A Multidisciplinary Approach to End-Stage Limb Salvage in the Highly Comorbid Atraumatic Population: An Observational Study
Abstract
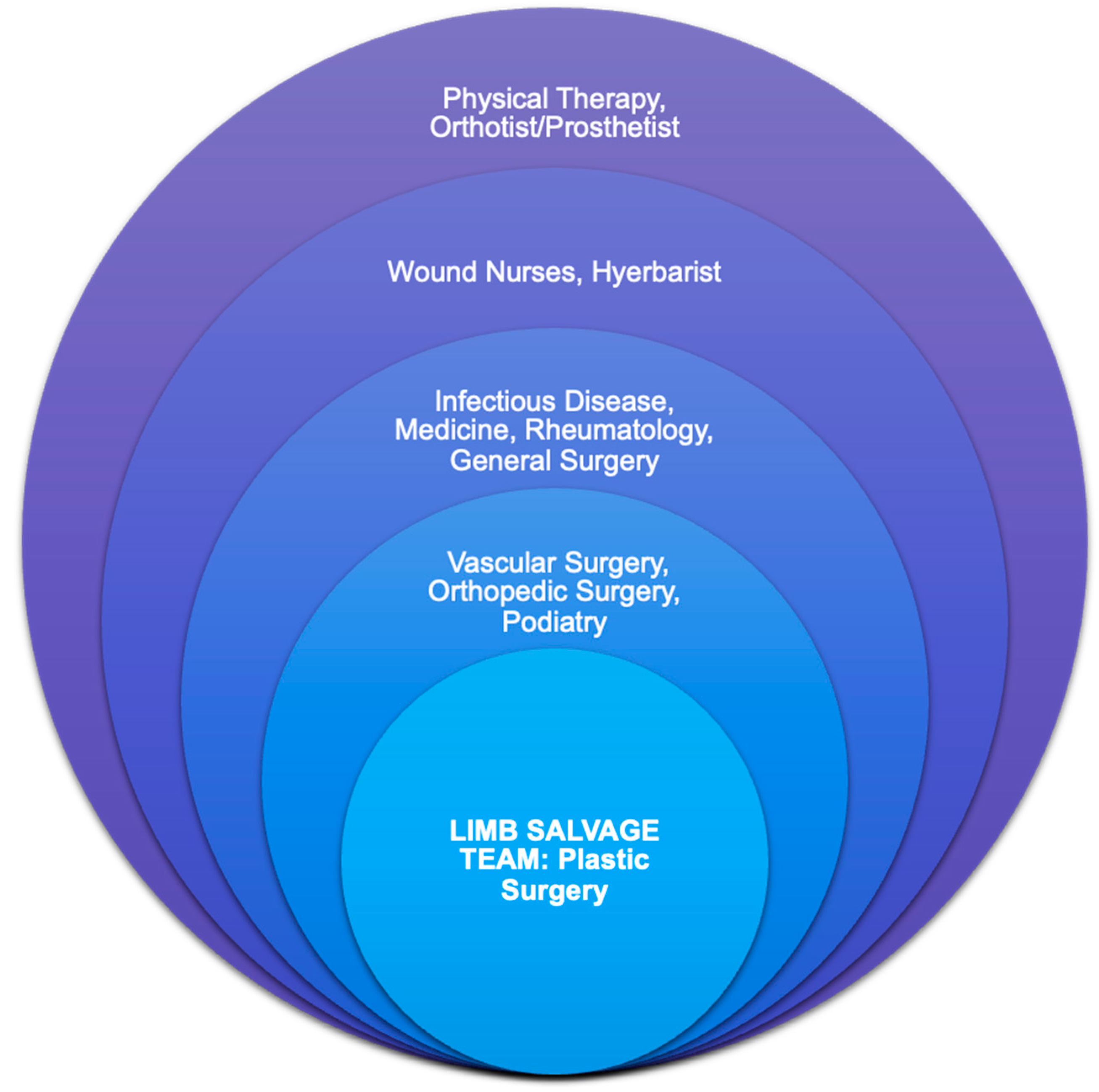
:1. Introduction
2. Methods
2.1. Preoperative Management
2.2. Intraoperative Management
2.3. Postoperative Management
2.4. Statistical Analysis
3. Results
3.1. Demographics
3.2. Preoperative Details
3.3. Operative Details
3.4. Postoperative Complications and Long-Term Outcomes
4. Discussion
4.1. Managing Patient Comorbidities
4.2. Infection Control
4.3. Optimizing for Diseased Vasculature
4.4. Long-Term Limb Salvage Outcomes
4.5. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dillingham, T.R.; Pezzin, L.E.; Shore, A.D. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputations. Arch. Phys. Med. Rehabil. 2005, 86, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Meshkin, D.H.; Zolper, E.G.; Chang, K.; Bryant, M.; Bekeny, J.C.; Evans, K.K.; Attinger, C.E.; Fan, K.L. Long-term Mortality After Nontraumatic Major Lower Extremity Amputation: A Systematic Review and Meta-analysis. J. Foot Ankle Surg. 2021, 60, 567–576. [Google Scholar] [CrossRef]
- Singh, G.; Chawla, S. Amputation in Diabetic Patients. Med. J. Armed Forces India 2006, 62, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Carlson, T.; Reed, J.F., 3rd. A case-control study of the risk factors for toe amputation in a diabetic population. Int. J. Low. Extrem. Wounds 2003, 2, 19–21. [Google Scholar] [CrossRef]
- Miyajima, S.; Shirai, A.; Yamamoto, S.; Okada, N.; Matsushita, T. Risk factors for major limb amputations in diabetic foot gangrene patients. Diabetes Res. Clin. Pract. 2006, 71, 272–279. [Google Scholar] [CrossRef]
- Calle-Pascual, A.L.; Garcia-Torre, N.; Moraga, I.; Diaz, J.A. Epidemiology of nontraumatic lower-extremity amputation in area 7, Madrid, between 1989 and 1999: A population-based study. Diabetes Care 2001, 24, 1686–1689. [Google Scholar] [CrossRef]
- Holstein, P.; Ellitsgaard, N.; Olsen, B.B.; Ellitsgaard, V. Decreasing incidence of major amputations in people with diabetes. Diabetologia 2000, 43, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Bui, D.T.; Cordeiro, P.G.; Hu, Q.Y.; Disa, J.J.; Pusic, A.; Mehrara, B.J. Free flap reexploration: Indications, treatment, and outcomes in 1193 free flaps. Plast. Reconstr. Surg. 2007, 119, 2092–2100. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.R.; Patil, R.K.; Nahar, S. Unfavourable results in free tissue transfer. Indian J. Plast. Surg. 2013, 46, 247–255. [Google Scholar] [CrossRef]
- Pohlenz, P.; Klatt, J.; Schön, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int. J. Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef]
