Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy and Inclusion/Exclusion Criteria
2.2. Data Extraction and Outcomes
2.3. Risk of Bias Assessment
2.4. Statistical Analysis
3. Results
3.1. Subsection Characteristics of Included Studies
3.2. Risk of Bias
3.3. Effectiveness of Antiviral Therapy on Post-Acute Sequelae of COVID-19
3.4. Effectiveness of Antiviral Therapy on Hospitalization or Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Health Organization Coronavirus (COVID-19) Dashboard; World Health Organization: Geneva, Switzerland, 2022; Available online: https://covid19.who.int/ (accessed on 26 July 2023).
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Michelen, M.; Manoharan, L.; Elkheir, N.; Cheng, V.; Dagens, A.; Hastie, C.; O’Hara, M.; Suett, J.; Dahmash, D.; Bugaeva, P.; et al. Characterising long COVID: A living systematic review. BMJ Glob. Health 2021, 6, e005427. [Google Scholar] [CrossRef]
- Raman, B.; Cassar, M.P.; Tunnicliffe, E.M.; Filippini, N.; Griffanti, L.; Alfaro-Almagro, F.; Okell, T.; Sheerin, F.; Xie, C.; Mahmod, M.; et al. Medium-term effects of SARS-CoV-2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post-hospital discharge. EClinicalmedicine 2021, 31, 100683. [Google Scholar] [CrossRef] [PubMed]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long covid-mechanisms, risk factors, and management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. NICE guideline on long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef]
- Cutler, D.M. The costs of long COVID. JAMA Health Forum 2022, 3, e221809. [Google Scholar] [CrossRef] [PubMed]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Chilunga, F.P.; Appelman, B.; van Vugt, M.; Kalverda, K.; Smeele, P.; van Es, J.; Wiersinga, W.J.; Rostila, M.; Prins, M.; Stronks, K.; et al. Differences in incidence, nature of symptoms, and duration of long COVID among hospitalised migrant and non-migrant patients in the Netherlands: A retrospective cohort study. Lancet Reg. Health Eur. 2023, 29, 100630. [Google Scholar] [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk factors associated with post-COVID-19 condition: A systematic review and meta-analysis. JAMA Intern. Med. 2023, 183, 566–580. [Google Scholar] [CrossRef]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network—United States, March–June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Pitre, T.; Van Alstine, R.; Chick, G.; Leung, G.; Mikhail, D.; Cusano, E.; Khalid, F.; Zeraatkar, D. Antiviral drug treatment for nonsevere COVID-19: A systematic review and network meta-analysis. CMAJ 2022, 194, E969–E980. [Google Scholar] [CrossRef]
- COVID-19 Long Hauler Advocacy Project & Long Covid Community Responds to the Lack of Long Covid Awareness & Education in President Biden’s State of the Union. Available online: https://www.longhauler-advocacy.org/c-19lap-responds-to-sotu (accessed on 26 July 2023).
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. [Google Scholar] [CrossRef]
