PEEP-Induced Lung Recruitment Maneuver Combined with Prone Position for ARDS: A Single-Center, Prospective, Randomized Clinical Trial
Abstract
:1. Introduction
2. Method
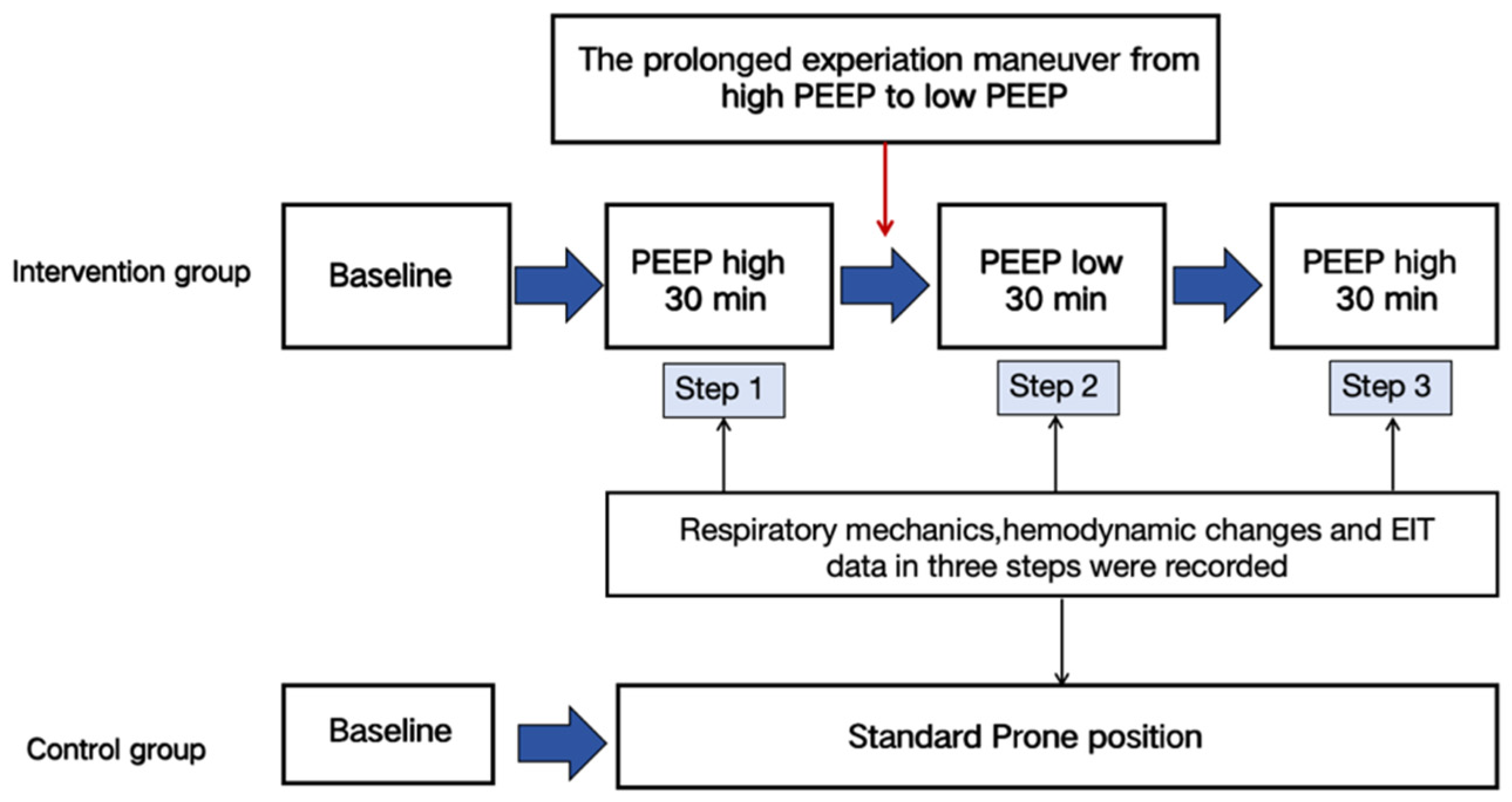
2.1. Study Design
2.2. Patients Selection
2.3. Data Collection
2.4. Intervention
2.5. PEEP-Induced Recruitment Maneuver
2.6. Electric Impedance Tomography
2.7. Recruitability
2.8. Statistical Analysis
3. Results
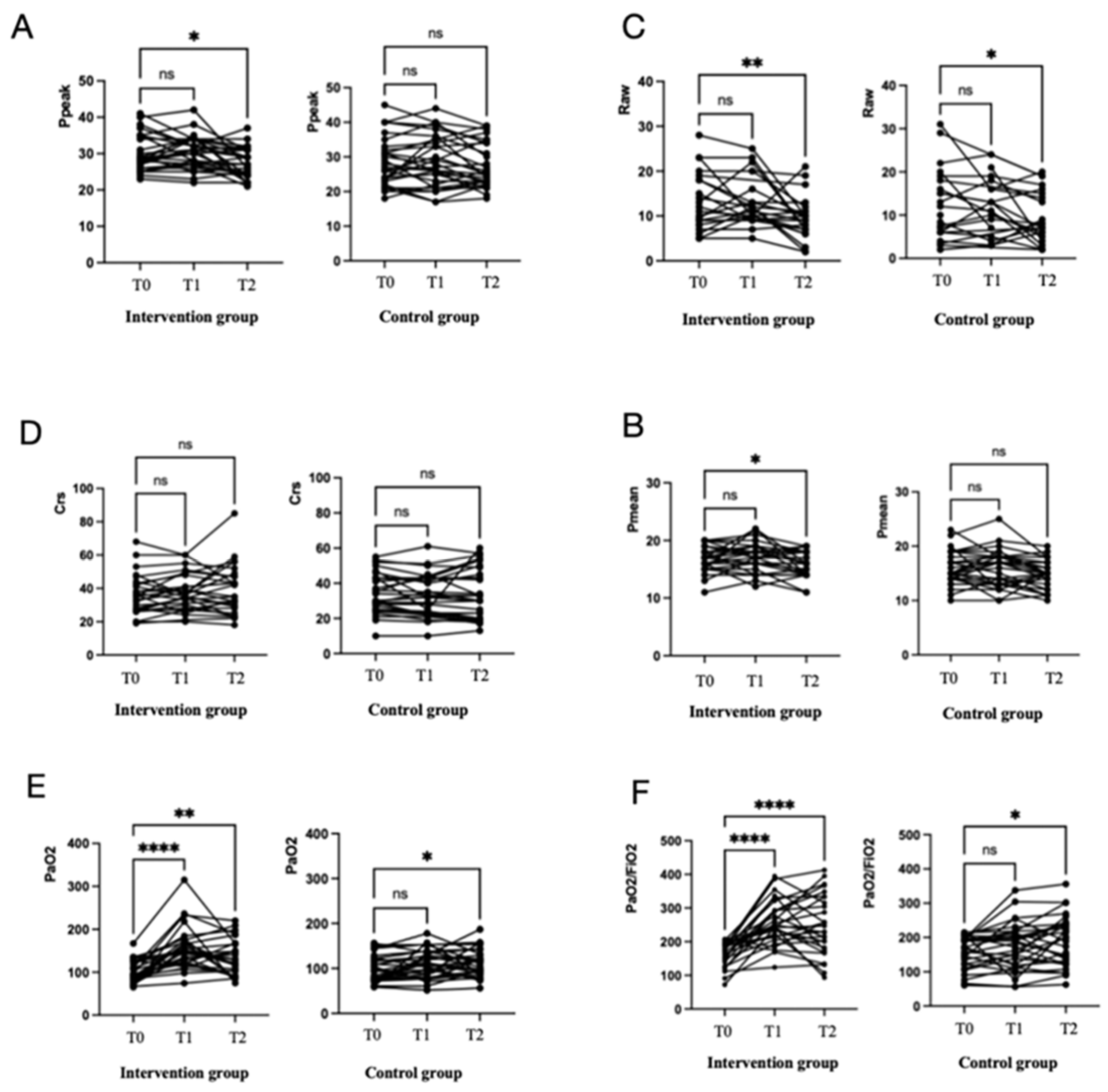
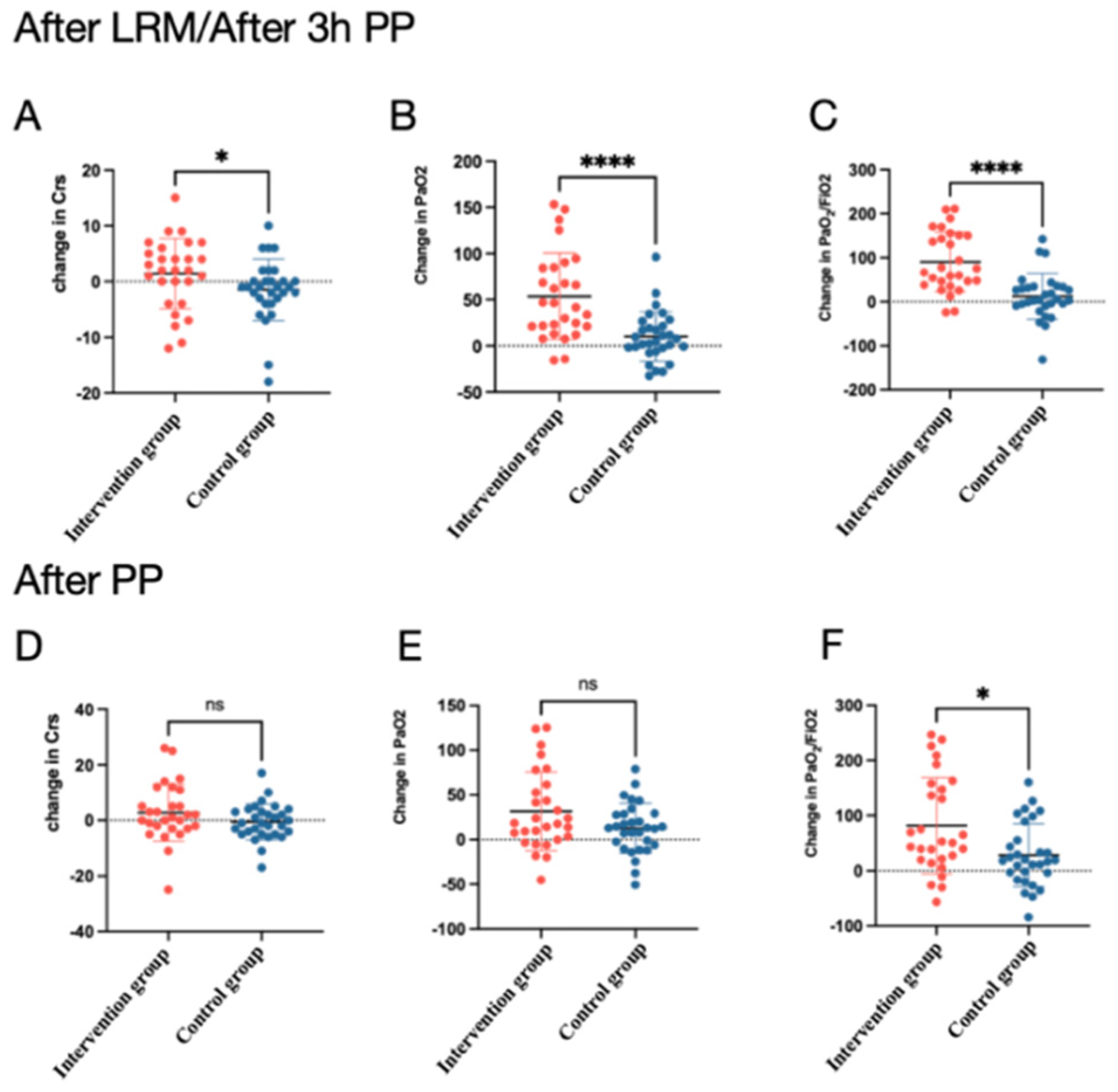
3.1. Respiratory Mechanics and Gas Change
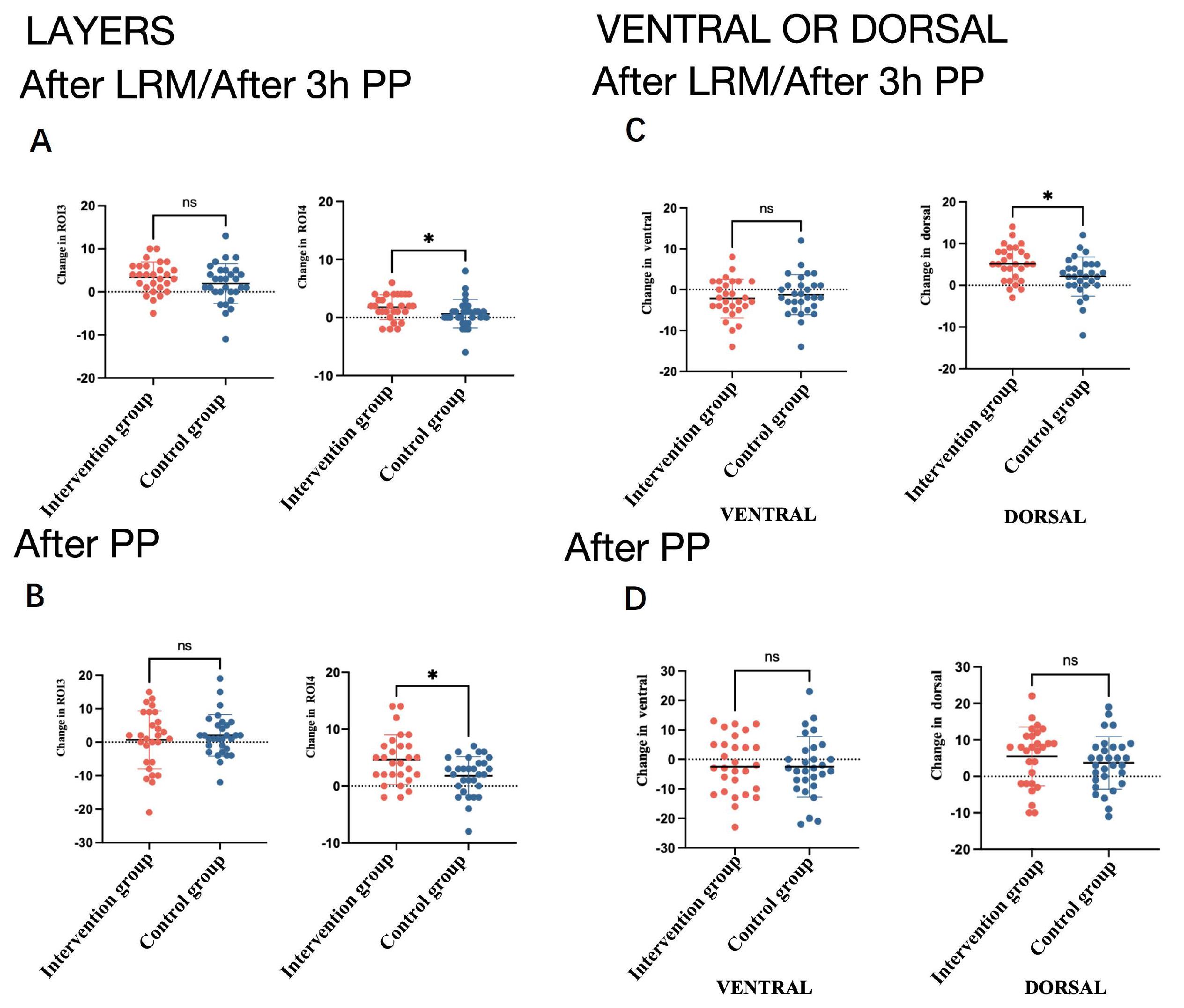
3.2. EIT Data
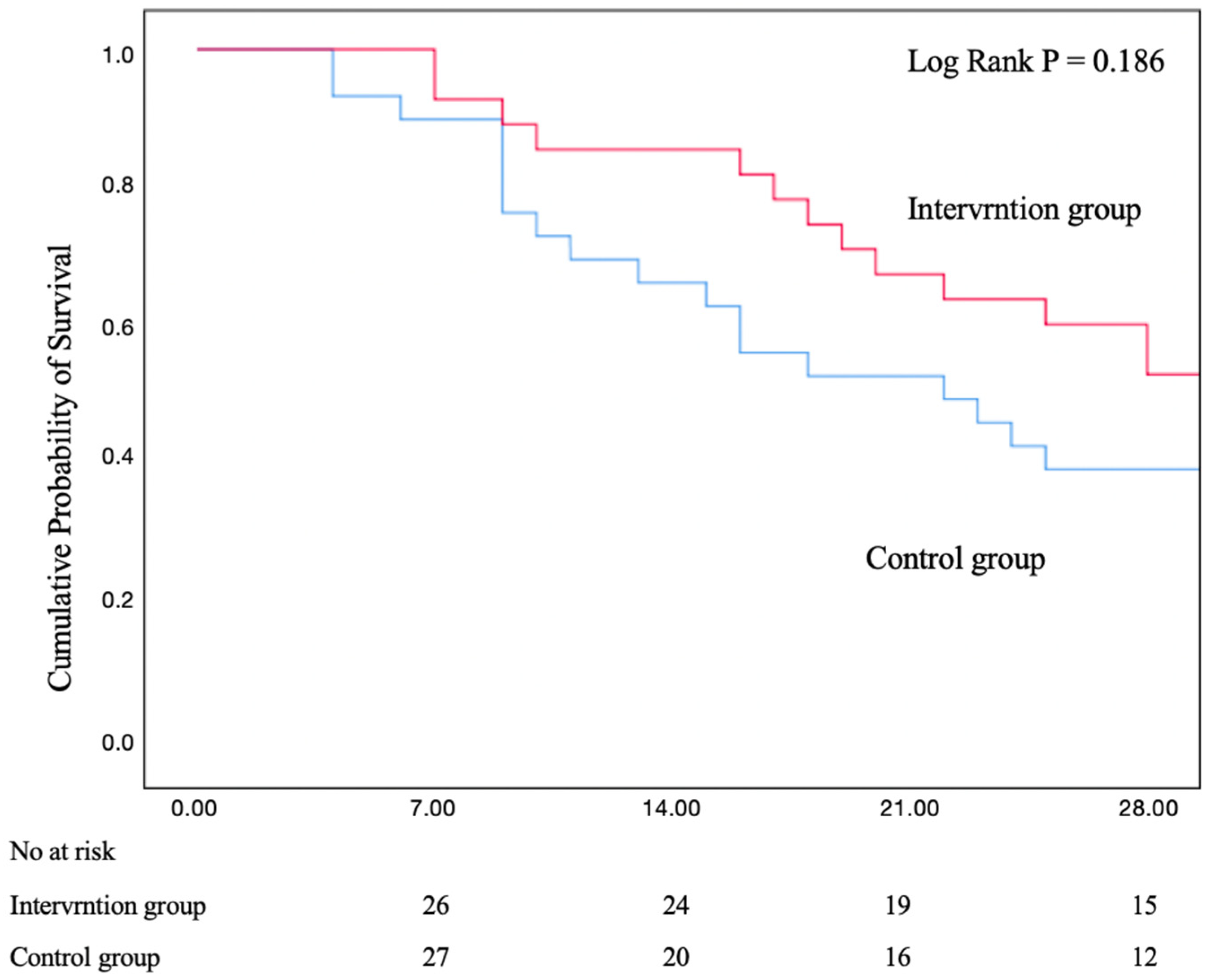
3.3. Mortality
3.4. R/I Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Gorman, E.A.; O’Kane, C.M.; McAuley, D.F. Acute respiratory distress syndrome in adults: Diagnosis, outcomes, long-term sequelae, and management. Lancet 2022, 400, 1157–1170. [Google Scholar] [CrossRef] [PubMed]
- Meyer, N.J.; Gattinoni, L.; Calfee, C.S. Acute respiratory distress syndrome. Lancet 2021, 398, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Bos, L.D.J.; Ware, L.B. Acute respiratory distress syndrome: Causes, pathophysiology, and phenotypes. Lancet 2022, 400, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Rampon, G.L.; Simpson, S.Q.; Agrawal, R. Prone Positioning for Acute Hypoxemic Respiratory Failure and ARDS: A Review. Chest 2023, 163, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Pelosi, P.; Suter, P.M.; Pedoto, A.; Vercesi, P.; Lissoni, A. Acute respiratory distress syndrome caused by pulmonary and extrapulmonary disease. Different syndromes? Am. J. Respir. Crit. Care Med. 1998, 158, 3–11. [Google Scholar] [CrossRef]
- Muscedere, J.G.; Mullen, J.B.; Gan, K.; Slutsky, A.S. Tidal ventilation at low airway pressures can augment lung injury. Am. J. Respir. Crit. Care Med. 1994, 149, 1327–1334. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Piehl, M.A.; Brown, R.S. Use of extreme position changes in acute respiratory failure. Crit. Care Med. 1976, 4, 13–14. [Google Scholar] [CrossRef]
- Thompson, A.E.; Ranard, B.L.; Wei, Y.; Jelic, S. Prone Positioning in Awake, Nonintubated Patients With COVID-19 Hypoxemic Respiratory Failure. JAMA Intern. Med. 2020, 180, 1537–1539. [Google Scholar] [CrossRef]
