Effects of Combination Therapy with Intravitreal Ranibizumab and Tissue Plasminogen Activator for Neovascular Age-Related Macular Degeneration
Abstract
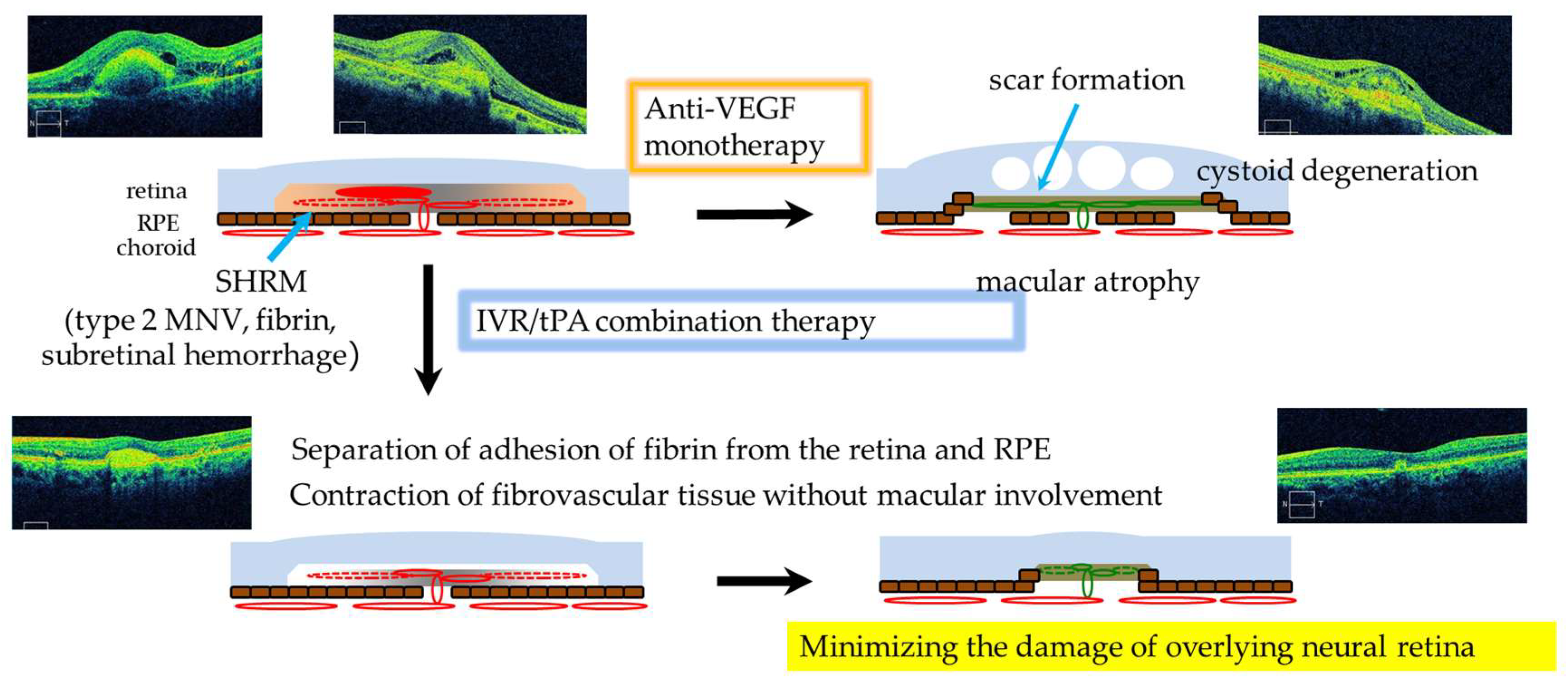
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics
2.2. Patients
2.3. Research and Analysis
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
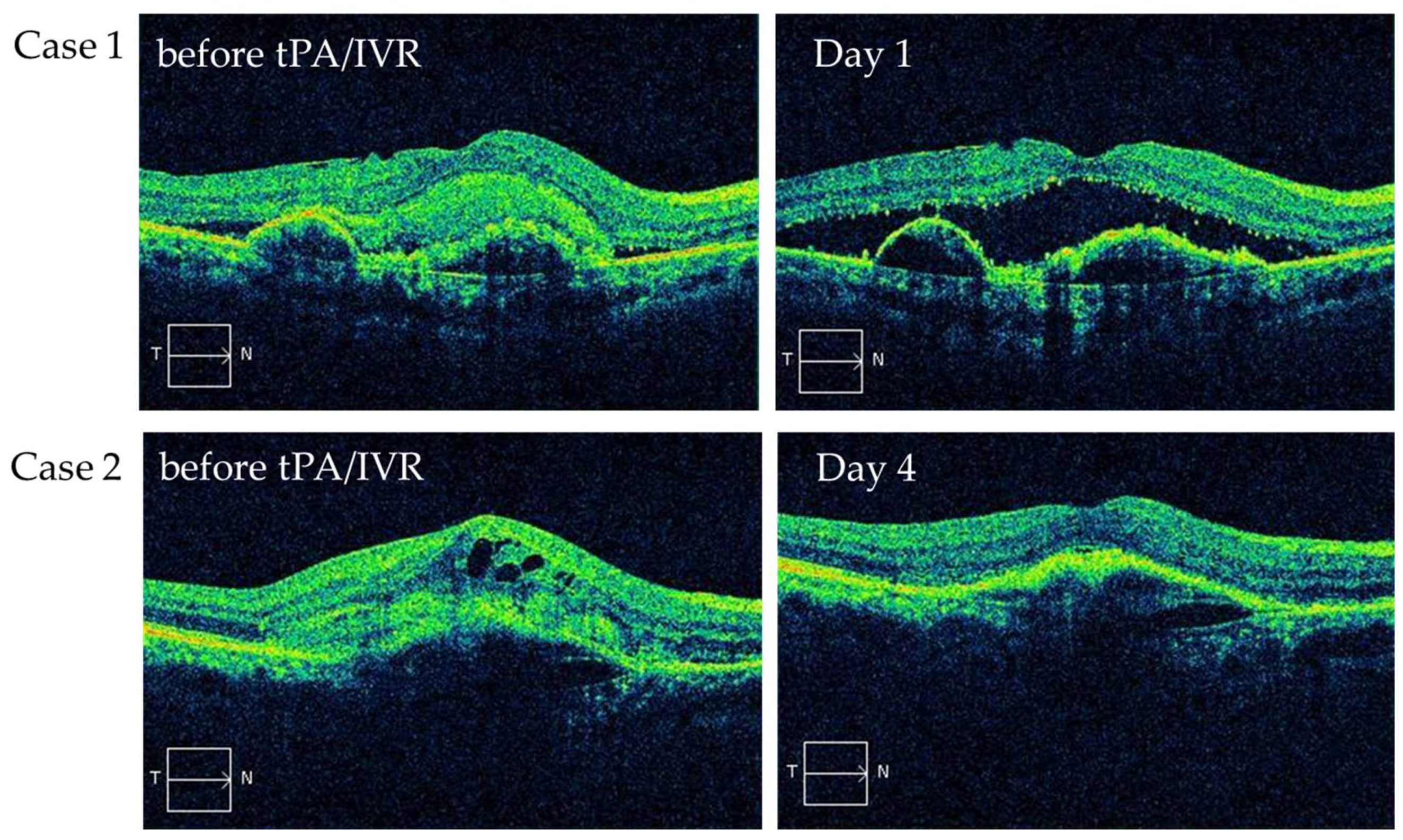
3.2. Status of SHRM Immediately after Treatmen
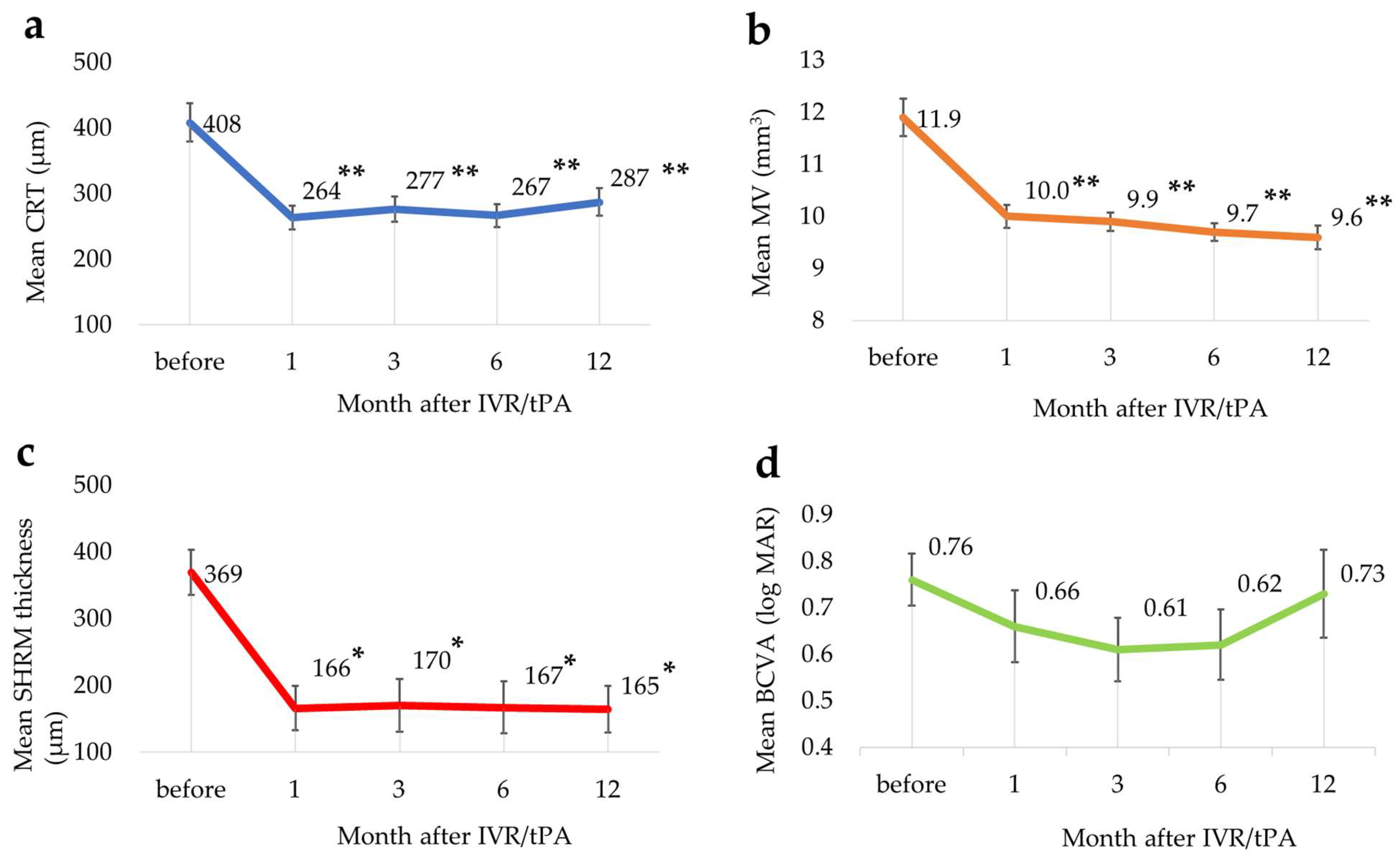
3.3. Changes in Each Parameter
3.4. Additional Treatments
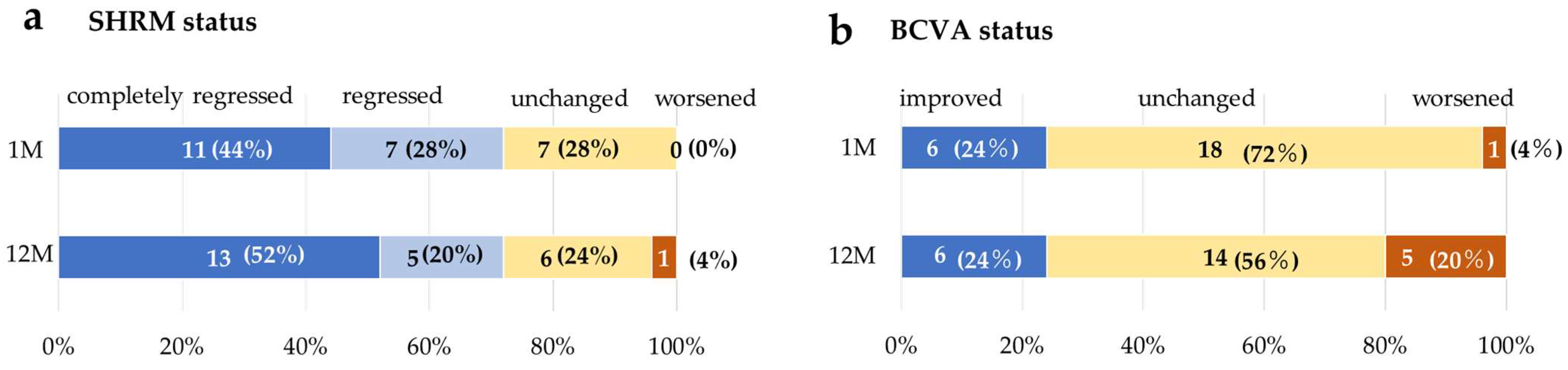
3.5. Change in SHRM and BCVAtatus
3.6. Distribution of BCVA at Month 12
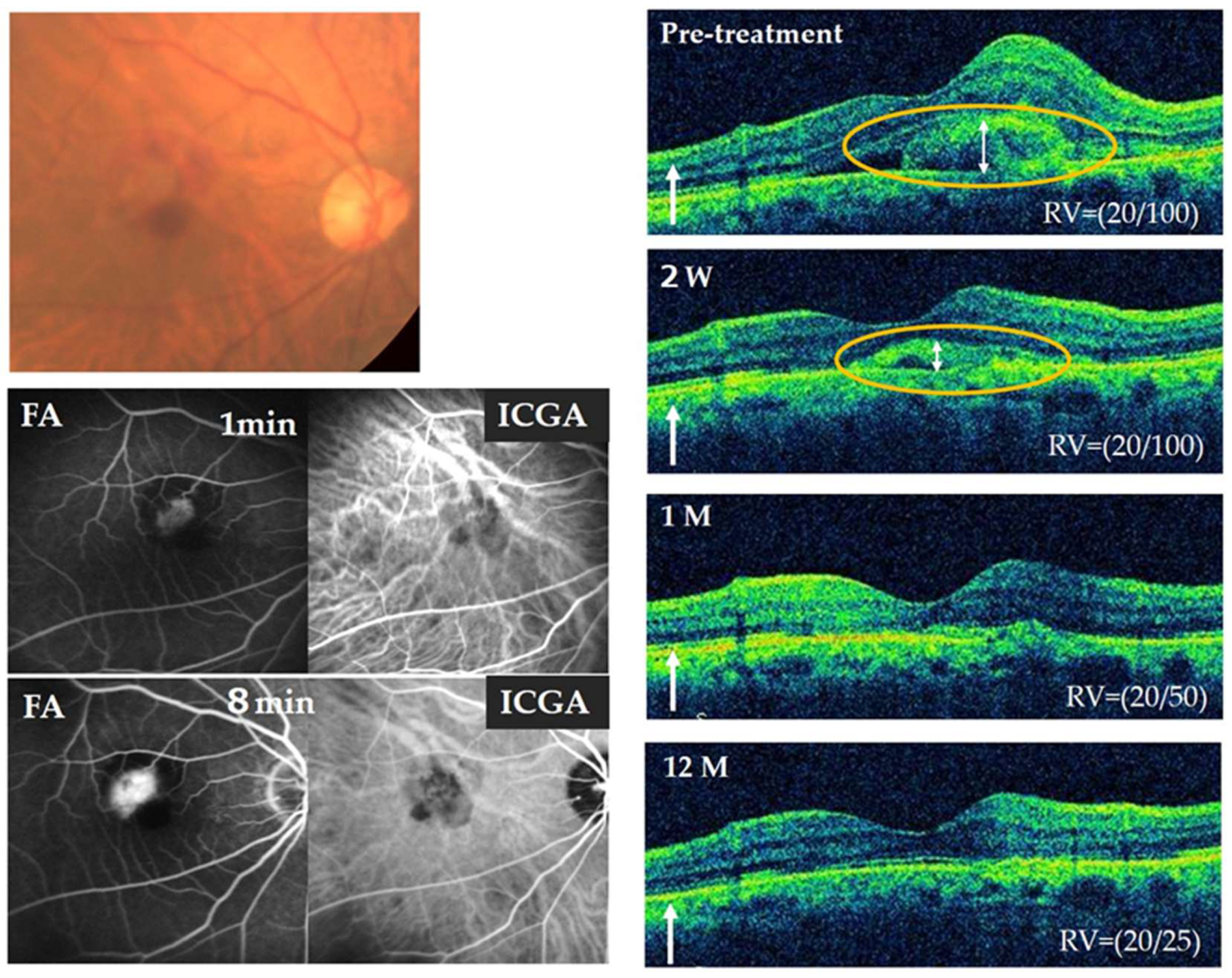
3.7. Representative Case
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Kiyohara, Y.; Hata, Y.; Arakawa, S.; Yonemoto, K.; Doi, Y.; Iida, M.; Ishibashi, T. Nine-year incidence and risk factors for age-related macular degeneration in a defined Japanese population the Hisayama study. Ophthalmology 2009, 116, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Morizane, Y.; Morimoto, N.; Fujiwara, A.; Kawasaki, R.; Yamashita, H.; Ogura, Y.; Shiraga, F. Incidence and causes of visual impairment in Japan: The first nation-wide complete enumeration survey of newly certified visually impaired individuals. Jpn. J. Ophthalmol. 