- Guan, Y.; Puenpatom, A.; Johnson, M.G.; Zhang, Y.; Zhao, Y.; Surber, J.; Weinberg, A.; Brotons, C.; Kozlov, R.; Lopez, R.; et al. Impact of molnupiravir treatment on patient-reported coronavirus disease 2019 (COVID-19) symptoms in the Phase 3 MOVe-OUT trial: A randomized, placebo-controlled trial. Clin. Infect. Dis. 2023, ciad409. [Google Scholar] [CrossRef] [PubMed]
- Cumpston, M.; Li, T.; Page, M.J.; Chandler, J.; Welch, V.A.; Higgins, J.P.; Thomas, J. Updated guidance for trusted systematic reviews: A new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst. Rev. 2019, 10, ED000142. [Google Scholar] [CrossRef]
- Bertuccio, P.; Degli Antoni, M.; Minisci, D.; Amadasi, S.; Castelli, F.; Odone, A.; Quiros-Roldan, E. The impact of early therapies for COVID-19 on death, hospitalization and persisting symptoms: A retrospective study. Infection 2023, 51, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.H.; Wu, J.Y.; Liu, T.H.; Hsu, W.H.; Tsai, Y.W.; Huang, P.Y.; Lai, C.C. Efficacy of nirmatrelvir and ritonavir for post-acute COVID-19 sequelae beyond 3 months of SARS-CoV-2 infection. J. Med. Virol. 2023, 95, e28750. [Google Scholar] [CrossRef]
- Liu, T.H.; Wu, J.Y.; Huang, P.Y.; Tsai, Y.W.; Lai, C.C. The effect of nirmatrelvir-ritonavir on the long-term risk of neuropsychiatric sequelae following COVID-19. J. Med. Virol. 2023, 95, e28951. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of treatment with nirmatrelvir and the risk of post–COVID-19 condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Molnupiravir and risk of post-acute sequelae of COVID-19: Cohort study. BMJ 2023, 381, e074572. [Google Scholar] [CrossRef] [PubMed]
- Mirin, A.A.; Dimmock, M.E.; Jason, L.A. Updated ME/CFS prevalence estimates reflecting post-COVID increases and associated economic costs and funding implications. Fatigue Biomed. Health Behav. 2022, 10, 83–93. [Google Scholar] [CrossRef]
- Jason, L.A.; Mirin, A.A. Updating the National Academy of Medicine ME/CFS prevalence and economic impact figures to account for population growth and inflation. Fatigue Biomed. Health Behav. 2021, 9, 9–13. [Google Scholar] [CrossRef]
- Mirin, A.A. A preliminary estimate of the economic impact of long COVID in the United States. Fatigue Biomed. Health Behav. 2022, 10, 190–199. [Google Scholar] [CrossRef]
- Goggins, S. Contesting public forgetting: Memory and policy learning in the era of COVID-19. Mem. Stud. 2023. [Google Scholar] [CrossRef]
- Song, W.J.; Hui, C.K.M.; Hull, J.H.; Birring, S.S.; McGarvey, L.; Mazzone, S.B.; Chung, K.F. Confronting COVID-19-associated cough and the post-COVID syndrome: Role of viral neurotropism, neuroinflammation, and neuroimmune responses. Lancet Respir. Med. 2021, 9, 533–544. [Google Scholar] [CrossRef] [PubMed]
- Trypsteen, W.; Van Cleemput, J.; Snippenberg, W.V.; Gerlo, S.; Vandekerckhove, L. LOn the whereabouts of SARS-CoV-2 in the human body: A systematic review. PLOS Pathog. 2020, 16, e1009037. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef]