- Coppo, A.; Bellani, G.; Winterton, D.; Di Pierro, M.; Soria, A.; Faverio, P.; Cairo, M.; Mori, S.; Messinesi, G.; Contro, E.; et al. Feasibility and physiological effects of prone positioning in non-intubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): A prospective cohort study. Lancet Respir. Med. 2020, 8, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Guérin, C.; Reignier, J.; Richard, J.-C.; Beuret, P.; Gacouin, A.; Boulain, T.; Mercier, E.; Badet, M.; Mercat, A.; Baudin, O.; et al. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013, 368, 2159–2168. [Google Scholar] [CrossRef]
- Lamm, W.J.; Graham, M.M.; Albert, R.K. Mechanism by which the prone position improves oxygenation in acute lung injury. Am. J. Respir. Crit. Care Med. 1994, 150, 184–193. [Google Scholar] [CrossRef]
- Mentzelopoulos, S.D.; Roussos, C.; Zakynthinos, S.G. Prone position reduces lung stress and strain in severe acute respiratory distress syndrome. Eur. Respir. J. 2005, 25, 534–544. [Google Scholar] [CrossRef]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14, S280–S288. [Google Scholar] [CrossRef]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute Respiratory Distress Syndrome: Advances in Diagnosis and Treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef]
- Fan, E.; Wilcox, M.E.; Brower, R.G.; Stewart, T.E.; Mehta, S.; Lapinsky, S.E.; Meade, M.O.; Ferguson, N.D. Recruitment maneuvers for acute lung injury: A systematic review. Am. J. Respir. Crit. Care Med. 2008, 178, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, C.; Goligher, E.C.; Young, M.E.; Keating, J.L.; Holland, A.E.; Romero, L.; Bradley, S.J.; Tuxen, D. Recruitment manoeuvres for adults with acute respiratory distress syndrome receiving mechanical ventilation. Cochrane Database Syst. Rev. 2016, 11, CD006667. [Google Scholar] [CrossRef] [PubMed]
- Shono, A.; Kotani, T. Clinical implication of monitoring regional ventilation using electrical impedance tomography. J. Intensive Care 2019, 7, 4. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, A.H.; Alcala, G.C.; Pavlovsky, B.; Roca, O.; Spadaro, S.; Scaramuzzo, G.; Chen, L.; Dianti, J.; Sousa, M.L.d.A.; Sklar, M.C.; et al. Lung Recruitment Assessed by Electrical Impedance Tomography (RECRUIT): A Multicenter Study of COVID-19 Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2023, 208, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Argiras, E.P.; Blakeley, C.R.; Dunnill, M.S.; Otremski, S.; Sykes, M.K. High PEEP decreases hyaline membrane formation in surfactant deficient lungs. Br. J. Anaesth. 1987, 59, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Broccard, A.; Shapiro, R.S.; Schmitz, L.L.; Adams, A.B.; Nahum, A.; Marini, J.J. Prone positioning attenuates and redistributes ventilator-induced lung injury in dogs. Crit. Care Med. 2000, 28, 295–303. [Google Scholar] [CrossRef]