2019, 63, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444.e41. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G. HAWK and HARRIER: Phase 3, Multicenter, Randomized, Double-Masked Trials of Brolucizumab for Neovascular Age-Related Macular Degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Khanani, A.M.; Guymer, R.H.; Basu, K.; Boston, H.; Heier, J.S.; Korobelnik, J.F.; Kotecha, A.; Lin, H.; Silverman, D.; Swaminathan, B.; et al. TENAYA and LUCERNE: Rationale and Design for the Phase 3 Clinical Trials of Faricimab for Neovascular Age-Related Macular Degeneration. Ophthalmol. Sci. 2021, 1, 100076. [Google Scholar] [CrossRef]
- Lalwani, G.A.; Rosenfeld, P.J.; Fung, A.E.; Dubovy, S.R.; Michels, S.; Feuer, W.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. A variable-dosing regimen with intravitreal ranibizumab for neovascular age-related macular degeneration: Year 2 of the PrONTO Study. Am. J. Ophthalmol. 2009, 148, 43–58.e41. [Google Scholar] [CrossRef]
- Gupta, O.P.; Shienbaum, G.; Patel, A.H.; Fecarotta, C.; Kaiser, R.S.; Regillo, C.D. A treat and extend regimen using ranibizumab for neovascular age-related macular degeneration clinical and economic impact. Ophthalmology 2010, 117, 2134–2140. [Google Scholar] [CrossRef]