- Quan, H.; Li, B.; Couris, C.M.; Fushimi, K.; Graham, P.; Hider, P.; Januel, J.M.; Sundararajan, V. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am. J. Epidemiol. 2011, 173, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; DeFazio, M.V.; Lakhiani, C.; Abboud, M.; Penzler, M.; Elmarsafi, T.; Kim, P.J.; Attinger, C.E.; Evans, K.K. Limb Salvage and Functional Outcomes following Free Tissue Transfer for the Treatment of Recalcitrant Diabetic Foot Ulcers. J. Reconstr. Microsurg. 2019, 35, 117–123. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, M.V.; Economides, J.M.; Anghel, E.L.; Tefera, E.A.; Evans, K.K. Lower Extremity Free Tissue Transfer in the Setting of Thrombophilia: Analysis of Perioperative Anticoagulation Protocols and Predictors of Flap Failure. J. Reconstr. Microsurg. 2019, 35, 270–286. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, M.V.; Hung, R.W.; Han, K.D.; Bunting, H.A.; Evans, K.K. Lower Extremity Flap Salvage in Thrombophilic Patients: Managing Expectations in the Setting of Microvascular Thrombosis. J. Reconstr. Microsurg. 2016, 32, 431–444. [Google Scholar] [CrossRef] [PubMed]
- DeFazio, M.V.; Han, K.D.; Akbari, C.M.; Evans, K.K. Free tissue transfer after targeted endovascular reperfusion for complex lower extremity reconstruction: Setting the stage for success in the presence of mutlivessel disease. Ann. Vasc. Surg. 2015, 29, 1316.e7–1316.e15. [Google Scholar] [CrossRef] [PubMed]
- Janhofer, D.E.; Lakhiani, C.; Kim, P.J.; Akbari, C.; Naz, I.; Tefera, E.A.; Attinger, C.E.; Evans, K.K. The Utility of Preoperative Arteriography for Free Flap Planning in Patients with Chronic Lower Extremity Wounds. Plast. Reconstr. Surg. 2019, 143, 604–613. [Google Scholar] [CrossRef]
- Black, C.K.; Zolper, E.G.; Ormiston, L.D.; Schwitzer, J.A.; Luvisa, K.; Attinger, C.E.; Fan, K.L.; Evans, K.K. Free Anterolateral Thigh Versus Vastus Lateralis Muscle Flaps for Coverage of Lower Extremity Defects in Chronic Wounds. Ann. Plast. Surg. 2020, 85 (Suppl. S1), S54–S59. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Fan, K.L.; Defazio, M.V.; Luvisa, K.; Reynolds, K.; Kotha, V.S.; Attinger, C.E.; Evans, K.K. Limb Salvage Rates and Functional Outcomes Using a Longitudinal Slit Arteriotomy End-to-Side Anastomosis for Limb-Threatening Defects in a High-Risk Patient Population. Plast. Reconstr. Surg. 2020, 145, 1302–1312. [Google Scholar] [CrossRef]
- DeFazio, M.V.; Fan, K.L.; Evans, K.K. Greater Saphenous Vein-Patch Interposition to Facilitate Flow-Sparing Microanastomosis of Calcified Arteries in the Distal Lower Extremity. Plast. Reconstr. Surg. 2019, 144, 340e–341e. [Google Scholar] [CrossRef]
- Dekker, P.K.; Abdou, S.A.; Youn, R.; Bekeny, J.C.; Kim, K.G.; Zolper, E.G.; Fan, K.L.; Evans, K.K. Saphenous Vein Interposition Grafts in Lower Extremity Reconstruction: Appraisal of Technique and Case Series. Plast. Reconstr. Surg. Glob. Open 2022, 10, e4536. [Google Scholar] [CrossRef]
- Lenz, Y.; Gross, R.; Penna, V.; Bannasch, H.; Stark, G.B.; Eisenhardt, S.U. Evaluation of the Implantable Doppler Probe for Free Flap Monitoring in Lower Limb Reconstruction. J. Reconstr. Microsurg. 2018, 34, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Rozen, W.M.; Enajat, M.; Whitaker, I.S.; Lindkvist, U.; Audolfsson, T.; Acosta, R. Postoperative monitoring of lower limb free flaps with the Cook-Swartz implantable Doppler probe: A clinical trial. Microsurgery 2010, 30, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Schmulder, A.; Gur, E.; Zaretski, A. Eight-year experience of the Cook-Swartz Doppler in free-flap operations: Microsurgical and reexploration results with regard to a wide spectrum of surgeries. Microsurgery 2011, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nigam, M.; Zolper, E.G.; Sharif-Askary, B.; Abdou, S.A.; Charipova, K.; Bekeny, J.C.; Fan, K.L.; Steinberg, J.S.; Attinger, C.E.; Evans, K.K. Expanding Criteria for Limb Salvage in Comorbid Patients with Nonhealing Wounds: The MedStar Georgetown Protocol and Lessons Learned after 200 Lower Extremity Free Flaps. Plast. Reconstr. Surg. 2022, 150, 197–209. