Effect of a Physical Exercise Program on the Inflammatory Response, Cardiac Functions, Functional Capacity, and Quality of Life in Patients with Sickle Cell Disease
Abstract
1. Introduction
2. Methods
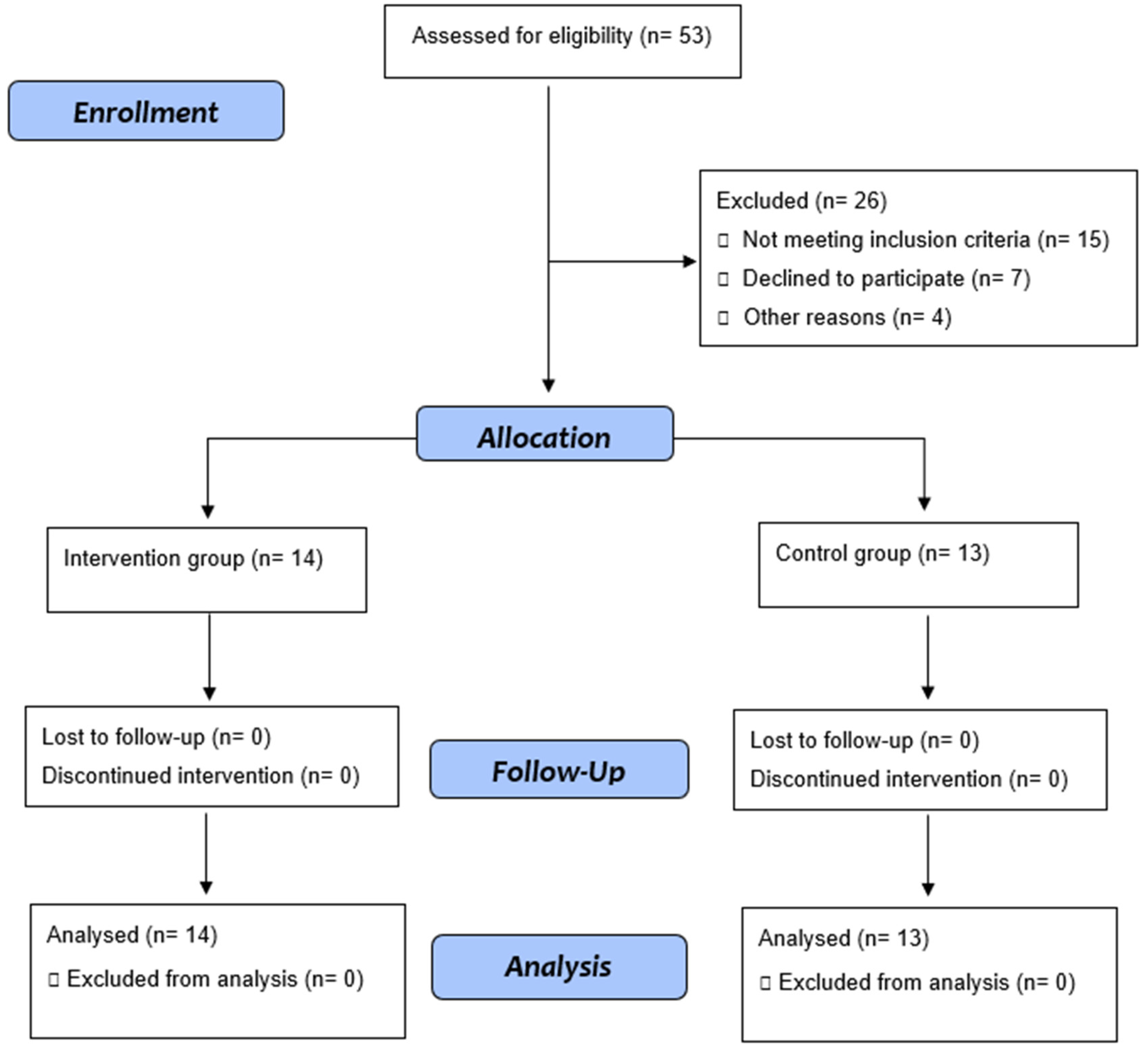
2.1. Study Design, Setting, and Participants
2.2. Interventions
2.2.1. Exercise Group
- (a)
- Initial phase: calisthenics and flexibility exercises, lasting approximately 10 min.