- Rosenberg, D.; Deonarain, D.M.; Gould, J.; Sothivannan, A.; Phillips, M.R.; Sarohia, G.S.; Sivaprasad, S.; Wykoff, C.C.; Cheung, C.M.G.; Sarraf, D.; et al. Efficacy, safety, and treatment burden of treat-and-extend versus alternative anti-VEGF regimens for nAMD: A systematic review and meta-analysis. Eye 2023, 37, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Yoneda, K.; Takeuchi, M.; Yasukawa, T.; Terasaki, H.; Yamamoto, Y.; Jujo, T.; Wakuta, M.; Matsubara, H.; Mitamura, Y.; Kato, A.; et al. Anti-VEGF Treatment Strategies for 3 Subtypes of Neovascular Age-Related Macular Degeneration in a Clinical Setting: A Multicenter Cohort Study in Japan. Ophthalmol. Retin. 2023, 7, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Rakoczy, E.P.; Lai, C.M.; Magno, A.L.; Wikstrom, M.E.; French, M.A.; Pierce, C.M.; Schwartz, S.D.; Blumenkranz, M.S.; Chalberg, T.W.; Degli-Esposti, M.A.; et al. Gene therapy with recombinant adeno-associated vectors for neovascular age-related macular degeneration: 1 year follow-up of a phase 1 randomised clinical trial. Lancet 2015, 386, 2395–2403. [Google Scholar] [CrossRef] [PubMed]
- Heier, J.S.; Kherani, S.; Desai, S.; Dugel, P.; Kaushal, S.; Cheng, S.H.; Delacono, C.; Purvis, A.; Richards, S.; Le-Halpere, A.; et al. Intravitreous injection of AAV2-sFLT01 in patients with