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Choi, M.S.; Jeon, S.B.; Lee, J.H. Predictive factors for successful limb salvage surgery in diabetic foot patients. BMC Surg. 2014, 14, 113. [Google Scholar] [CrossRef]
- McEwen, L.N.; Ylitalo, K.R.; Munson, M.; Herman, W.H.; Wrobel, J.S. Foot Complications and Mortality: Results from Translating Research Into Action for Diabetes (TRIAD). J. Am. Podiatr. Med. Assoc. 2016, 106, 7–14. [Google Scholar] [CrossRef]
- Oh, T.S.; Lee, H.S.; Hong, J.P. Diabetic foot reconstruction using free flaps increases 5-year-survival rate. J. Plast. Reconstr. Aesthet. Surg. 2013, 66, 243–250. [Google Scholar] [CrossRef]
- Singh, N.; Armstrong, D.G.; Lipsky, B.A. Preventing foot ulcers in patients with diabetes. JAMA 2005, 293, 217–228. [Google Scholar] [CrossRef]
- Stern, J.R.; Wong, C.K.; Yerovinkina, M.; Spindler, S.J.; See, A.S.; Panjaki, S.; Loven, S.L.; D’Andrea, R.F., Jr.; Nowygrod, R. A Meta-analysis of Long-term Mortality and Associated Risk Factors following Lower Extremity Amputation. Ann. Vasc. Surg. 2017, 42, 322–327. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of medical care in diabetes—2010. Diabetes Care 2010, 33 (Suppl. S1), S11–S61. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Brownrigg, J.R.; Apelqvist, J.; Bakker, K.; Schaper, N.C.; Hinchliffe, R.J. Evidence-based management of PAD & the diabetic foot. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Ndosi, M.; Wright-Hughes, A.; Brown, S.; Backhouse, M.; Lipsky, B.A.; Bhogal, M.; Reynolds, C.; Vowden, P.; Jude, E.B.; Nixon, J.; et al. Prognosis of the infected diabetic foot ulcer: A 12-month prospective observational study. Diabet. Med. 2018, 35, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Reiber, G.E. The epidemiology of diabetic foot problems. Diabet. Med. 1996, 13 (Suppl. S1), S6–S11. [Google Scholar] [CrossRef] [PubMed]
- Endara, M.; Masden, D.; Goldstein, J.; Gondek, S.; Steinberg, J.; Attinger, C. The role of chronic and perioperative glucose management in high-risk surgical closures: A case for tighter glycemic control. Plast. Reconstr. Surg. 2013, 132, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, M.K.; Heit, J.A.; Ashrani, A.A.; Leibson, C.L.; Petterson, T.M.; Bailey, K.R. Is progestin an independent risk factor for incident venous thromboembolism? A population-based case-control study. Thromb. Res. 2010, 126, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Edmonds, M.J.; Crichton, T.J.; Runciman, W.B.; Pradhan, M. Evidence-based risk factors for postoperative deep vein thrombosis. ANZ J. Surg. 2004, 74, 1082–1097. [Google Scholar] [CrossRef]