- (b)
- Main phase: aerobic exercise, with walking guidance and gradual increase in intensity and duration, with prescription as follows: In the first two weeks: 35 min of walking between 60% and 70% of maximum heart rate (HR max) achieved in the treadmill test; third and fourth week: 40 min of walking between 60% and 70% of HR max; fifth and sixth week: 40 min of walking between 65% and 75% of HR max; seventh and eighth week: 50 min of walking between 65% and 75% of HR max.
- (c)
- Final phase: relaxation phase, 10–15 min of calisthenics and flexibility exercises. This exercise protocol was based on previous studies and current recommendations that advocate low-intensity activities for individuals with sickle cell anemia, as high-intensity exercises can trigger painful crises. Current recommendations for maintaining health in the general population include moderate-intensity aerobic exercise, 150 min/week, but emphasize that sedentary individuals can benefit from lower volumes and intensities and with individualized progression [30,31]. The physical education teacher monitored activities carried out weekly via telephone.
2.2.2. Control Group
3. Outcomes
3.1. Primary
3.2. Secondary
4. Measurements
4.1. Clinical Evaluation
4.2. Anthropometric Assessment
4.3. Analysis of Inflammatory Biomarkers
4.4. Doppler Echocardiographic Assessment
4.5. Functional Capacity Assessment—Ergometric Test
4.6. Degree of Physical Activity Assessment
4.7. Quality of Life Assessment
4.8. Statistical Methods
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ashley-Koch, A.; Yang, Q.; Olney, R.S. Sickle Hemoglobin (Hb S) Allele and Sickle Cell Disease: A HuGE Review. Am. J. Epidemiol. 2000, 151, 839–845. [Google Scholar] [CrossRef]
- Bunn, H.F. Pathogenesis and Treatment of Sickle Cell Disease. N. Engl. J. Med. 1997, 337, 762–769. [Google Scholar] [CrossRef]
- Roseff, S.D. Sickle cell disease: A review. Immunohematology 2009, 25, 67–74. [Google Scholar] [CrossRef]
- Ware, R.E.; de Montalembert, M.; Tshilolo, L.; Abboud, M.R. Sickle cell disease. Lancet 2017, 390, 311–323. [Google Scholar] [CrossRef]
- Gladwin, M.T.; Sachdev, V. Cardiovascular Abnormalities in Sickle Cell Disease. J. Am. Coll. Cardiol. 2012, 59, 1123–1133. [Google Scholar] [CrossRef]
- Sparkenbaugh, E.; Pawlinski, R. Interplay between coagulation and vascular inflammation in sickle cell disease. Br. J. Haematol. 2013, 162, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Platt, O.S.; Brambilla, D.J.; Rosse, W.F.; Milner, P.F.; Castro, O.; Steinberg, M.H.; Klug, P.P. Mortality In Sickle Cell Disease—Life Expectancy and Risk Factors for Early Death. N. Engl. J. Med. 1994, 330, 1639–1644. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.K.; Ahmed, S. Pulmonary manifestations of sickle cell disease. Postgrad. Med. J. 2003, 79, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Castro, O.; Gladwin, M.T. Pulmonary Hypertension in Sickle Cell Disease: Mechanisms, Diagnosis, and Management. Hematol. Clin. N. Am. 2005, 19, 881–896. [Google Scholar] [CrossRef]
- Chirico, E.N.; Faës, C.; Connes, P.; Canet-Soulas, E.; Martin, C.; Pialoux, V. Role of Exercise-Induced Oxidative Stress in Sickle Cell Trait and Disease. Sports Med. 2015, 46, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Gladwin, M.T. Cardiovascular complications in patients with sickle cell disease. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 423–430. [Google Scholar] [CrossRef]
- Machado, R.F.; Hildesheim, M.; Mendelsohn, L.; Remaley, A.T.; Kato, G.; Gladwin, M.T. NT-pro brain natriuretic peptide levels and the risk of death in the cooperative study of sickle cell disease. Br. J. Haematol. 2011, 154, 512–520. [Google Scholar] [CrossRef]
- Makis, A.C.; Hatzimichael, E.C.; Bourantas, K.L. The role of cytokines in sickle cell disease. Ann. Hematol. 2000, 79, 407–413. [Google Scholar] [CrossRef]