advanced neovascular age-related macular degeneration: A phase 1, open-label trial. Lancet 2017, 390, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Watanabe, A.; Kurimoto, Y.; Hirami, Y.; Morinaga, C.; Daimon, T.; Fujihara, M.; Akimaru, H.; Sakai, N.; Shibata, Y.; et al. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 376, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Sugita, S.; Mandai, M.; Hirami, Y.; Takagi, S.; Maeda, T.; Fujihara, M.; Matsuzaki, M.; Yamamoto, M.; Iseki, K.; Hayashi, N.; et al. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med. 2020, 9, 2217. [Google Scholar] [CrossRef]
- Eichenbaum, D.A.; Ahmed, A.; Hiya, F. Ranibizumab port delivery system: A clinical perspective. BMJ Open Ophthalmol. 2022, 7, e001104. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, G.J.; Ying, G.S.; Toth, C.A.; Daniel, E.; Grunwald, J.E.; Martin, D.F.; Maguire, M.G. Macular Morphology and Visual Acuity in Year Five of the Comparison of Age-related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 252–260.e1845. [Google Scholar] [CrossRef] [PubMed]
- Fung, A.E.; Lalwani, G.A.; Rosenfeld, P.J.; Dubovy, S.R.; Michels, S.; Feuer, W.J.; Puliafito, C.A.; Davis, J.L.; Flynn, H.W., Jr.; Esquiabro, M. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am. J. Ophthalmol. 2007, 143, 566–583. [Google Scholar] [CrossRef]
