Micro-Structural and Biomechanical Evaluation of Bioresorbable and Conventional Bone Cements for Augmentation of the Proximal Femoral Nail
Abstract
:1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. Biomechanics
3.1.1. PMMA-Cement
3.1.2. Bioresorbable-Cement
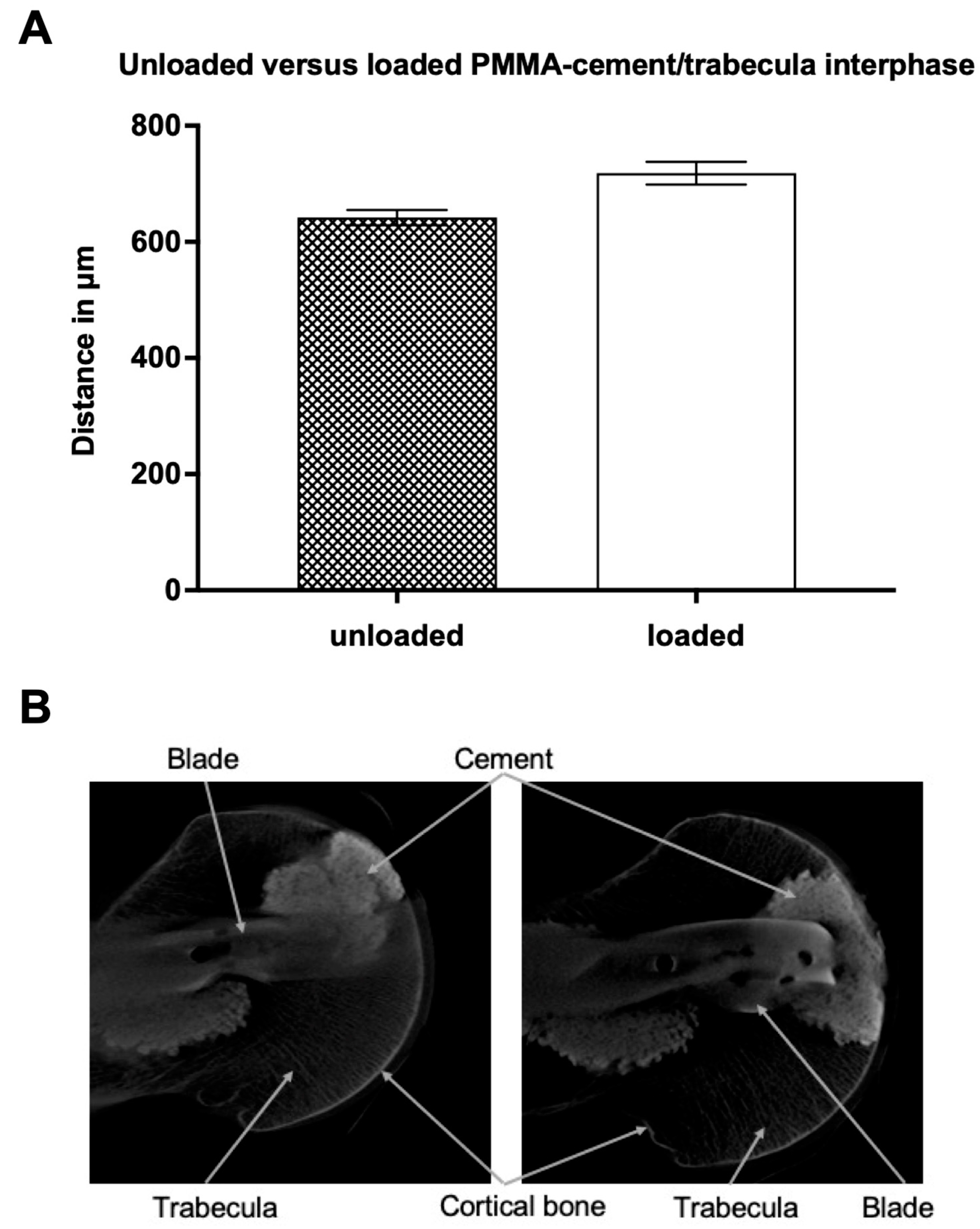
3.2. Micro-Computed Tomography
3.2.1. Unloaded PMMA-Cement vs. Bioresorbable-Cement
3.2.2. Loaded PMMA-Cement vs. Bioresorbable-Cement
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedman, S.M.; Mendelson, D.A. Epidemiology of fragility fractures. Clin. Geriatr. Med. 2014, 30, 175–181. [Google Scholar] [CrossRef]
- Kammerlander, C.; Gosch, M.; Kammerlander-Knauer, U.; Luger, T.J.; Blauth, M.; Roth, T. Long-term functional outcome in geriatric hip fracture patients. Arch. Orthop. Trauma Surg. 2011, 131, 1435–1444. [Google Scholar] [CrossRef]
- Neuerburg, C.; Gosch, M.; Blauth, M.; Bocker, W.; Kammerlander, C. Augmentation techniques on the proximal femur. Unfallchirurg 2015, 118, 755–764. [Google Scholar] [CrossRef] [PubMed]
- Vigano, M.; Pennestri, F.; Listorti, E.; Banfi, G. Proximal hip fractures in 71,920 elderly patients: Incidence, epidemiology, mortality and costs from a retrospective observational study. BMC Public Health 2023, 23, 1963. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Xu, X.; Yang, X.; Chen, X.; Wang, L.; Liu, C.; Lin, P. Intramedullary nails versus sliding hip screws for AO/OTA 31-A2 trochanteric fractures in adults: A meta-analysis. Int. J. Surg. 2017, 43, 67–74. [Google Scholar] [CrossRef]
- Shen, L.; Zhang, Y.; Shen, Y.; Cui, Z. Antirotation proximal femoral nail versus dynamic hip screw for intertrochanteric fractures: A meta-analysis of randomized controlled studies. Orthop. Traumatol. Surg. Res. 2013, 99, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shen, Y.; Zong, Z.; Zhao, Y.; Liu, H.; Hua, X.; Chen, H. Percutaneous compression plate versus proximal femoral nail anti-rotation in treating elderly patients with intertrochanteric fractures: A prospective randomized study. J. Orthop. Sci. 2013, 18, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ariza-Vega, P.; Kristensen, M.T.; Martin-Martin, L.; Jimenez-Moleon, J.J. Predictors of long-term mortality in older people with hip fracture. Arch. Phys. Med. Rehabil. 2015, 96, 1215–1221. [Google Scholar] [CrossRef]
- Fensky, F.; Nuchtern, J.V.; Kolb, J.P.; Huber, S.; Rupprecht, M.; Jauch, S.Y.; Sellenschloh, K.; Puschel, K.; Morlock, M.M.; Rueger, J.M.; et al. Cement augmentation of the proximal femoral nail antirotation for the treatment of osteoporotic pertrochanteric fractures--a biomechanical cadaver study. Injury 2013, 44, 802–807. [Google Scholar] [CrossRef]
