp/pi nanofiber membrane for lithium ion battery separator prepared by a bicomponent cross-electrospinning method. Mater. Lett. 2014, 133, 67–70. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, J.-H.; Choi, E.-S.; Kim, J.H.; Lee, S.-Y. Nanoporous polymer scaffold-embedded nonwoven composite separator membranes for high-rate lithium-ion batteries. RSC Adv. 2014, 4, 54312–54321. [Google Scholar] [CrossRef]
- Padmaraj, O.; Rao, B.N.; Jena, P.; Venkateswarlu, M.; Satyanarayana, N. Electrospun nanocomposite fibrous polymer electrolyte for secondary lithium battery applications. In AIP Conference Proceedings; American Institute of Physics: College Park, MD, USA, 2014; Volume 1591, pp. 1723–1725. [Google Scholar]
- Raja, M.; Angulakshmi, N.; Thomas, S.; Kumar, T.P.; Stephan, A.M. Thin, flexible and thermally stable ceramic membranes as separator for lithium-ion batteries. J. Membr. Sci. 2014, 471, 103–109. [Google Scholar] [CrossRef]
- Zaccaria, M.; Fabiani, D.; Cannucciari, G.; Gualandi, C.; Focarete, M.L.; Arbizzani, C.; De Giorgio, F.; Mastragostino, M. Effect of silica and tin oxide nanoparticles on properties of nanofibrous electrospun separators. J. Electrochem. Soc. 2015, 162, A915–A920. [Google Scholar] [CrossRef]
- Raja, M.; Kumar, T.P.; Sanjeev, G.; Zolin, L.; Gerbaldi, C.; Stephan, A.M. Montmorillonite-based ceramic membranes as novel lithium-ion battery separators. Ionics 2014, 20, 943–948. [Google Scholar] [CrossRef]
- Xiao, W.; Gao, Z.; Wang, S.; Liu, J.; Yan, C. A novel naa-type zeolite-embedded composite separator for lithium-ion battery. Mater. Lett. 2015, 145, 177–179. [Google Scholar] [CrossRef]
- Yang, C.-C.; Lian, Z.-Y. Electrochemical performance of lini1/3co1/3mn1/302 lithium polymer battery based on pvdf-hfp/m-sba15 composite polymer membranes. Ceram. Trans. 2014, 246, 18–202. [Google Scholar]
- Yang, C.-C.; Lian, Z.-Y.; Lin, S.J.; Shih, J.-Y.; Chen, W.-H. Preparation and application of pvdf-hfp composite polymer electrolytes in lini0.5co0.2mn0.3o2 lithium-polymer batteries. Electrochim. Acta 2014, 134, 258–265. [Google Scholar] [CrossRef]
- Kuo, P.L.; Tsao, C.H.; Hsu, C.H.; Chen, S.T.; Hsu, H.M. A new strategy for preparing oligomeric ionic liquid gel polymer electrolytes for high-performance and nonflammable lithium ion batteries. J. Membr. Sci. 2016, 499, 462–469. [Google Scholar] [CrossRef]
- Wang, H.; Gao, H. A sandwich-like composite nonwoven separator for li-ion batteries. Electrochim. Acta 2016, 215, 525–534. [Google Scholar] [CrossRef]
- Xiao, W.; Gong, Y.; Wang, H.; Liu, J.; Yan, C. Organic–inorganic binary nanoparticle-based composite separators for high performance lithium-ion batteries. New J. Chem. 2016, 40, 8778–8785. [Google Scholar] [CrossRef]
- Yu, B.; Zhao, X.M.; Jiao, X.N.; Qi, D.Y. Composite nanofiber membrane for lithium-ion batteries prepared by electrostatic spun/spray deposition. J. Electrochem. Energy Convers. Storage 2016, 13, 1–6. [Google Scholar] [CrossRef]
- Wang, M.; Chen, X.; Wang, H.; Wu, H.; Jin, X.; Huang, C. Improved performances of lithium-ion batteries with a separator based on inorganic fibers. J. Mater. Chem. A 2017, 5, 311–318. [Google Scholar] [CrossRef]
- Suriyakumar, S.; Raja, M.; Angulakshmi, N.; Nahm, K.S.; Stephan, A.M. A flexible zirconium oxide based-ceramic membrane as a separator for lithium-ion batteries. RSC Adv. 2016, 6, 92020–92027. [Google Scholar] [CrossRef]
- Solarajan, A.K.; Murugadoss, V.; Angaiah, S. Dimensional stability and electrochemical behaviour of zro2 incorporated electrospun pvdf-hfp based nanocomposite polymer membrane electrolyte for li-ion capacitors. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, J.; Wu, D.; Zhang, M.; Meng, J.; Ni, P. Development of plasma-treated polypropylene nonwoven-based composites for high-performance lithium-ion battery separators. Electrochim. Acta 2015, 167, 396–403. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Ma, Y.; Yang, W. Improved performance of lithium ion battery separator enabled by co-electrospinnig polyimide/poly(vinylidene fluoride-co-hexafluoropropylene) and the incorporation of tio2-(2-hydroxyethyl methacrylate). J. Power Sources 2015, 273, 1127–1135. [Google Scholar] [CrossRef]