- Sarray, S.; Saleh, L.R.; Saldanha, L.; Al-Habboubi, H.H.; Mahdi, N.; Almawi, W.Y. Serum IL-6, IL-10 and TNF levels in pediatric sickle cell disease patients during vasooclusive crises and steady state condition. Cytokine 2015, 72, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Lanaro, C.; Franco-Penteado, C.F.; Albuquerque, D.M.; Saad, S.T.; Conran, N.; Costa, F.F. Altered levels of Cytokines and inflammatory mediators in plasma and leukocytes of sickle cell anemia patients and effects of hydoxyurea therapy. J. Leukoc. Biol. 2009, 85, 235–242. [Google Scholar] [CrossRef]
- Pierrot-Gallo, B.S.; Vicari, P.; Matsuda, S.S.; Adegoke, S.A.; Mecabo, G.; Figueiredo, M.S. Haptoglobin gene polymorphisms and interleukin-6 and -8 levels in patients with sickle cell anemia. Rev. Bras. Hematol. Hemoter. 2015, 37, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Nader, E.; Romana, M.; Connes, P. The Red Blood Cell—Inflammation Vicious Circle in Sickle Cell Disease. Front. Immunol. 2020, 11, 454. [Google Scholar] [CrossRef]
- Sundd, P.; Gladwin, M.T.; Novelli, E.M. Pathophysiology of Sickle Cell Disease. Annu. Rev. Pathol. 2019, 14, 263–292. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Pialoux, V.; Faes, C.; Charrin, E.; Skinner, S.; Connes, P. Does Physical Activity Increase or Decrease the Risk of Sickle Cell Disease Complications? Br. J. Sports Med. 2018, 52, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Connes, P.; Machado, R.; Hue, O.; Reid, H. Exercise limitation, exercise testing and exercise recommendations in sickle cell anemia. Clin. Hemorheol. Microcirc. 2011, 49, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, C.G.; Saltin, B. Cardiovascular Adaptations to Physical Training. Annu. Rev. Physiol. 1983, 45, 169–189. [Google Scholar] [CrossRef]
- Murias, J.M.; Kowalchuk, J.M.; Paterson, D.H. Time course and mechanisms of adaptations in cardiorespiratory fitness with endurance training in older and young men. J. Appl. Physiol. 2010, 108, 621–627. [Google Scholar] [CrossRef]
- Grau, M.; Nader, E.; Jerke, M.; Schenk, A.; Renoux, C.; Dietz, T.; Collins, B.; Bizjak, D.A.; Joly, P.; Bloch, W.; et al. Impact of A Six Week Training Program on Ventilatory Efficiency, Red Blood Cell Rheological Parameters and Red Blood Cell Nitric Oxide Signaling in Young Sickle Cell Anemia Patients: A Pilot Study. J. Clin. Med. 2019, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- Merlet, A.; Messonnier, L.A.; Coudy-Gandilhon, C.; Bechet, D.; Gellen, B.; Rupp, T.; Galacteros, F.; Bartolucci, P.; Féasson, L. Beneficial effects of endurance exercise training on skeletal muscle microvasculature in sickle cell disease patients. Blood 2019, 134, 2233–2241. [Google Scholar] [CrossRef]
- Pedersen, B.K. Anti-inflammatory effects of exercise: Role in diabetes and cardiovascular disease. Eur. J. Clin. Investig. 2017, 47, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Pedersen, B.K. Exercise as a Mean to Control Low-Grade Systemic Inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef]
- Petersen, A.M.W.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.-M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Précoma, D.B.; De Oliveira, G.M.M.; Simão, A.F.; Dutra, O.P.; Coelho, O.R.; Izar, M.C.D.O.; Póvoa, R.M.D.S.; Giuliano, I.D.C.B.; Filho, A.C.D.A.; Machado, C.A.; et al. Updated Cardiovascular Prevention Guideline of the Brazilian Society of Cardiology—2019. Arq. Bras. Cardiol. 2019, 113, 787–891. [Google Scholar] [CrossRef] [PubMed]
- Garrow, J.S.; Webster, J. Quetelet's index (W/H2) as a measure of fatness. Int. J. Obes. 1985, 9, 147–153. [Google Scholar] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Chairman, A.S.; Chan, D.B.K.; Cujec, B.; Dumesnil, J.G.; Honos, G.; Munt, B. Guidelines for the Provision of Echocardiography in Canada. Recommendations of a Joint Canadian Cardiovascular Society and Canadian Society of Echocardiography Consensus Panel. 2004. Available online: https://ccs.ca/app/uploads/2020/12/Echo_STDP_2004.pdf (accessed on 19 July 2019).