- Rival, G.; Patry, C.; Floret, N.; Navellou, J.C.; Belle, E.; Capellier, G. Prone position and recruitment manoeuvre: The combined effect improves oxygenation. Crit. Care 2011, 15, R125. [Google Scholar] [CrossRef] [PubMed]
- Oczenski, W.; Hörmann, C.; Keller, C.; Lorenzl, N.; Kepka, A.; Schwarz, S.; Fitzgerald, R.D. Recruitment maneuvers during prone positioning in patients with acute respiratory distress syndrome. Crit. Care Med. 2005, 33, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Del Sorbo, L.; Grieco, D.L.; Junhasavasdikul, D.; Rittayamai, N.; Soliman, I.; Sklar, M.C.; Rauseo, M.; Ferguson, N.D.; Fan, E.; et al. Potential for Lung Recruitment Estimated by the Recruitment-to-Inflation Ratio in Acute Respiratory Distress Syndrome. A Clinical Trial. Am. J. Respir. Crit. Care Med. 2020, 201, 178–187. [Google Scholar] [CrossRef]
- Fan, E.; Del Sorbo, L.; Goligher, E.C.; Hodgson, C.L.; Munshi, L.; Walkey, A.J.; Adhikari, N.K.J.; Amato, M.B.P.; Branson, R.; Brower, R.G.; et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2017, 195, 1253–1263. [Google Scholar] [CrossRef]
- Pettenuzzo, T.; Sella, N.; Lorenzoni, G.; Calore, A.; Zarantonello, F.; Andreatta, G.; De Cassai, A.; Gregori, D.; Boscolo, A.; Navalesi, P. The Recruitment-to-Inflation Ratio Is Correlated with EIT-Derived Collapse and Overdistention in COVID-19 ARDS. Am. J. Respir. Crit. Care Med. 2022, 206, 1284–1286. [Google Scholar] [CrossRef]
- Cornejo, R.A.; Díaz, J.C.; Tobar, E.A.; Bruhn, A.R.; Ramos, C.A.; González, R.A.; Repetto, C.A.; Romero, C.M.; Gálvez, L.R.; Llanos, O.; et al. Effects of prone positioning on lung protection in patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2013, 188, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Grieco, D.L.; Delle Cese, L.; Menga, L.S.; Rosà, T.; Michi, T.; Lombardi, G.; Cesarano, M.; Giammatteo, V.; Bello, G.; Carelli, S.; et al. Physiological effects of awake prone position in acute hypoxemic respiratory failure. Crit. Care 2023, 27, 315. [Google Scholar] [CrossRef]
- Gainnier, M.; Michelet, P.; Thirion, X.; Arnal, J.-M.; Sainty, J.-M.; Papazian, L. Prone position and positive end-expiratory pressure in acute respiratory distress syndrome. Crit. Care Med. 2003, 31, 2719–2726. [Google Scholar] [CrossRef]
- Pelosi, P.; Gama de Abreu, M.; Rocco, P.R.M. New and conventional strategies for lung recruitment in acute respiratory distress syndrome. Crit. Care 2010, 14, 210. [Google Scholar] [CrossRef]