- Sweetland, S.; Parkin, L.; Balkwill, A.; Green, J.; Reeves, G.; Beral, V. Smoking, surgery, and venous thromboembolism risk in women: United Kingdom cohort study. Circulation 2013, 127, 1276–1282. [Google Scholar] [CrossRef]
- Heit, J.A.; Melton, L.J., III; Lohse, C.M.; Petterson, T.M.; Silverstein, M.D.; Mohr, D.N.; O’fallon, W.M. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin. Proc. 2001, 76, 1102–1110. [Google Scholar] [CrossRef]
- Deldar, R.; Gupta, N.; Bovill, J.D.; Zolper, E.G.; Kim, K.G.; Fan, K.L.; Evans, K.K. Risk-Stratified Anticoagulation Protocol Increases Success of Lower Extremity Free Tissue Transfer in the Setting of Thrombophilia. Plast. Reconstr. Surg. 2023, 152, 653–666. [Google Scholar] [CrossRef]
- Zolper, E.G.; Lavin, C.V.; Deldar, R.; Bekeny, J.C.; Fan, K.L.; Evans, K.K. Implementation of a Stratified Anticoagulation Protocol Increases Lower Extremity Free Tissue Transfer Success in the Setting of Thrombophilia. Plast. Reconstr. Surg. Glob. Open. 2020, 8 (Suppl. S9), 76. [Google Scholar] [CrossRef]
- Patel, M.S.; Carson, J.L. Anemia in the preoperative patient. Med. Clin. N. Am. 2009, 93, 1095–1104. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.I.; Boyko, E.J.; Ahroni, J.H.; Smith, D.G. Lower-extremity amputation in diabetes. The independent effects of peripheral vascular disease, sensory neuropathy, and foot ulcers. Diabetes Care 1999, 22, 1029–1035. [Google Scholar] [CrossRef]
- Leibson, C.L.; Ransom, J.E.; Olson, W.; Zimmerman, B.R.; O’Fallon, W.M.; Palumbo, P.J. Peripheral arterial disease, diabetes, and mortality. Diabetes Care 2004, 27, 2843–2849. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
- Afonso, A.C.; Oliveira, D.; Saavedra, M.J.; Borges, A.; Simões, M. Biofilms in Diabetic Foot Ulcers: Impact, Risk Factors and Control Strategies. Int. J. Mol. Sci. 2021, 22, 8278. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Soares, M.; Boyko, E.J.; Ribeiro, J.; Ribeiro, I.; Dinis-Ribeiro, M. Predictive factors for diabetic foot ulceration: A systematic review. Diabetes/Metab. Res. Rev. 2012, 28, 574–600. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Moura, J.; Carvalho, E.; Empadinhas, N. Microbiota of Chronic Diabetic Wounds: Ecology, Impact, and Potential for Innovative Treatment Strategies. Front. Microbiol. 2017, 8, 1791. [Google Scholar] [CrossRef]
- James, G.A.; Swogger, E.; Wolcott, R.; Pulcini, E.D.; Secor, P.; Sestrich, J.; Costerton, J.W.; Stewart, P.S. Biofilms in chronic wounds. Wound Repair Regen. 2008, 16, 37–44. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L. Slough and biofilm: Removal of barriers to wound healing by desloughing. J Wound Care 2015, 24, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-F.; Ni, P.-W.; Huang, Y.; Xie, T. Therapeutic strategies for chronic wound infection. Chin. J. Traumatol. 2022, 25, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; On, E.; Hadas, N.; Halperin, N.; Hofman, S.; Boldur, I. Microbiologic flora contaminating open fractures: Its significance in the choice of primary antibiotic agents and the likelihood of deep wound infection. J. Orthop. Trauma. 1989, 3, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kanuri, A.; O’Kelly, N.D.; Shuck, J.; Kim, P.; Evans, K.K.; Attinger, C.E. The Effect of Positive Postdebridement Cultures on Local Muscle Flap Reconstruction of the Lower Extremity. Plast. Reconstr. Surg. Glob. Open 2018, 6, e1864. [Google Scholar] [CrossRef] [PubMed]