- McInnis, K.J.; Balady, G.J.; Weiner, D.A.; Ryan, T.J. Comparison of ischemic and physiologic responses during exercise tests in men using the standard and modified bruce protocols. Am. J. Cardiol. 1992, 69, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Trabulo, M.; Mendes, M.; Mesquita, A.; Seabra-Gomes, R. Does the modified Bruce protocol induce physiological stress equal to that of the Bruce protocol? Revista Portuguesa de Cardiologia: Orgao Oficial da Sociedade Portuguesa de Cardiologia. Port. J. Cardiol. Off. J. Port. Soc. Cardiol. 1994, 13, 753–760. [Google Scholar]
- McInnis, K.J.; Balady, G.J. Comparison of submaximal exercise responses using the Bruce vs modified Bruce protocols. Med. Sci. Sports Exerc. 1994, 26, 103–107. [Google Scholar] [CrossRef]
- Matsudo, S.; Araújo, T.; Matsudo, V.; Andrade, D.; Andrade, E.; Oliveira, L.C. Questionário Internacional de Atividade Física (IPAQ): Estudo de validade e reprodutibilidade no Brasil. Rev. Bras. Ativ. Fís. Saúde 2001, 6, 5–12. [Google Scholar]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-Item Short Form Health Survey (SF-36). Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Taylor, S.C.; Shacks, S.J.; Mitchell, R.A.; Banks, A. Serum Interleukin-6 Levels in the Steady State of Sickle Cell Disease. J. Interf. Cytokine Res. 1995, 15, 1061–1064. [Google Scholar] [CrossRef]
- Qari, M.H.; Dier, U.; Mousa, S.A. Biomarkers of Inflammation, Growth Factor, and Coagulation Activation in Patients with Sickle Cell Disease. Clin. Appl. Thromb. Hemost. 2011, 18, 195–200. [Google Scholar] [CrossRef]
- Keikhaei, B.; Mohseni, A.R.; Norouzirad, R.; Alinejadi, M.; Ghanbari, S.; Shiravi, F.; Solgi, G. Altered levels of pro-inflammatory cytokines in sickle cell disease patients during vaso-occlusive crises and the steady state condition. Eur. Cytokine Netw. 2013, 24, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Kader, S.M.; Al-Shreef, F.M. Impact of aerobic exercises on selected inflammatory markers and immune system response among patients with sickle cell anemia in asymptomatic steady state. Afr. Health Sci. 2018, 18, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cronin, O.; Keohane, D.; Molloy, M.; Shanahan, F. The effect of exercise interventions on inflammatory biomarkers in healthy, physically inactive subjects: A systematic review. Qjm Int. J. Med. 2017, 110, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Tucker, W.J.; Beaudry, R.I.; Liang, Y.; Clark, A.M.; Tomczak, C.R.; Nelson, M.D.; Ellingsen, O.; Haykowsky, M.J. Meta-analysis of Exercise Training on Left Ventricular Ejection Fraction in Heart Failure with Reduced Ejection Fraction: A 10-year Update. Prog. Cardiovasc. Dis. 2019, 62, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Linke, A.; Schoene, N.; Gielen, S.; Hofer, J.; Erbs, S.; Schuler, G.; Hambrecht, R. Endothelial dysfunction in patients with chronic heart failure: Systemic effects of lower-limb exercise training. J. Am. Coll. Cardiol. 2001, 37, 392–397. [Google Scholar] [CrossRef]