- Albert, S.P.; DiRocco, J.; Allen, G.B.; Bates, J.H.T.; Lafollette, R.; Kubiak, B.D.; Fischer, J.; Maroney, S.; Nieman, G.F. The role of time and pressure on alveolar recruitment. J Appl Physiol (1985) 2009, 106, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Attaway, A.H.; Scheraga, R.G.; Bhimraj, A.; Biehl, M.; Hatipoğlu, U. Severe COVID-19 pneumonia: Pathogenesis and clinical management. BMJ 2021, 372, n436. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Chiumello, D.; Caironi, P.; Busana, M.; Romitti, F.; Brazzi, L.; Camporota, L. COVID-19 pneumonia: Different respiratory treatments for different phenotypes? Intensive Care Med. 2020, 46, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 Does Not Lead to a “Typical” Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Caironi, P.; Cressoni, M.; Chiumello, D.; Ranieri, V.M.; Quintel, M.; Russo, S.; Patroniti, N.; Cornejo, R.; Bugedo, G. Lung recruitment in patients with the acute respiratory distress syndrome. N. Engl. J. Med. 2006, 354, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Fossali, T.; Pavlovsky, B.; Ottolina, D.; Colombo, R.; Basile, M.C.; Castelli, A.; Rech, R.; Borghi, B.; Ianniello, A.; Flor, N.; et al. Effects of Prone Position on Lung Recruitment and Ventilation-Perfusion Matching in Patients With COVID-19 Acute Respiratory Distress Syndrome: A Combined CT Scan/Electrical Impedance Tomography Study. Crit. Care Med. 2022, 50, 723–732. [Google Scholar] [CrossRef]
- Grasso, S.; Stripoli, T.; De Michele, M.; Bruno, F.; Moschetta, M.; Angelelli, G.; Munno, I.; Ruggiero, V.; Anaclerio, R.; Cafarelli, A.; et al. ARDSnet ventilatory protocol and alveolar hyperinflation: Role of positive end-expiratory pressure. Am. J. Respir. Crit. Care Med. 2007, 176, 761–767. [Google Scholar] [CrossRef]
- Wendel Garcia, P.D.; Caccioppola, A.; Coppola, S.; Pozzi, T.; Ciabattoni, A.; Cenci, S.; Chiumello, D. Latent class analysis to predict intensive care outcomes in Acute Respiratory Distress Syndrome: A proposal of two pulmonary phenotypes. Crit. Care 2021, 25, 154. [Google Scholar] [CrossRef]
- Del Sorbo, L.; Tonetti, T.; Ranieri, V.M. Alveolar recruitment in acute respiratory distress syndrome: Should we open the lung (no matter what) or may accept (part of) the lung closed? Intensive Care Med. 2019, 45, 1436–1439. [Google Scholar] [CrossRef]
- Zerbib, Y.; Lambour, A.; Maizel, J.; Kontar, L.; De Cagny, B.; Soupison, T.; Bradier, T.; Slama, M.; Brault, C. Respiratory effects of lung recruitment maneuvers depend on the recruitment-to-inflation ratio in patients with COVID-19-related acute respiratory distress syndrome. Crit. Care 2022, 26, 12. [Google Scholar] [CrossRef]