- Bahr, C. CVI and PAD: A review of venous and arterial disease. JAAPA 2007, 20, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Cushman, M. Epidemiology and risk factors for venous thrombosis. Semin. Hematol. 2007, 44, 62–69. [Google Scholar] [CrossRef]
- Shabani Varaki, E.; Gargiulo, G.D.; Penkala, S.; Breen, P.P. Peripheral vascular disease assessment in the lower limb: A review of current and emerging non-invasive diagnostic methods. Biomed. Eng. Online 2018, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, V.; Vincent, G.; Azdad, K.; Hubermont, G.; Ledent, G.; Ngongang, C.; Filimon, A.M. A reliable approach to diabetic neuroischemic foot wounds: Below-the-knee angiosome-oriented angioplasty. J. Endovasc. Ther. 2011, 18, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Apelqvist, J.A.; Lepäntalo, M.J. The ulcerated leg: When to revascularize. Diabetes/Metab. Res. Rev. 2012, 28 (Suppl. S1), 30–35. [Google Scholar] [CrossRef] [PubMed]
- Elbadawy, A.; Ali, H.; Saleh, M.; Hasaballah, A. Editor’s Choice—A Prospective Study to Evaluate Complete Wound Healing and Limb Salvage Rates after Angiosome Targeted Infrapopliteal Balloon Angioplasty in Patients with Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2018, 55, 392–397. [Google Scholar] [CrossRef]
- Ji, D.; Zhang, T.; Li, C.; Liu, Y.; Wang, F. Evaluation of angiosome-targeted infrapopliteal endovascular revascularization in critical diabetic limb ischemia. J. Interv. Med. 2018, 1, 176–181. [Google Scholar] [CrossRef]
- Sumpio, B.E.; Lee, T.; Blume, P.A. Vascular evaluation and arterial reconstruction of the diabetic foot. Clin. Podiatr. Med. Surg. 2003, 20, 689–708. [Google Scholar] [CrossRef]
- Davies, M.G.; Anaya-Ayala, J.E. Endovascular techniques in limb salvage: Cutting, cryo, brachy, and drug-eluting balloons. Methodist DeBakey Cardiovasc. J. 2013, 9, 69–72. [Google Scholar] [CrossRef]
- Werk, M.; Langner, S.; Reinkensmeier, B.; Boettcher, H.F.; Tepe, G.; Dietz, U.; Hosten, N.; Hamm, B.; Speck, U.; Ricke, J. Inhibition of restenosis in femoropopliteal arteries: Paclitaxel-coated versus uncoated balloon: Femoral paclitaxel randomized pilot trial. Circulation 2008, 118, 1358–1365. [Google Scholar] [CrossRef]
- Kubo, T.; Yano, K.; Hosokawa, K. Management of flaps with compromised venous outflow in head and neck microsurgical reconstruction. Microsurgery 2002, 22, 391–395. [Google Scholar] [CrossRef]
- Riot, S.; Herlin, C.; Mojallal, A.; Garrido, I.; Bertheuil, N.; Filleron, T.; Somda, S.; Grolleau, J.L.; Lopez, R.; Chaput, B. A Systematic Review and Meta-Analysis of Double Venous Anastomosis in Free Flaps. Plast. Reconstr. Surg. 2015, 136, 1299–1311. [Google Scholar] [CrossRef]
- Demirkan, F.; Wei, F.C.; Lutz, B.S.; Cher, T.S.; Chen, I.H. Reliability of the venae comitantes in venous drainage of the free radial forearm flaps. Plast. Reconstr. Surg. 1998, 102, 1544–1548. [Google Scholar] [CrossRef]