- Barbeau, P.; Woods, K.F.; Ramsey, L.T.; Litaker, M.S.; Pollock, D.M.; Pollock, J.S.; Callahan, L.-A.; Kutlar, A.; Mensah, G.; Gutin, B. Exercise in Sickle Cell Anemia: Effect on Inflammatory and Vasoactive Mediators. Endothelium 2001, 8, 147–155. [Google Scholar] [CrossRef]
- Chatel, B.; Messonnier, L.A.; Bendahan, D. Do we have to consider acidosis induced by exercise as deleterious in sickle cell disease? Exp. Physiol. 2018, 103, 1213–1220. [Google Scholar] [CrossRef]
- Rees, D.C.; Williams, T.N.; Gladwin, M.T. Sickle-cell disease. Lancet 2010, 376, 2018–2031. [Google Scholar] [CrossRef]
- Jesus, A.C.D.S.; Konstantyner, T.; Lôbo, I.K.V.; Braga, J.A.P. Socioeconomic and nutritional characteristics of children and adolescents with sickle cell anemia: A systematic review. Rev. Paul Pediatr. 2018, 36, 491–499. [Google Scholar] [CrossRef]
| Variables. | Control Group (n = 13) | Exercise Group (n = 14) | p |
|---|---|---|---|
| Age (years) | 25 (22.5–36.5) | 27.5 (22.6–36) | 1.0 |
| Female | 5 (38.5%) | 9 (64.3%) | 0.339 |
| Weight (kg) | 57.6 ± 14.5 | 60.0 ± 13.3 | 0.403 |
| SAH, n (%) | 0 | 1 (7.1%) | 1.0 |
| Obesity, n (%) | 0 | 1 (7.1%) | 1.0 |
| Dyspnea, n (%) | 3 (23.1%) | 6 (42.9%) | 0.42 |
| Folic acid, n (%) | 13 (100%) | 12 (85.7%) | 0.48 |
| Hydroxyurea, n (%) | 3 (23.1%) | 5 (35.7%) | 0.678 |
| Variables | Control Group (n = 13) | Exercise Group (n = 14) | p | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M2-M1 | M1 | M2 | M2-M1 | ||
| Hemoglobin (g/dL) | 9.70 (7.50–10.4) | 10.2 (8.65–11.0) | 0.70 (0.42–1.15) | 9.30 (7.95–10.6) | 9.90 (9.05–11.1) | 0.60 (−0.28–1.53) | 0.942 |
| Hematocrit (%) | 30.2 (23.5–31.4) | 29.1 (23.8–35.0) | 0.90 (−1.15–3.50) | 30.5 (24.5–36.0) | 31.1 (25.6–34.4) | 1.00 (−1.60–2.00) | 0.645 |
| Platelets (mil/mm3) | 343 (239–485) | 322 (255–453) | 4.00 (−43.5–29.0) | 493 (388–546) | 464 (385–566) | −0.5 (−35.8–37.8) | 0.923 |
| White blood cells (cells/mm3) | 13,300 (8800–20,350) | 12,500 (9700–15,350) | 0.30 (−1.00–1.05) | 13,250 (11,575–17,175) | 13,350 (9700–17,025) | 0.45 (−0.50–1.60) | 0.451 |
| Variables | Control Group (n = 13) | Exercise Group (n = 14) | p | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M2-M1 | M1 | M2 | M2-M1 | ||
| TNF-alpha (pg/mL) | 9.87 (9.18–11.1) | 10.5 (9.41–10.8) | 0.53 (0.16–0.90) | 9.60 (8.73–10.6) | 10.6 (9.03–11.1) | 1.03 (−1.36–2.12) | 0.482 |