- Ehrnthaller, C.; Olivier, A.C.; Gebhard, F.; Durselen, L. The role of lesser trochanter fragment in unstable pertrochanteric A2 proximal femur fractures—Is refixation of the lesser trochanter worth the effort? Clin. Biomech. 2017, 42, 31–37. [Google Scholar] [CrossRef]
- Kammerlander, C.; Doshi, H.; Gebhard, F.; Scola, A.; Meier, C.; Linhart, W.; Garcia-Alonso, M.; Nistal, J.; Blauth, M. Long-term results of the augmented PFNA: A prospective multicenter trial. Arch. Orthop. Trauma Surg. 2014, 134, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, C.; Gebhard, F.; Meier, C.; Lenich, A.; Linhart, W.; Clasbrummel, B.; Neubauer-Gartzke, T.; Garcia-Alonso, M.; Pavelka, T.; Blauth, M. Standardised cement augmentation of the PFNA using a perforated blade: A new technique and preliminary clinical results. A prospective multicentre trial. Injury 2011, 42, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, C.; Hem, E.S.; Klopfer, T.; Gebhard, F.; Sermon, A.; Dietrich, M.; Bach, O.; Weil, Y.; Babst, R.; Blauth, M. Cement augmentation of the Proximal Femoral Nail Antirotation (PFNA)—A multicentre randomized controlled trial. Injury 2018, 49, 1436–1444. [Google Scholar] [CrossRef]
- Boner, V.; Kuhn, P.; Mendel, T.; Gisep, A. Temperature evaluation during PMMA screw augmentation in osteoporotic bone--an in vitro study about the risk of thermal necrosis in human femoral heads. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 90, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Sermon, A.; Slock, C.; Coeckelberghs, E.; Seys, D.; Panella, M.; Bruyneel, L.; Nijs, S.; Akiki, A.; Castillon, P.; Chipperfield, A.; et al. Quality indicators in the treatment of geriatric hip fractures: Literature review and expert consensus. Arch. Osteoporos. 2021, 16, 152. [Google Scholar] [CrossRef]
- Rauschmann, M.; Vogl, T.; Verheyden, A.; Pflugmacher, R.; Werba, T.; Schmidt, S.; Hierholzer, J. Bioceramic vertebral augmentation with a calcium sulphate/hydroxyapatite composite (Cerament SpineSupport): In vertebral compression fractures due to osteoporosis. Eur. Spine J. 2010, 19, 887–892. [Google Scholar] [CrossRef]
- Abramo, A.; Geijer, M.; Kopylov, P.; Tagil, M. Osteotomy of distal radius fracture malunion using a fast remodeling bone substitute consisting of calcium sulphate and calcium phosphate. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 281–286. [Google Scholar] [CrossRef]
- Nusselt, T.; Hofmann, A.; Wachtlin, D.; Gorbulev, S.; Rommens, P.M. CERAMENT treatment of fracture defects (CERTiFy): Protocol for a prospective, multicenter, randomized study investigating the use of CERAMENT™ BONE VOID FILLER in tibial plateau fractures. Trials 2014, 15, 75. [Google Scholar] [CrossRef]