- Ichinose, A.; Terashi, H.; Nakahara, M.; Sugimoto, I.; Hashikawa, K.; Nomura, T.; Ogata, N.; Yokoo, S.; Tahara, S. Do multiple venous anastomoses reduce risk of thrombosis in free-flap transfer? Efficacy of dual anastomoses of separate venous systems. Ann. Plast. Surg. 2004, 52, 61–63. [Google Scholar] [CrossRef]
- Matthews, J.L.K.; Alolabi, N.; Farrokhyar, F.; Voineskos, S.H. One versus 2 Venous Anastomoses in Free Flap Surgery: A Systematic Review and Meta-Analysis. Plast. Surg. 2018, 26, 91–98. [Google Scholar] [CrossRef]
- Ahmadi, I.; Herle, P.; Rozen, W.M.; Leong, J. One versus two venous anastomoses in microsurgical free flaps: A meta-analysis. J. Reconstr. Microsurg. 2014, 30, 413–418. [Google Scholar] [CrossRef]
- Enajat, M.; Rozen, W.M.; Whitaker, I.S.; Smit, J.M.; Acosta, R. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: Investigating the effect on venous congestion and flap survival. Microsurgery 2010, 30, 185–191. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Kocak, E.; Ogunleye, O.; Hartley, C.J.; Miller, M.J. One versus two venous anastomoses in microvascular free flap surgery. Plast. Reconstr. Surg. 2010, 126, 1548–1557. [Google Scholar] [CrossRef]
- Heidekrueger, P.I.; Ehrl, D.; Heine-Geldern, A.; Ninkovic, M.; Broer, P.N. One versus two venous anastomoses in microvascular lower extremity reconstruction using gracilis muscle or anterolateral thigh flaps. Injury 2016, 47, 2828–2832. [Google Scholar] [CrossRef]
- Bigdeli, A.K.; Gazyakan, E.; Schmidt, V.J.; Bauer, C.; Germann, G.; Radu, C.A.; Kneser, U.; Hirche, C. Long-Term Outcome after Successful Lower Extremity Free Flap Salvage. J. Reconstr. Microsurg. 2019, 35, 263–269. [Google Scholar] [CrossRef]
- Moellhoff, N.; Gernert, C.; Frank, K.; Giunta, R.E.; Ehrl, D. The 72-Hour Microcirculation Dynamics in Viable Free Flap Reconstructions. J. Reconstr. Microsurg. 2022, 38, 637–646. [Google Scholar] [CrossRef]
- Lin, S.J.; Nguyen, M.-D.; Chen, C.; Colakoglu, S.; Curtis, M.S.; Tobias, A.M.; Lee, B.T. Tissue oximetry monitoring in microsurgical breast reconstruction decreases flap loss and improves rate of flap salvage. Plast. Reconstr. Surg. 2011, 127, 1080–1085. [Google Scholar] [CrossRef]
- Abdou, S.A.; Sharif-Askary, B.; Zolper, E.G.; Evans, K.K. Intraoperative Utility of the Implantable Doppler in Lower Extremity Reconstruction: A Matched Case-control Study. Plast. Reconstr. Surg. Glob. Open 2020, 8, e3229. [Google Scholar] [CrossRef]
- Schaper, N.C.; van Netten, J.J.; Apelqvist, J.; Bus, S.A.; Hinchliffe, R.J.; Lipsky, B.A. Practical Guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36 (Suppl. S1), e3266. [Google Scholar] [CrossRef]
- Attinger, C.; Venturi, M.; Kim, K.; Ribiero, C. Maximizing length and optimizing biomechanics in foot amputations by avoiding cookbook recipes for amputation. Semin. Vasc. Surg. 2003, 16, 44–66. [Google Scholar] [CrossRef]
- Caravaggi, C.M.; Sganzaroli, A.B.; Galenda, P.; Balaudo, M.; Gherardi, P.; Simonetti, D.; Ferraresi, R.; Farnetti, A.; Morandi, A. Long-term follow-up of tibiocalcaneal arthrodesis in diabetic patients with early chronic Charcot osteoarthropathy. J. Foot Ankle Surg. 2012, 51, 408–411. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Bevilacqua, N.J.; Habershaw, G. Surgical off-loading of the diabetic foot. J. Vasc. Surg. 2010, 52 (Suppl. S3), 44s–58s. [Google Scholar] [CrossRef] [PubMed]