| IL-6 (pg/mL) | 3.81 ± 1.45 | 3.46 ± 0.55 | −0.36 ± 1.23 | 3.53 ± 0.83 | 3.09 ± 0.53 | −0.44 ± 0.80 | 0.829 |
| IL-1 (pg/mL) | 5.29 ± 0.38 | 5.23 ± 0.56 | −0.06 ± 0.49 | 5.23 ± 0.51 | 5.07 ± 0.35 | −0.16 ± 0.58 | 0.656 |
| IL-10 (pg/mL) | 8.88 (6.91–10.2) | 9.13 (7.72–11.2) | 0.36 (−0.66–3.24) | 7.47 (6.86–8.54) | 8.07 (7.58–9.57) | 0.97 (−0.28–2.20) | 0.981 |
| CRP (ng/mL) | 122.1 ± 47.1 | 130.4 ± 42.4 | 8.32 ± 37.3 | 116.7 ± 47.0 | 98.9 ± 51.5 | −17.7 ± 46.1 | 0.121 |
| BNP (pg/mL) | 125 (120–155) | 127 (117–136) | −4.01 (−18.7–3.07) | 130 (120–136) | 125 (122–130) | −3.58 (−14.2–10.3) | 0.827 |
| Variables | Control Group (n = 13) | Exercise Group (n = 14) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M2-M1 | M1 | M2 | M2-M1 | ||
| Morphological | |||||||
| LVDD (mm) | 50.0 ± 5.66 | 51.3 ± 3.99 | 1.31 ± 3.28 | 51.8 ± 6.78 | 52.6 ± 5.93 | 0.79 ± 4.26 | 0.726 |
| LVSD (mm) | 33.2 ± 7.68 | 34.5 ± 8.42 | 1.23 ± 2.35 | 30.6 ± 5.49 | 31.4 ± 5.98 | 0.79 ± 2.52 | 0.640 |
| PW (mm) | 8.96 ± 1.56 | 8.65 ± 1.30 | −0.31 ± 0.81 | 8.69 ± 1.40 | 9.05 ± 1.80 | 0.36 ± 1.36 | 0.139 |
| IVS (mm) | 9.02 ± 1.76 | 8.81 ± 1.49 | −0.22 ± 0.81 | 8.36 ± 1.31 | 9.30 ± 1.68 | 0.94 ± 1.58 | 0.027 |
| LVMI (g/m2) | 103.2 ± 40.0 | 100.4 ± 28.8 | −2.81 ± 17.4 | 94.9 ± 22.8 | 106.7 ± 32.5 | 11.8 ± 23.8 | 0.082 |
| Systolic function | |||||||
| EF (%) | 61.0 ± 10.1 | 63.0 ± 9.34 | 2.08 ± 4.44 | 66.8 ± 2.62 | 74.0 ± 6.40 | 7.21 ± 6.17 | 0.021 |
| S wave (cm/s) | 9.00 ± 2.27 | 8.61 ± 1.85 | −0.39 ± 1.64 | 9.24 ± 1.37 | 9.83 ± 1.58 | 0.59 ± 2.18 | 0.203 |
| St wave (cm/s) | 14.1 ± 3.24 | 13.2 ± 2.64 | −0.89 ± 2.58 | 14.1 ± 2.93 | 15.7 ± 3.10 | 1.65 ± 2.47 | 0.015 |
| Diastolic function | |||||||
| LAVI (mL/m2) | 32.4 ± 9.79 | 32.5 ± 10.2 | 0.14 ± 4.85 | 28.0 ± 8.41 | 31.9 ± 11.7 | 3.90 ± 4.90 | 0.056 |
| E wave (cm/s) | 94.1 ± 24.6 | 92.5 ± 21.0 | −1.54 ± 13.7 | 91.8 ± 22.8 | 96.1 ± 25.6 | 4.29± 20.6 | 0.398 |
| A wave (cm/s) | 53.2 ± 14.4 | 57.5 ± 15.9 | 4.31 ± 10.8 | 50.9 ± 17.9 | 53.7 ± 15.0 | 2.79 ± 8.80 | 0.691 |
| E/A | 1.82 ± 0.50 | 1.64 ± 0.24 | −0.19 ± 0.35 | 1.92 ± 0.64 | 1.88 ± 0.62 | −0.05 ± 0.52 | 0.422 |
| E′ wave (cm/s) | 12.0 (9.85–13.0) | 11.0 (10.0–11.7) | −0.92 ± 2.37 | 12.0 (10.6–13.0) | 13.5 (11.8–15.0) | 1.51 ± 3.58 | 0.050 |
| A′ wave (cm/s) | 7.0 (6.0–8.0) | 7.0 (7.0–8.0) | 0.0 (−1.0–1.0) | 8.5 (7.0–11.5) | 8.0 (7.0–12.0) | 0.0 (−1.0–1.0) | 0.766 |
| E/E’ | 8.19 ± 2.62 | 8.51 ± 2.03 | 0.32 ± 1.87 | 7.83 ± 2.24 | 7.11± 1.77 | −0.72 ± 2.65 | 0.253 |
| E′t wave (cm/s) | 14.6 ± 3.36 | 12.7 ± 2.59 | −1.94 ± 2.84 | 13.8 ± 3.31 | 15.0 ± 3.36 | 1.21 ± 4.67 | 0.046 |
| A′t wave (cm/s) | 9.0 (7.0–14.0) | 10.0 (9.0–11.5) | 0.4 (−1.0–1.5) | 9.0 (7.75–15.7) | 10.5 (7.0–13.8) | 0.0 (−2.93–3.25) | 0.788 |