- Keane, P.A.; Liakopoulos, S.; Chang, K.T.; Wang, M.; Dustin, L.; Walsh, A.C.; Sadda, S.R. Relationship between optical coherence tomography retinal parameters and visual acuity in neovascular age-related macular degeneration. Ophthalmology 2008, 115, 2206–2214. [Google Scholar] [CrossRef]
- Willoughby, A.S.; Ying, G.S.; Toth, C.A.; Maguire, M.G.; Burns, R.E.; Grunwald, J.E.; Daniel, E.; Jaffe, G.J. Subretinal Hyperreflective Material in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2015, 122, 1846–1853.e1845. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.; Ying, G.S.; Kim, B.J.; Toth, C.A.; Ferris, F., 3rd; Martin, D.F.; Grunwald, J.E.; Jaffe, G.J.; Dunaief, J.L.; Pan, W.; et al. Five-Year Follow-up of Nonfibrotic Scars in the Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2019, 126, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.B.; Stinnett, S.; Han, J.I.L.; Jaffe, G.J. Correlation of subretinal hyperreflective material morphology and visual acuity in neovascular age-related macular degeneration. Retina 2020, 40, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Yasukawa, T.; Ito, Y.; Takase, A.; Hirano, Y.; Yoshida, M.; Ogura, Y. Pneumatic displacement of submacular hemorrhage with or without tissue plasminogen activator. Graefe’s Arch. Clin. Exp. Ophthalmol. Albrecht Von Graefes Arch. Fur Klin. Und Exp. Ophthalmol. 