- Del Sorbo, L.; Tisminetzky, M.; Chen, L.; Brochard, L.; Arellano, D.; Brito, R.; Diaz, J.C.; Cornejo, R. Association of lung recruitment and change in recruitment-to-inflation ratio from supine to prone position in acute respiratory distress syndrome. Crit. Care 2023, 27, 140. [Google Scholar] [CrossRef]
- Grieco, D.L.; Bongiovanni, F.; Chen, L.; Menga, L.S.; Cutuli, S.L.; Pintaudi, G.; Carelli, S.; Michi, T.; Torrini, F.; Lombardi, G.; et al. Respiratory physiology of COVID-19-induced respiratory failure compared to ARDS of other etiologies. Crit. Care 2020, 24, 529. [Google Scholar] [CrossRef] [PubMed]
- Grasso, S.; Terragni, P.; Birocco, A.; Urbino, R.; Del Sorbo, L.; Filippini, C.; Mascia, L.; Pesenti, A.; Zangrillo, A.; Gattinoni, L.; et al. ECMO criteria for influenza A (H1N1)-associated ARDS: Role of transpulmonary pressure. Intensive Care Med. 2012, 38, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rothen, H.U.; Sporre, B.; Engberg, G.; Wegenius, G.; Hedenstierna, G. Re-expansion of atelectasis during general anaesthesia: A computed tomography study. Br. J. Anaesth. 1993, 71, 788–795. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.; Zheng, X.; Zhao, Z.; Zhong, M.; Jiang, L.; Huang, Y.; Liu, X.; Li, Y.; Zhang, D. Lung Recruitment, Individualized PEEP, and Prone Position Ventilation for COVID-19-Associated Severe ARDS: A Single Center Observational Study. Front Med. 2020, 7, 603943. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Palumbo, M.M.; Sverzellati, N.; Busana, M.; Malchiodi, L.; Bresciani, P.; Ceccarelli, P.; Sani, E.; Romitti, F.; Bonifazi, M.; et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022, 48, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Odenstedt, H.; Lindgren, S.; Olegård, C.; Erlandsson, K.; Lethvall, S.; Aneman, A.; Stenqvist, O.; Lundin, S. Slow moderate pressure recruitment maneuver minimizes negative circulatory and lung mechanic side effects: Evaluation of recruitment maneuvers using electric impedance tomography. Intensive Care Med. 2005, 31, 1706–1714. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar] [CrossRef]

| ALL (n = 58) | PP Combined LRM (n = 28) | PP (n = 30) | p | |
|---|---|---|---|---|
| Demographic data | ||||
| Age, mean (SD), years | 61.5 (15) | 60 (17.5) | 64 (12.8) | 0.16 |
| Female sex, n (%) | 11 (18.9%) | 4 (14.2) | 7 (23.3) | 0.38 |
| BMI, median (IQR), kg/m2 | 22.5 (20.7, 25.3) | 22.6 (21.1, 26.3) | 22.4 (19.8, 24.7) | 0.41 |
| APACHE II at ICU admission, median (IQR) | 23 (21, 27) | 24 (21, 28) | 23 (18.27) | 0.07 |