| Variables | Control Group (n = 13) | Exercise Group (n = 14) | p | ||||
|---|---|---|---|---|---|---|---|
| M1 | M2 | M2-M1 | M1 | M2 | M2-M1 | ||
| Level of physical activity | |||||||
| Job | 2380 (570–4870) | 2367 (764–4412) | 137 (−83.5-252) | 2779 (1109–5100) | 2954 (1250–4477) | 61.0 (−318–1111) | 0.790 |
| Transportation | 641 (396–801) | 785 (448–1079) | 117 (45–194) | 594 (211–1255) | 726 (211–1539) | 51.5 (0–211) | 0.273 |
| Housework | 2400 (1463–2576) | 2456 (1540–2655) | 150 (−56–363) | 1200 (485–3277) | 1293 (679–2886) | 105 (−180–204) | 0.452 |
| Leisure | 1045 (419–2835) | 1100 (624–2140) | −206.2 ± 1040 | 1249 (180–2400) | 2803 (1897–3784) | 1261 ± 860 | <0.001 |
| Walking | 1300 (644–2090) | 1568 (874–2240) | 114 (−84.5–267) | 1286 (528–2751) | 2442 (805–3501) | 544 (171–845) | 0.024 |
| Moderate activity | 6563 ± 4584 | 6680 ± 4145 | 117 ± 1491 | 6769 ± 3691 | 7152 ± 4003 | 382 ± 1607 | 0.660 |
| Vigorous activity | 0.0 (0.0–441) | 0.0 (0.0–0.0) | 0.0 (−360–0.0) | 0.0 (0.0–480) | 0.0 (0.0–356) | 0.0 (−34–113) | 0.331 |
| Activity level rating | 3.0 (3.0–4.0) | 3.5 (3.0–4.0) | 0.0 (0.0–0.8) | 4.0 (4.0–5.0) | 5.0 (4.0–5.0) | 1.0 (0.0–1.0) | 0.111 |
| Total activity | 12,974 (9305–23,191) | 14,830 (10,224–19,497) | 584 (−1785–1263) | 14,940 (9524–22,038) | 16,155 (12,675–22,292) | 1945 (957–4175) | 0.021 |
| Functional capacity | |||||||
| VO2 peak (ml/kg/min) | 35.0 (28.6–35.1) | 35.0 (24.2–35.3) | 0.06 (−0.55–0.57) | 35.6 (35.0–47.3) | 48.3 (40.2–59.9) | 9.40 (1.78–16.9) | <0.001 |
| Distance (m) | 552.8 ± 160.1 | 586.7 ± 217.4 | 33.92 ± 97.2 | 809.1 ± 232.2 | 1020.7 ± 252.9 | 211.6 ± 130.7 | <0.001 |
| Time (min) | 9.91 ± 2.13 | 10.3 ± 2.29 | 0.36 ± 0.87 | 12.9 ± 2.62 | 14.8 ± 2.13 | 1.88 ± 1.55 | 0.005 |
| HR rest (bpm) | 76.3 ± 10.6 | 75.9 ± 6.78 | −0.39 ± 7.34 | 80.1 ± 14.2 | 72.1 ± 13.1 | −8.07 ± 10.1 | 0.034 |
| HR max. (bpm) | 163.2 ± 12.7 | 166.5 ± 13.1 | 3.39 ± 9.73 | 179.1 ± 10.6 | 182.1 ± 12.3 | 3.00 ± 10.6 | 0.923 |
| Quality of life | |||||||
| Functional capacity | 65.0 (52.5–70.0) | 70.0 (61.0–82.5) | 5.0 (2.5–11.5) | 71.0 (63.8–80.0) | 90.0 (82.5–95.0) | 13.5 (6.5–20.3) | 0.067 |
| Physical aspects | 60.0 (52.0–72.5) | 60.0 (49.5–70.0) | 0.0 (−4.0–5.0) | 54.5 (25.0–100.0) | 100.0 (59.3–100.0) | 8.50 (0.0–41.8) | 0.022 |
| Pain | 47.5 ± 20.2 | 54.8 ± 17.2 | 7.31 ± 9.38 | 53.4 ± 23.8 | 59.9 ± 29.5 | 6.54 ± 21.0 | 0.904 |
| General state | 50.2 ± 13.7 | 52.7 ± 15.4 | 2.54 ± 9.22 | 53.3 ± 19.7 | 59.6 ± 15.2 | 6.36 ± 18.0 | 0.500 |
| Vitality | 50.7 ± 10.1 | 51.9 ± 12.6 | 1.19 ± 8.00 | 56.8 ± 13.3 | 59.9 ± 12.2 | 3.14 ± 14.4 | 0.670 |