- Simon, S.R.; Tejwani, S.G.; Wilson, D.L.; Santner, T.J.; Denniston, N.L. Arthrodesis as an early alternative to nonoperative management of charcot arthropathy of the diabetic foot. J. Bone Jt. Surg. 2000, 82, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.C.; Crews, R.T.; Armstrong, D.G. The pivotal role of offloading in the management of neuropathic foot ulceration. Curr. Diab. Rep. 2005, 5, 423–429. [Google Scholar] [CrossRef] [PubMed]


| Total, n (%) | ||
|---|---|---|
| Age, mean ± SD | 55.88 ± 13.62 | |
| Sex | ||
| Male | 212 (70.7%) | |
| Female | 88 (29.3%) | |
| Race | ||
| White | 134 (44.8%) | |
| Black or African American | 131 (43.8%) | |
| Hispanic | 10 (3.3%) | |
| Asian | 6 (2.0%) | |
| Other/unknown | 18 (6.0%) | |
| BMI (kg/m2), median (IQR) | 28.5 (7.7) | |
| Hospital LOS, median (IQR) | 27 (16) | |
| Postop LOS, median (IQR) | 14 (8.5) | |
| Smoking | ||
| Never smoker | 191 (63.7%) | |
| Former | 69 (23%) | |
| Current | 40 (13.3%) | |
| CCI, median (IQR) | 4 (3) | |
| DM | 164 (54.7%) | |
| PVD | 105 (35%) | |
| ESRD | 15 (5%) | |
| VTE | 25 (8.3%) | |
| Transplant history | 9 (3%) | |
| MI | 12 (4%) | |
| CVA/TIA | 14 (4.67%) | |
| Malignancy history | 34 (11.33%) | |
| CKD | 47 (15.67%) | |
| CHF | 16 (5.33%) | |
| COPD | 9 (3%) | |
| Hypercoagulability | 230 (76.7%) | |
| Home AC | 20 (6.67%) | |
| Home AP | 114 (38%) | |
| Total | |
|---|---|
| WBC (×109/L), mean ± SD | 7.95 ± 2.71 |
| ESR (mm/h), mean ± SD | 66.28 ± 39.69 |
| CRP (mg/dL), median (IQR) | 16.5 (35.5) |
| HgbA1c (%), median (IQR) | 6.4 (2.6) |
| Albumin (g/dL), median (IQR) | 3.1 (1.0) |
| Prealbumin (mg/dL), mean ± SD | 19.0 ± 7.0 |
| Hgb on DOS (g/dL), mean ± SD | 9.98 ± 1.71 |
| Platelet count (/microL), median (IQR) | 274 (131) |
| Total, n (%) | ||
|---|---|---|
| Wound area (cm2), median (IQR) | 77.5 (72) | |
| Wound location | ||
| Forefoot | 55 (18.3%) | |
| Midfoot | 50 (16.7%) | |
| Hindfoot | 65 (21.7%) | |
| Ankle | 96 (32%) | |
| Lower leg | 61 (20.3%) | |
| Knee | 12 (4%) | |
| TMA site | 36 (12%) | |
| BKA stump | 4 (1.33%) | |
| Anterior leg | 54 (18%) | |
| Posterior leg | 43 (14.3%) | |
| Plantar foot | 58 (19.3%) | |
| Dorsal foot | 47 (15.7%) | |
| Medial leg | 55 (18.3%) | |
| Lateral leg | 59 (19.7%) | |
| Charcot arthropathy | 27 (9%) | |
| Total debridements, median (IQR) | 3 (1) | |
| Time from initial DBT to FTT (days), median (IQR) | 10 (8.5) | |
| Total, n (%) | ||
|---|---|---|
| Preoperative LE angiogram * | 294 (98.0%) | |
| Time from angiogram to FTT (days), median (IQR) | 8 (9) | |
| Anterior tibial artery | ||
| Patent | 214 (73.0%) | |
| Occluded | 52 (17.8%) | |
| Reconstituted | 27 (9.2%) | |
| Posterior tibial artery | ||
| Patent | 222 (75.77%) | |
| Occluded | 55 (18.77%) | |
| Reconstituted | 16 (5.46%) | |
| Peroneal artery | ||
| Patent | 255 (87.03%) | |
| Occluded | 26 (8.87%) | |
| Reconstituted | 12 (4.1%) | |
| Dorsalis pedis | ||
| Patent | 236 (89.06%) | |