| RASS at ICU admission, median (IQR) | −4 (−4.5, −3) | −4(−4, −3) | −4(−4, −3) | 0.24 |
| Days intubated prior to randomization, median (IQR) | 2(1, 4) | 2(1, 3) | 2(2, 4) | 0.33 |
| Etiology of ARDS, n (%) | ||||
| COVID-19 | 28 (48.2) | 14 (50.0) | 14 (46.7) | 0.80 |
| Bacterial pneumonia | 15 (25.9) | 8 (28.6) | 7 (23.3) | 0.65 |
| Extrapulmonary | 15 (25.9) | 6 (21.4) | 9 (30.0) | 0.46 |
| Comorbidities, n (%) | ||||
| Hypertension | 24 (41.3) | 14 (50.0) | 10 (33.3) | 0.45 |
| Diabetes mellitus | 10 (18.9) | 3 (10.7) | 7 (23.3) | 0.30 |
| Renal insufficiency | 27 (46.5) | 15 (53.5) | 12 (40.0) | 0.30 |
| Hepatic insufficiency | 22 (37.9) | 10 (35.7) | 12 (40.0) | 0.73 |
| Hemodynamics, median (IQR) | ||||
| Heart rate (bpm) | 98 (78, 116) | 100 (82, 125) | 90 (76, 115) | 0.31 |
| SpO2 (%) | 99 (97, 100) | 98 (96, 100) | 99 (98, 100) | 0.20 |
| Mean arterial pressure (mmHg) | 85 (75.97) | 89 (76, 101) | 85 (72, 94) | 0.15 |
| Baseline ventilator Settings in supine position, median (IQR) | ||||
| Tidal volume (mL) | 449 (411, 492) | 445 (410.491) | 447 (302, 489) | 0.79 |
| Respiratory rate, breaths/min | 20 (18, 25) | 20 (18, 22) | 22 (18, 28) | 0.31 |
| PEEP (cmH2O) | 10 (8, 12) | 10 (10, 12) | 10 (6, 10) | 0.09 |
| Crs (mL/cmH2O) | 35 (23, 43) | 36 (27, 42) | 35 (25, 43) | 0.37 |
| Ppeak (cmH2O) | 27 (24, 33) | 27 (24, 32) | 30 (24, 39) | 0.78 |
| Pplat (cmH2O) | 22 (19, 27) | 23 (19, 26) | 24 (21, 32) | 0.48 |
| Pmean (cmH2O) | 16 (13, 18) | 16 (14, 18) | 15 (12, 19) | 0.29 |
| Raw(cmH2O) | 11 (7, 16) | 10 (7, 19) | 13 (6, 15) | 0.93 |
| Driving preassure (cmH2O) | 11 (5.5, 15.5) | 11 (9, 15) | 13 (7.5, 16) | 0.75 |
| Arterial blood gas, median (IQR) | ||||
| pH | 7.35 (7.29, 7.41) | 7.34 (7.29, 7.4) | 7.37 (7.29, 7.44) | 0.38 |
| PaO2 (mmHg) | 94.7 (80.5, 120.5) | 88.8 (75.2, 121.9) | 99.1 (82.0, 119.4) | 0.45 |
| PaCO2 (mmHg) | 43.6 (37.8, 52.5) | 44 (38.9, 51.3) | 42.7 (36.5, 55.3) | 0.65 |
| HCO3− (mmol/L) | 23.9 (21.4, 25.85) | 23.2 (20.9, 25.8) | 24.3 (21.7, 26.2) | 0.49 |
| Lactate (mmol/L) | 1.7 (1.3, 2.1) | 1.6 (1.2, 2.0) | 1.7 (1.4, 2.2) | 0.21 |
| Base excess (mmol/L) | −1.2 (−3.7, 1.6) | −1.9 (−4.5, 2) | −0.1 (−3.3, 1.6) | 0.71 |
| PaO2/FiO2 (mmHg) | 108 (86, 144) | 112 (89, 142) | 105 (83, 149) | 0.73 |
| Parameters, Median (IQR) | Prone Position Combined with PEEP-Induce LRM (28) | Prone Position (30) | ||||||
|---|---|---|---|---|---|---|---|---|
| T0 | T1 | T2 | p | T0 | T1 | T2 | p | |
| Resperatory mechanics | ||||||||
| PEEP (cmH2O) | 8 (6, 10) | 8 (6, 10) | 8 (6, 10) | 0.68 | 8.5 (7, 10) | 8.5 (7, 10) | 8.5 (7, 10) | 0.39 |