- Hofmann, A.; Gorbulev, S.; Guehring, T.; Schulz, A.P.; Schupfner, R.; Raschke, M.; Huber-Wagner, S.; Rommens, P.M.; Group, C.E.S. Autologous Iliac Bone Graft Compared with Biphasic Hydroxyapatite and Calcium Sulfate Cement for the Treatment of Bone Defects in Tibial Plateau Fractures: A Prospective, Randomized, Open-Label, Multicenter Study. J. Bone Jt. Surg. Am. 2020, 102, 179–193. [Google Scholar] [CrossRef]
- Dong, C.; Klimek, P.; Abacherli, C.; De Rosa, V.; Krieg, A.H. Percutaneous cyst aspiration with injection of two different bioresorbable bone cements in treatment of simple bone cyst. J. Child. Orthop. 2020, 14, 76–84. [Google Scholar] [CrossRef]
- Masala, S.; Nano, G.; Marcia, S.; Muto, M.; Fucci, F.P.; Simonetti, G. Osteoporotic vertebral compression fracture augmentation by injectable partly resorbable ceramic bone substitute (Cerament|SPINESUPPORT): A prospective nonrandomized study. Neuroradiology 2012, 54, 1245–1251. [Google Scholar] [CrossRef]
- Marcia, S.; Boi, C.; Dragani, M.; Marini, S.; Marras, M.; Piras, E.; Anselmetti, G.C.; Masala, S. Effectiveness of a bone substitute (CERAMENT™) as an alternative to PMMA in percutaneous vertebroplasty: 1-year follow-up on clinical outcome. Eur. Spine J. 2012, 21 (Suppl. 1), S112–S118. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Min. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Ogawa, T.; Banno, M.; Watanabe, J.; Noda, T.; Schermann, H.; Ozaki, T. Cement augmentation of internal fixation for trochanteric fracture: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2022, 48, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, K.; Eickhoff, A.; Roderer, G.; Gebhard, F.; Richter, P.H. Osteoporotic Bone: When and How to Use Augmentation? J. Orthop. Trauma 2019, 33 (Suppl. 8), S21–S26. [Google Scholar] [CrossRef]
- Mattie, R.; Brar, N.; Tram, J.T.; McCormick, Z.L.; Beall, D.P.; Fox, A.; Saltychev, M. Vertebral Augmentation of Cancer-Related Spinal Compression Fractures: A Systematic Review and Meta-Analysis. Spine 2021, 46, 1729–1737. [Google Scholar] [CrossRef]
- Joeris, A.; Kabiri, M.; Galvain, T.; Vanderkarr, M.; Holy, C.E.; Plaza, J.Q.; Tien, S.; Schneller, J.; Kammerlander, C. Cost-Effectiveness of Cement Augmentation Versus No Augmentation for the Fixation of Unstable Trochanteric Fractures. J. Bone Jt. Surg. Am. 2022, 104, 2026–2034. [Google Scholar] [CrossRef]
- Schuetze, K.; Ehinger, S.; Eickhoff, A.; Dehner, C.; Gebhard, F.; Richter, P.H. Cement augmentation of the proximal femur nail antirotation: Is it safe? Arch. Orthop. Trauma Surg. 2021, 141, 803–811. [Google Scholar] [CrossRef]