| Social aspects | 52.3 ± 21.5 | 52.9 ± 18.7 | 0.60 ± 8.01 | 64.9 ± 20.2 | 67.7 ± 19.6 | 2.81 ± 17.3 | 0.679 |
| Emotional aspects | 67.0 (52.5–80.8) | 70.0 (51.3–86.0) | 3.00 (−8.80–7.95) | 66.8 (33.5–100.0) | 100.0 (62.5–100.0) | 1.5 (0.0–36.3) | 0.302 |
| Mental health | 52.7 ± 18.3 | 52.0 ± 16.7 | −0.62 ± 5.84 | 69.1 ± 13.7 | 67.6 ± 14.7 | −1.43± 9.08 | 0.786 |
| Variables | Time | Distance Walked | VO2 Peak | |||
|---|---|---|---|---|---|---|
| Correlation Coefficient | p-Value | Correlation Coefficient | p-Value | Correlation Coefficient | p | |
| IL-10 | −0.188 | 0.344 | −0.220 | 0.266 | −0.228 | 0.259 |
| TNF-alpha | −0.101 | 0.614 | −0.123 | 0.536 | −0.130 | 0.524 |
| BNP | −0.334 | 0.088 | −0.343 | 0.080 | −0.362 | 0.069 |
| IL-1 | −0.156 | 0.433 | −0.233 | 0.240 | −0.286 | 0.155 |
| IL-6 | −0.367 | 0.059 | −0.444 | 0.020 | −0.480 | 0.013 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli Rossi, D.A.; De Araujo Junior, J.A.; Luvizutto, G.J.; Bazan, R.; Salmazo, P.S.; Modolo, G.P.; Hueb, J.C.; Nunes, H.R.d.C.; Hokama, N.K.; Minicucci, M.F.; et al. Effect of a Physical Exercise Program on the Inflammatory Response, Cardiac Functions, Functional Capacity, and Quality of Life in Patients with Sickle Cell Disease. J. Clin. Med. 2023, 12, 3952. https://doi.org/10.3390/jcm12123952
Antonelli Rossi DA, De Araujo Junior JA, Luvizutto GJ, Bazan R, Salmazo PS, Modolo GP, Hueb JC, Nunes HRdC, Hokama NK, Minicucci MF, et al. Effect of a Physical Exercise Program on the Inflammatory Response, Cardiac Functions, Functional Capacity, and Quality of Life in Patients with Sickle Cell Disease. Journal of Clinical Medicine. 2023; 12(12):3952. https://doi.org/10.3390/jcm12123952
Chicago/Turabian StyleAntonelli Rossi, Daniele Andreza, Jonas Alves De Araujo Junior, Gustavo José Luvizutto, Rodrigo Bazan, Péricles Sidnei Salmazo, Gabriel Pinheiro Modolo, João Carlos Hueb, Hélio Rubens de Carvalho Nunes, Newton Key Hokama, Marcos Ferreira Minicucci, and et al. 2023. "Effect of a Physical Exercise Program on the Inflammatory Response, Cardiac Functions, Functional Capacity, and Quality of Life in Patients with Sickle Cell Disease" Journal of Clinical Medicine 12, no. 12: 3952. https://doi.org/10.3390/jcm12123952
APA StyleAntonelli Rossi, D. A., De Araujo Junior, J. A., Luvizutto, G. J., Bazan, R., Salmazo, P. S., Modolo, G. P., Hueb, J. C., Nunes, H. R. d. C., Hokama, N. K., Minicucci, M. F., Roscani, M. G., & Zanati Bazan, S. G. (2023). Effect of a Physical Exercise Program on the Inflammatory Response, Cardiac Functions, Functional Capacity, and Quality of Life in Patients with Sickle Cell Disease. Journal of Clinical Medicine, 12(12), 3952. https://doi.org/10.3390/jcm12123952