| Ppeak (cmH2O) | 28.5 (26.25, 34.75) | 30.5 (27, 34) | 26.5 (24, 31) b (p = 0.01) | 0.002 * | 26.50 (23, 32.25) | 27.5 (22.75, 35.25) | 25.0 (22.75, 34) | 0.15 |
| Pplat (cmH2O) | 23.5 (20, 26) | 24 (20.25, 25.75) | 22.5 (20, 26) | 0.38 | 21.00 (17.75, 26) | 22.5 (18, 27.25) | 21.0 (17.75, 27.25) | 0.45 |
| Pmean (cmH2O) | 17 (15, 18) | 17 (16, 19) | 16 (14, 18) b (p = 0.02) | 0.02 * | 15 (14, 18.25) | 16.5 (13, 18) | 15 (12.75, 17) | 0.04 |
| Tidal volume, (mL) | 446.5 (419.3, 489.5) | 460.5 (428.3, 502.8) | 464 (435, 502.5) | 0.24 | 431.5 (402.8, 491.3) | 442.5 (396.8, 487.8) | 447 (385.3, 515.8) | 0.16 |
| MV (L/min) | 9.21 (8.26, 11.23) | 10.3 (8.3, 13.1) | 9.89 (8.2, 12.5) | 0.31 | 10 (8.28, 11.33) | 10.55 (8.57, 11.83) | 10.12 (8.09, 11.88) | 0.90 |
| Crs (mL/cmH2O) | 35 (27, 42.75) | 35.5 (28.5, 46.25) | 35 (28.25, 47) | 0.26 | 30 (25, 43) | 32 (22.75, 43) | 30 (20, 46) | 0.46 |
| Raw (cmH2O) | 11 (8, 18) | 11 (9, 16) | 9(7, 11) b (p = 0.02) | 0.004 * | 11 (6, 18) | 12 (4.25, 17.5) | 7.55 (4.75, 15.25) b (p = 0.03) | 0.06 |
| PaO2 (mmHg) | 95.4 (81.5, 126.2) | 149.1 (120.9, 173.2) a (p < 0.0001) | 125.6 (94.2, 166.5) b (p = 0.002) | <0.0001 * | 94.7 (75.98, 122.4) | 107 (79.48, 131) | 113.6 (93.7, 129.9) b (p = 0.04) | 0.04 |
| PaCO2 (mmHg) | 45.05 (40.48, 48.38) | 42.7 (40.15, 47.45) | 43.3 (39.65, 48.78) | 0.53 | 46.4 (40.1, 52.75) | 46.4 (40.7, 52.48) | 44.9 (38.7, 51.43) | 0.09 |
| PaO2/FiO2, mmHg | 177 (141.6, 194.8) | 242.9 (214, 292.5) a (p < 0.0001) | 238.3 (176.4, 328.7) b (p < 0.0001) | <0.0001 * | 172.3 (122.5, 195.1) | 177.8 (116.7, 213.2) | 193.3 (122.8, 238.0) b (p = 0.02) | 0.03 |
| Hemodynamic parameters | ||||||||
| Heart rate (bpm) | 87 (74.25, 98.75) | 84 (77.5, 94) | 80.5 (69.25, 91) | 0.39 | 85.5 (74.75, 100.3) | 80.5 (69.5, 98) | 82 (69, 96.25) | 0.50 |
| SpO2 (%) | 100 (98, 100) | 99 (98, 99) | 100 (99, 100) | 0.66 | 100 (98, 100) | 99 (98, 99) | 100 (99, 100) | 0.83 |
| MAP (mmHg) | 97 (82.25, 103.8) | 89.5 (76.5, 99.5) | 86 (86, 99.25) | 0.08 | 84 (76, 93.5) | 85.5 (76.75, 93.75) | 81.5 (77, 90) | 0.20 |
| Prone Position Combined LRM (n = 28) | PP (n = 30) | p | |
|---|---|---|---|
| Mortality | |||
| 28-day mortality | 13 (46.4%) | 18 (60.0%) | 0.30 |
| 90-day mortality | 16 (57.1%) | 20 (66.6%) | 0.46 |
| Overall mortality | 19 (67.9%) | 22 (73.3%) | 0.65 |
| Length of stay, d | |||
| Intensive care unit, | 22 (17, 38) | 16 (9, 25) | 0.041 * |
| Hospital | 38 (21, 48) | 24 (15, 32) | 0.044 * |
| Mechanical ventilation, d | 19 (10, 27.5) | 15 (9, 23) | 0.13 |
| Adverse event, n% | |||