- Dai, M.; Shen, J.; Zhang, J.; Li, G. A novel separator material consisting of zeoliticimidazolate framework-4 (zif-4) and its electrochemical performance for lithium-ions battery. J. Power Sources 2017, 369, 27–34. [Google Scholar] [CrossRef]
- Liu, J.; He, C.; He, J.; Cui, J.; Liu, H.; Wu, X. An enhanced poly(vinylidene fluoride) matrix separator with high density polyethylene for good performance lithium ion batteries. J. Solid State Electrochem. 2017, 21, 919–925. [Google Scholar] [CrossRef]
- Li, H.; Niu, D.-H.; Zhou, H.; Chao, C.-Y.; Wu, L.-J.; Han, P.-L. Preparation and characterization of pvdf separators for lithium ion cells using hydroxyl-terminated polybutadiene grafted methoxyl polyethylene glycol (htpb-g-mpeg) as additive. Appl. Surf. Sci. 2018, 440, 186–192. [Google Scholar] [CrossRef]
- Xiao, S.Y.; Yang, Y.Q.; Li, M.X.; Wang, F.X.; Chang, Z.; Wu, Y.P.; Liu, X. A composite membrane based on a biocompatible cellulose as a host of gel polymer electrolyte for lithium ion batteries. J. Power Sources 2014, 270, 53–58. [Google Scholar] [CrossRef]
- Arbizzani, C.; Colò, F.; De Giorgio, F.; Guidotti, M.; Mastragostino, M.; Alloin, F.; Bolloli, M.; Molméret, Y.; Sanchez, J.Y. A non-conventional fluorinated separator in high-voltage graphite/lini0.4mn1.6o4 cells. J. Power Sources 2014, 246, 299–304. [Google Scholar] [CrossRef]
- Arbizzani, C.; De Giorgio, F.; Mastragostino, M. Characterization tests for plug-in hybrid electric vehicle application of graphite/lini0.4mn1.6o4 cells with two different separators and electrolytes. J. Power Sources 2014, 266, 170–174. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Liang, H.Q.; Gu, L.; Yu, Y.; Huang, Y.Q.; Xu, Z.K. Pvdf/pan blend separators via thermally induced phase separation for lithium ion batteries. Polymer 2016, 107, 54–60. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, M.; Liu, H.; Na, B.; Lv, R.; Wang, B.; Huang, Y. Modification and characterization of electrospun poly (vinylidene fluoride)/poly (acrylonitrile) blend separator membranes. Composites Part B 2017, 112, 31–37. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yang, Q.; Wang, S.; Hu, G.; Xiong, C. Properties of gel polymer electrolytes based on poly(butyl acrylate) semi-interpenetrating polymeric networks toward li-ion batteries. Ionics 2017, 23, 2319–2325. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Liang, Z.-Y.; Chen, Y.-M.; Zhu, B.-K.; Zhu, L.-P. Preparation and properties of poly (vinylidene fluoride)/poly(dimethylsiloxane) graft (poly(propylene oxide)-block-poly(ethylene oxide)) blend porous separators and corresponding electrolytes. Electrochim. Acta 2014, 116, 413–420. [Google Scholar] [CrossRef]
- Kim, K.M.; Poliquit, B.Z.; Lee, Y.-G.; Won, J.; Ko, J.M.; Cho, W.I. Enhanced separator properties by thermal curing of poly(ethylene glycol)diacrylate-based gel polymer electrolytes for lithium-ion batteries. Electrochim. Acta 2014, 120, 159–166. [Google Scholar] [CrossRef]
- La Monaca, A.; Arbizzani, C.; De Giorgio, F.; Focarete, M.L.; Fabiani, D.; Zaccaria, M. Electrospun membranes based on pvdf-peo blends for lithium batteries. ECS Trans. 2016, 73, 75–81. [Google Scholar] [CrossRef]
- Monaca, A.L.; Giorgio, F.D.; Focarete, M.L.; Fabiani, D.; Zaccaria, M.; Arbizzani, C. Polyvinylidene difluoride–polyethyleneoxide blends for electrospun separators in li-ion batteries. J. Electrochem. Soc. 2017, 164, A6431–A6439. [Google Scholar] [CrossRef]