2011, 249, 1153–1157. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Johnson, M.W.; Schneiderman, T.E.; Regillo, C.D.; Tornambe, P.E.; Poliner, L.S.; Blodi, B.A.; Elner, S.G. Management of submacular hemorrhage with intravitreous tissue plasminogen activator injection and pneumatic displacement. Ophthalmology 1999, 106, 1900–1906, discussion 1906–1907. [Google Scholar] [CrossRef] [PubMed]
- Kadonosono, K.; Arakawa, A.; Yamane, S.; Inoue, M.; Yamakawa, T.; Uchio, E.; Yanagi, Y. Displacement of submacular hemorrhages in age-related macular degeneration with subretinal tissue plasminogen activator and air. Ophthalmology 2015, 122, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Morizane, Y.; Hosokawa, M.; Shiode, Y.; Kawata, T.; Doi, S.; Matoba, R.; Hosogi, M.; Fujiwara, A.; Inoue, Y.; et al. Submacular hemorrhage in polypoidal choroidal vasculopathy treated by vitrectomy and subretinal tissue plasminogen activator. Am. J. Ophthalmol. 2015, 159, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Olivier, S.; Chow, D.R.; Packo, K.H.; MacCumber, M.W.; Awh, C.C. Subretinal recombinant tissue plasminogen activator injection and pneumatic displacement of thick submacular