- Nilsson, M.; Zheng, M.H.; Tagil, M. The composite of hydroxyapatite and calcium sulphate: A review of preclinical evaluation and clinical applications. Expert. Rev. Med. Devices 2013, 10, 675–684. [Google Scholar] [CrossRef]
- Pesch, S.; Hanschen, M.; Greve, F.; Zyskowski, M.; Seidl, F.; Kirchhoff, C.; Biberthaler, P.; Huber-Wagner, S. Treatment of fracture-related infection of the lower extremity with antibiotic-eluting ceramic bone substitutes: Case series of 35 patients and literature review. Infection 2020, 48, 333–344. [Google Scholar] [CrossRef]
- Hoelscher-Doht, S.; Heilig, M.; von Hertzberg-Boelch, S.P.; Jordan, M.C.; Gbureck, U.; Meffert, R.H.; Heilig, P. Experimental magnesium phosphate cement paste increases torque of trochanteric fixation nail advanced blades in human femoral heads. Clin. Biomech. 2023, 109, 106088. [Google Scholar] [CrossRef] [PubMed]
- Hettwer, W.; Horstmann, P.F.; Bischoff, S.; Gullmar, D.; Reichenbach, J.R.; Poh, P.S.P.; van Griensven, M.; Gras, F.; Diefenbeck, M. Establishment and effects of allograft and synthetic bone graft substitute treatment of a critical size metaphyseal bone defect model in the sheep femur. APMIS 2019, 127, 53–63. [Google Scholar] [CrossRef] [PubMed]




| Test load | 200 N | 400 N | ||
|---|---|---|---|---|
| Cement | PMMA | Bio-Cement | PMMA | Bio-Cement |
| Fracture displacement in mm | 1.13 ± 0.39 | 1.02 ± 0.32 | 1.22 ± 0.31 | 1.20 ± 0.26 |
| Axial bone stiffness in N/mm | 31.13 ± 12.75 | 41.73 ± 16.66 | 30.16 ± 2.34 | 31.08 ± 3.60 |
| Iliotibial tract force in N | 345.00 ± 33.76 | 334.80 ± 8.03 | 740.80 ± 53.80 | 695.00 ± 13.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linhart, C.; Kistler, M.; Saller, M.; Greiner, A.; Lampert, C.; Kassube, M.; Becker, C.A.; Böcker, W.; Ehrnthaller, C. Micro-Structural and Biomechanical Evaluation of Bioresorbable and Conventional Bone Cements for Augmentation of the Proximal Femoral Nail. J. Clin. Med. 2023, 12, 7202. https://doi.org/10.3390/jcm12237202
Linhart C, Kistler M, Saller M, Greiner A, Lampert C, Kassube M, Becker CA, Böcker W, Ehrnthaller C. Micro-Structural and Biomechanical Evaluation of Bioresorbable and Conventional Bone Cements for Augmentation of the Proximal Femoral Nail. Journal of Clinical Medicine. 2023; 12(23):7202. https://doi.org/10.3390/jcm12237202
Chicago/Turabian StyleLinhart, Christoph, Manuel Kistler, Maximilian Saller, Axel Greiner, Christopher Lampert, Matthias Kassube, Christopher A. Becker, Wolfgang Böcker, and Christian Ehrnthaller. 2023. "Micro-Structural and Biomechanical Evaluation of Bioresorbable and Conventional Bone Cements for Augmentation of the Proximal Femoral Nail" Journal of Clinical Medicine 12, no. 23: 7202. https://doi.org/10.3390/jcm12237202