- Iwasaki, M.; Saito, J.; Zhao, H.; Sakamoto, A.; Hirota, K.; Ma, D. Inflammation triggered by SARS-CoV-2 and ACE2 augment drives multiple organ failure of severe COVID-19: Molecular mechanisms and implications. Inflammation 2021, 44, 13–34. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of molnupiravir-induced SARS-CoV-2 mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Kausar, S.; Said Khan, F.; Ishaq Mujeeb Ur Rehman, M.; Akram, M.; Riaz, M.; Rasool, G.; Hamid Khan, A.; Saleem, I.; Shamim, S.; Malik, A. A review: Mechanism of action of antiviral drugs. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211002621. [Google Scholar] [CrossRef]
- Zhou, H.; Tada, T.; Dcosta, B.M.; Landau, N.R. Neutralization of SARS-CoV-2 omicron BA.2 by Therapeutic Monoclonal Antibodies. Biorxiv 2022. [Google Scholar] [CrossRef]
- Cully, M. A tale of two antiviral targets-and the COVID-19 drugs that bind them. Nat. Rev. Drug Discov. 2022, 21, 3–5. [Google Scholar] [CrossRef]
- Rahmah, L.; Abarikwu, S.O.; Arero, A.G.; Essouma, M.; Jibril, A.T.; Fal, A.; Flisiak, R.; Makuku, R.; Marquez, L.; Mohamed, K.; et al. Oral antiviral treatments for COVID-19: Opportunities and challenges. Pharmacol. Rep. 2022, 74, 1255–1278. [Google Scholar] [CrossRef]
- Libster, R.; Pérez Marc, G.; Wappner, D.; Coviello, S.; Bianchi, A.; Braem, V.; Esteban, I.; Caballero, M.T.; Wood, C.; Berrueta, M.; et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N. Engl. J. Med. 2021, 384, 610–618. [Google Scholar] [CrossRef]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early remdesivir to prevent progression to severe COVID-19 in outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following mild SARS-CoV-2 infection: Characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Nevalainen, O.P.O.; Horstia, S.; Laakkonen, S.; Rutanen, J.; Mustonen, J.M.J.; Kalliala, I.E.J.; Ansakorpi, H.; Kreivi, H.R.; Kuutti, P.; Paajanen, J.; et al. Effect of remdesivir post hospitalization for COVID-19 infection from the randomized SOLIDARITY Finland trial. Nat. Commun. 2022, 13, 6152. [Google Scholar] [CrossRef]


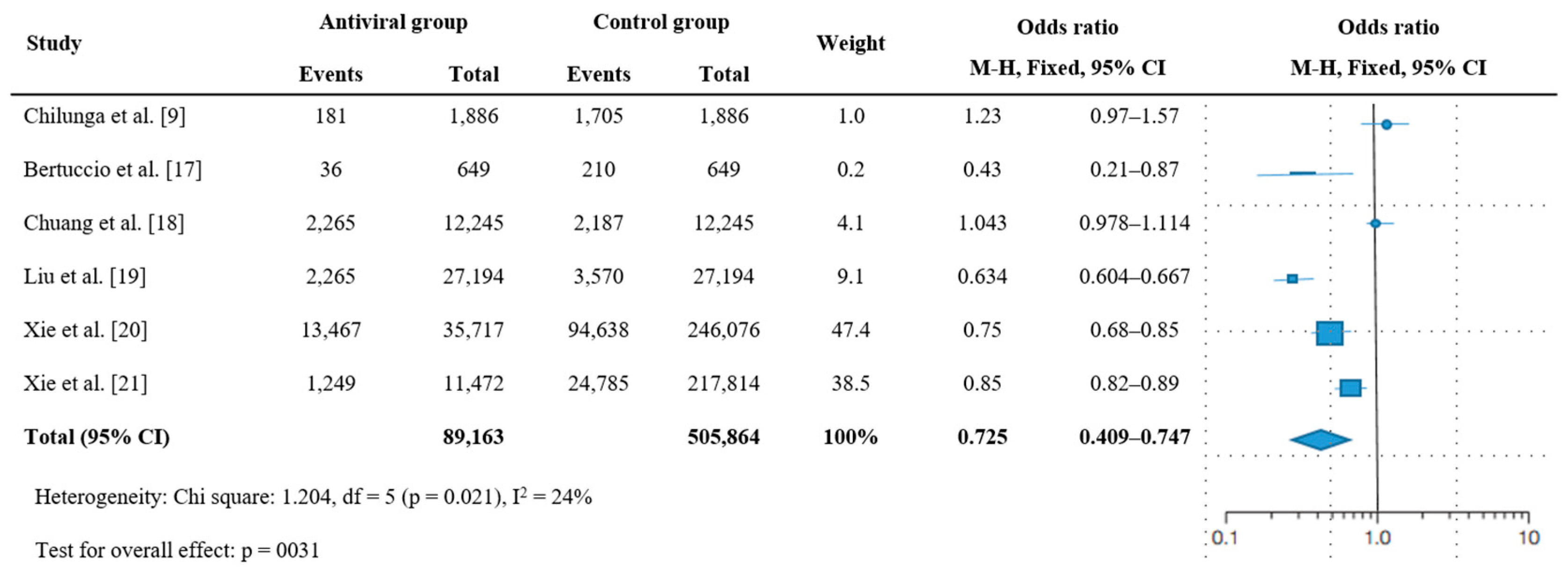
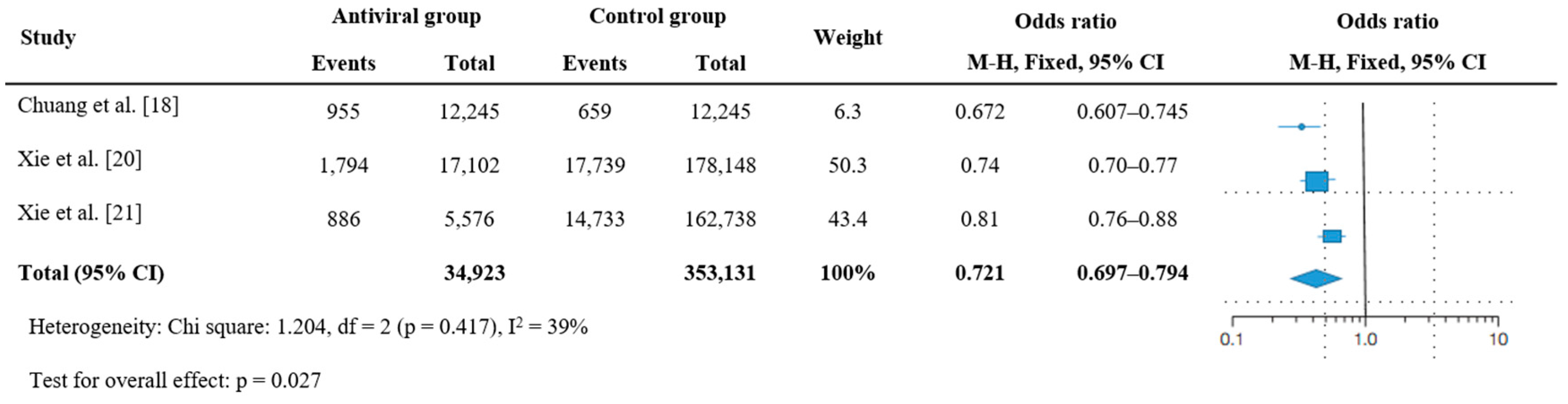
| Study | Design | Population | No. | Male (%) | Median (Range) or Mean (SD) Age | Nation | Follow-Up Duration | PASC | Antiviral Therapy | Death or Hospitalization | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Definition | No. | Drug | No. (%) | Intervals from Diagnosis to Treatment | |||||||||
| Chilunga et al. [9] | Retrospective cohort | ≥18 years old, either a confirmed positive SARS-CoV-2 PCR or high clinical suspicion for COVID-19 based on clinical presentation and computed tomography imaging | 1886 | 56.5 | 62 (59–71) | The Netherlands | 12 weeks | NICE guideline [2] | 483 | Remdesivir | 181 (9.6) | - | . |