| Occluded | 18 (6.79%) | |
| Reconstituted | 11 (4.15%) | |
| Vessel run-off, initial | ||
| 3 | 166 (56.46%) | |
| 2 | 79 (26.87%) | |
| 1 | 39 (13.27%) | |
| 0 | 10 (3.40%) | |
| Endovascular interventions | 56 (18.67%) | |
| Balloon angioplasty | 55 (98.21%) | |
| Stent placement | 3 (5.36%) | |
| Vascular bypass | 4 (1.33%) | |
| Time from bypass to FTT (days), median (IQR) | 16 (1.5%) | |
| Time from intervention to FTT (days), median (IQR) | 9.5 (8) | |
| Venous Mapping ** | 233 (77.7%) | |
| Venous reflux | ||
| None | 45 (24.9%) | |
| Deep | 54 (29.8%) | |
| Superficial | 20 (11.1%) | |
| Both | 62 (34.3%) | |
| Venous thrombosis | ||
| None | 192 (83.1%) | |
| Deep | 12 (5.2%) | |
| Superficial | 23 (10.0%) | |
| Both | 4 (1.7%) | |
| Total | |||
|---|---|---|---|
| Flap type | |||
| ALT/AMT | 158 (52.7%) | ||
| Vastus lateralis | 76 (25.3%) | ||
| Gracilis | 3 (1.0%) | ||
| Rectus Femoris | 3 (1.0%) | ||
| Radial Forearm | 6 (2.0%) | ||
| Latissimus Dorsi | 14 (4.7%) | ||
| Parascapular | 1 (0.3%) | ||
| MSAP | 4 (1.3%) | ||
| MFC | 1 (0.3%) | ||
| Rectus Abdominis | 4 (1.3%) | ||
| Free Fibular | 1 (0.3%) | ||
| SCIP | 1 (0.3%) | ||
| Flap tissue composition | |||
| Chimeric | 28 (9.3%) | ||
| Adipofascial | 7 (2.3%) | ||
| Fasciocutaneous | 163 (54.3%) | ||
| Muscle | 97 (32.3%) | ||
| Myocutaneous | 6 (2.0%) | ||
| Total, n (%) | |
|---|---|
| Immediate flap success | 289 (96.3%) |
| Takeback (POD0–7) | 18.0 (6.0%) |
| Time to takeback (days), median (IQR) | 1.5 (7) |
| Flap salvage | 11 (3.7%) |
| Partial flap necrosis (POD0–12) | 10 (3.3%) |
| Hematoma | 16 (5.3%) |
| Dehiscence | 47 (15.7%) |
| Infection | 42 (14.0%) |
| Donor site complication | 23 (7.7%) |
| Postoperative ipsilateral amputation | 38 (12.7%) |
| Time to amputation (days), median (IQR) | 169 (220) |
| Postoperative contralateral amputation | 4 (1.3%) |
| Follow-up duration (months), median (IQR) | 14.95 (24.3) |
| Time to ambulation (months), median (IQR) | 3.2 (6.0) |
| Ambulatory | 253 (84.3%) |
| Mortality | 22 (7.4%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.R.; Lava, C.X.; Neughebauer, M.B.; Rohrich, R.N.; Atves, J.; Steinberg, J.; Akbari, C.M.; Youn, R.C.; Attinger, C.E.; Evans, K.K. A Multidisciplinary Approach to End-Stage Limb Salvage in the Highly Comorbid Atraumatic Population: An Observational Study. J. Clin. Med. 2024, 13, 2406. https://doi.org/10.3390/jcm13082406
Li KR, Lava CX, Neughebauer MB, Rohrich RN, Atves J, Steinberg J, Akbari CM, Youn RC, Attinger CE, Evans KK. A Multidisciplinary Approach to End-Stage Limb Salvage in the Highly Comorbid Atraumatic Population: An Observational Study. Journal of Clinical Medicine. 2024; 13(8):2406. https://doi.org/10.3390/jcm13082406
Chicago/Turabian StyleLi, Karen R., Christian X. Lava, Monique B. Neughebauer, Rachel N. Rohrich, Jayson Atves, John Steinberg, Cameron M. Akbari, Richard C. Youn, Christopher E. Attinger, and Karen K. Evans. 2024. "A Multidisciplinary Approach to End-Stage Limb Salvage in the Highly Comorbid Atraumatic Population: An Observational Study" Journal of Clinical Medicine 13, no. 8: 2406. https://doi.org/10.3390/jcm13082406