| Pneumothorax | 1 (3.3%) | 0 | 0.48 |
| Change in R/I > 0.5 after LRM (n = 16) | Change in R/I < 0.5 after LRM (n = 12) | p-Value | Change in R/I > 0.5 after pp (n = 16) | Change in R/I < 0.5 after pp (n = 12) | p-Value | |
|---|---|---|---|---|---|---|
| ∆tidal volume, (mL) | 10.0 (−10.0, 42.25) | 4.5 (−21.75, 57.25) | 0.53 | −1.0 (−16.5, 19.0) | 4.0 (−17.0, 38.0) | 0.36 |
| ∆MV (L/min) | 0.1 (−0.76, 1.13) | 0.0 (−0.61, 2.2) | 0.50 | −0.1 (−0.67, 0.47) | −0.03 (−0.65, 0.99) | 0.39 |
| ∆Crs (mL/cmH2O) | 3.5 (0.25, 7.0) | 1.0 (−6.25, 4.0) | 0.18 | 2.5 (−1.5, 10.3) | -0.5 (−3.0, 9.5) | 0.55 |
| ∆PaO2 (mmHg) | 76.05 (28.95, 117.9) | 21.45 (12.23, 43.4) | 0.01 * | 90.85 (14.05, 125.3) | 50.3 (21.2, 79.2) | 0.53 |
| ∆PaO2/FiO2 (mmHg) | 140.5 (55.4, 170.6) | 47.6 (25.85, 87.2) | 0.02 * | 51.65 (15.7, 161.8) | 54.85 (24.55, 144.9) | 0.83 |
| EIT data | ||||||
| ∆TV ROI 1 layers (%) | −2.0 (−3.75, −1.0) | −0.5 (−2.75, 0.0) | 0.21 | −1.0 (−11.25, 7.5) | −5.5 (−14.75, −0.25) | 0.28 |
| ∆TV ROI 2 layers (%) | −4.0 (−7.0, 1.0) | 0.0 (−4.0, 2.0) | 0.14 | 4.0 (−0.75, 12.75) | 4.0 (−6.75, 6.75) | 0.32 |
| ∆TV ROI 3 layers (%) | 5.0 (0.75, 6.75) | 2.0 (1.0, 3.75) | 0.02 * | −6.0 (−15.25, 6.75) | 2.0 (−10.25, 8.25) | 0.28 |
| ∆TV ROI 4 layers (%) | 1.0 (0.25, 4.0) | 0.5 (−0.75, 2.0) | 0.26 | 2.0 (−1.75, 4.0) | 5.5 (0.25, 6.75) | 0.99 |
| ∆ventral of tidal image region (%) | −6.5 (−9.0, −0.25) | −2.5 (−4.75, 1.25) | 0.07 | 4.0 (−9.25, 13.5) | −5.5 (−12.75, 6.25) | 0.10 |
| ∆dorsal of tidal image region (%) | 6.0 (1.5, 9.0) | 4.0 (−0.75, 4.75) | 0.04 * | −2.5 (−11.25, 11.0) | 4.0 (−5.0, 10.5) | 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, L.; Ni, Y.; Zhou, Y.; Fu, L.; Wu, W.; Li, P.; Yu, H.; Liang, G.; Luo, F. PEEP-Induced Lung Recruitment Maneuver Combined with Prone Position for ARDS: A Single-Center, Prospective, Randomized Clinical Trial. J. Clin. Med. 2024, 13, 853. https://doi.org/10.3390/jcm13030853
Lan L, Ni Y, Zhou Y, Fu L, Wu W, Li P, Yu H, Liang G, Luo F. PEEP-Induced Lung Recruitment Maneuver Combined with Prone Position for ARDS: A Single-Center, Prospective, Randomized Clinical Trial. Journal of Clinical Medicine. 2024; 13(3):853. https://doi.org/10.3390/jcm13030853
Chicago/Turabian StyleLan, Lan, Yuenan Ni, Yubei Zhou, Linxi Fu, Wentao Wu, Ping Li, He Yu, Guopeng Liang, and Fengming Luo. 2024. "PEEP-Induced Lung Recruitment Maneuver Combined with Prone Position for ARDS: A Single-Center, Prospective, Randomized Clinical Trial" Journal of Clinical Medicine 13, no. 3: 853. https://doi.org/10.3390/jcm13030853