- Zhu, C.; Nagaishi, T.; Shi, J.; Lee, H.; Wong, P.Y.; Sui, J.; Hyodo, K.; Kim, I.S. Enhanced wettability and thermal stability of a novel polyethylene terephthalate-based poly(vinylidene fluoride) nanofiber hybrid membrane for the separator of lithium-ion batteries. ACS Appl. Mater. Interfaces 2017, 9, 26400–26406. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Son, C.W.; Lee, S.; Kim, D.Y.; Park, C.; Eom, K.S.; Fuller, T.F.; Joh, H.I.; Jo, S.M. Multicore-shell nanofiber architecture of polyimide/polyvinylidene fluoride blend for thermal and long-term stability of lithium ion battery separator. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yvonne, T.; Zhang, C.; Zhang, C.; Omollo, E.; Ncube, S. Properties of electrospun pvdf/pmma/ca membrane as lithium based battery separator. Cellulose 2014, 21, 2811–2818. [Google Scholar] [CrossRef]
- Li, H.; Lin, C.-E.; Shi, J.-L.; Ma, X.-T.; Zhu, B.-K.; Zhu, L.-P. Preparation and characterization of safety pvdf/p(mma-co-pegma) active separators by studying the liquid electrolyte distribution in this kind of membrane. Electrochim. Acta 2014, 115, 317–325. [Google Scholar] [CrossRef]
- Wu, X.L.; Lin, J.; Wang, J.Y.; Guo, H. Electrospun pvdf/pmma/sio2 membrane separators for rechargeable lithium-ion batteries. Key Eng. Mater. 2015, 645-646, 1201–1206. [Google Scholar] [CrossRef]
- He, T.; Jia, R.; Lang, X.; Wu, X.; Wang, Y. Preparation and electrochemical performance of pvdf ultrafine porous fiber separator-cum-electrolyte for supercapacitor. J. Electrochem. Soc. 2017, 164, 379–384. [Google Scholar] [CrossRef]
- Gören, A.; Costa, C.M.; Tamaño Machiavello, M.N.; Cíntora-Juárez, D.; Nunes-Pereira, J.; Tirado, J.L.; Silva, M.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S. Effect of the degree of porosity on the performance of poly(vinylidene fluoride-trifluoroethylene)/poly(ethylene oxide) blend membranes for lithium-ion battery separators. Solid State Ionics 2015, 280, 1–9. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Peng, B.; Su, Y.; Jiang, F.; Hsieh, Y.-L.; Wei, Q. Coaxial electrospun cellulose-core fluoropolymer-shell fibrous membrane from recycled cigarette filter as separator for high performance lithium-ion battery. ACS Sustain. Chem. Eng. 2015, 3, 932–940. [Google Scholar] [CrossRef]
- He, J.; Liu, J.; Li, J.; Lai, Y.; Wu, X. Enhanced ionic conductivity and electrochemical capacity of lithium ion battery based on pvdf-hfp/hdpe membrane. Mater. Lett. 2016, 170, 126–129. [Google Scholar] [CrossRef]
- Farooqui, U.R.; Ahmad, A.L.; Hamid, N.A. Effect of polyaniline (pani) on poly(vinylidene fluoride-co-hexaflouro propylene) (pvdf-co-hfp) polymer electrolyte membrane prepared by breath figure method. Polym. Test. 2017, 60, 124–131. [Google Scholar] [CrossRef]
- Kimura, N.; Sakumoto, T.; Mori, Y.; Wei, K.; Kim, B.-S.; Song, K.-H.; Kim, I.-S. Fabrication and characterization of reinforced electrospun poly(vinylidene fluoride-co-hexafluoropropylene) nanofiber membranes. Compos. Sci. Technol. 2014, 92, 120–125. [Google Scholar] [CrossRef]
- Ding, G.; Qin, B.; Liu, Z.; Zhang, J.; Zhang, B.; Hu, P.; Zhang, C.; Xu, G.; Yao, J.; Cui, G. A polyborate coated cellulose composite separator for high performance lithium ion batteries. J. Electrochem. Soc. 2015, 162, A834–A838. [Google Scholar] [CrossRef]
- Seidel, S.M.; Jeschke, S.; Vettikuzha, P.; Wiemhofer, H.D. Pvdf-hfp/ether-modified polysiloxane membranes obtained via airbrush spraying as active separators for application in lithium ion batteries. Chem. Commun. 2015, 51, 12048–12051. [Google Scholar] [CrossRef] [PubMed]
- Freitag, A.; Stamm, M.; Ionov, L. Separator for lithium-sulfur battery based on polymer blend membrane. J. Power Sources 2017, 363, 384–391. [Google Scholar] [CrossRef]
- Zhai, Y.; Xiao, K.; Yu, J.; Ding, B. Closely packed x-poly(ethylene glycol diacrylate) coated polyetherimide/poly(vinylidene fluoride) fiber separators for lithium ion batteries with enhanced thermostability and improved electrolyte wettability. J. Power Sources 2016, 325, 292–300. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).