| Bertuccio et al. [17] | Retrospective observational | >18 years old with positive SARS-CoV-2 nasopharyngeal swab, mild/moderate symptoms, at least one risk factor for COVID-19 progression | 649 | 51.6 | 67 (54–76) | Italy | 1/3 months | U.S. CDC COVID-19 symptom list [a] | 323 | Nirmatrelvir/ritonavir, molnupiravir, and remdesivir | 197 (30.4) | - | Death or hospitalization |
| Chuang et al. [18] | Retrospective cohort | ≥18 years old with positive test for SARS-CoV-2 or received a diagnosis of COVID-19 | 477,382 | 42.7 * | 55.9 (16.7) * | Taiwan | 90 to 180 days | Fatigue, pain, dizziness, headache, cognitive impairment, cardiopulmonary symptoms, abdominal symptoms, anxiety or depression, and sleep disorders | 4452 | Nirmatrelvir/ritonavir | 12,245 (2.6) | Within 5 days | Hospitalization or emergency room visits |
| Liu et al. [19] | Retrospective cohort | ≥18 years old with positive test for SARS-CoV-2 or received a diagnosis of COVID-19, at high risk of severe COVID-19 | 2,361,239 | 41.7 * | 59.4 (15.0) * | Taiwan | 90 days to 1 year | Neuropsychiatric sequela | 5835 | Nirmatrelvir/ritonavir | 27,194 (1.2) | - | . |
| Xie et al. [20] | Observational cohort | With positive SARS-CoV-2 test, at least one risk factor of progression to severe acute COVID-19 illness | 281,793 | 87.9 | 65.7 (13.4) | U.S. | 180 days | Incident ischemic heart disease, dysrhythmia, DVT, PE, fatigue/malaise, liver disease, acute kidney injury, muscle pain, diabetes, neurocognitive impairment, dysautonomia, shortness of breath, cough | 40,098 | Nirmatrelvir/ritonavir | 35,717 (12.7) | Within 5 days | Death or hospitalization |
| Xie et al. [21] | Observational cohort | Same as above | 229,286 | 91.6 | 69.8 (11.7) | U.S. | 180 days | Incident ischemic heart disease, dysrhythmia, DVT, PE, fatigue/malaise, liver disease, acute kidney injury, muscle pain, diabetes, neurocognitive impairment, dysautonomia, shortness of breath, cough | 29,743 | Molnupiravir | 13,007 (5.7) | Within 5 days | Death or hospitalization |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.J.; Seo, Y.B.; Seo, J.-W.; Lee, J.; Nham, E.; Seong, H.; Yoon, J.G.; Noh, J.Y.; Cheong, H.J.; Kim, W.J.; et al. Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 7375. https://doi.org/10.3390/jcm12237375
Choi YJ, Seo YB, Seo J-W, Lee J, Nham E, Seong H, Yoon JG, Noh JY, Cheong HJ, Kim WJ, et al. Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(23):7375. https://doi.org/10.3390/jcm12237375
Chicago/Turabian StyleChoi, Yu Jung, Yu Bin Seo, Jun-Won Seo, Jacob Lee, Eliel Nham, Hye Seong, Jin Gu Yoon, Ji Yun Noh, Hee Jin Cheong, Woo Joo Kim, and et al. 2023. "Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 23: 7375. https://doi.org/10.3390/jcm12237375
APA StyleChoi, Y. J., Seo, Y. B., Seo, J.-W., Lee, J., Nham, E., Seong, H., Yoon, J. G., Noh, J. Y., Cheong, H. J., Kim, W. J., Kim, E. J., & Song, J. Y. (2023). Effectiveness of Antiviral Therapy on Long COVID: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(23), 7375. https://doi.org/10.3390/jcm12237375