hemorrhage in Age-Related macular degeneration. Ophthalmology 2004, 111, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.S.; Mehta, N.; Wu, C.Y.; Barash, A.; Deobhakta, A.A.; Rosen, R.B. Outcomes of pars plana vitrectomy with subretinal tissue plasminogen activator injection and pneumatic displacement of fovea-involving submacular haemorrhage. BMJ Open Ophthalmol. 2020, 5, e000394. [Google Scholar] [CrossRef]
- Kachi, I.; Yasukawa, T.; Kato, A.; Takase, N.; Morita, H.; Kubota, A.; Hirano, Y.; Uemura, A.; Ogura, Y. Combination therapy with intravitreal tissue plasminogen activator and ranibizumab for subfoveal type 2 choroidal neovascularization. Jpn. J. Ophthalmol. 2016, 60, 179–186. [Google Scholar] [CrossRef]
- Murakami, T.; Takagi, H.; Kita, M.; Nishiwaki, H.; Miyamoto, K.; Ohashi, H.; Watanabe, D.; Yoshimura, N. Intravitreal tissue plasminogen activator to treat macular edema associated with branch retinal vein occlusion. Am. J. Ophthalmol. 2006, 142, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Takagi, H.; Ohashi, H.; Kita, M.; Nishiwaki, H.; Miyamoto, K.; Watanabe, D.; Sakamoto, A.; Yamaike, N.; Yoshimura, N. Role of posterior vitreous detachment induced by intravitreal tissue plasminogen activator in macular edema with central retinal vein occlusion. Retina 2007, 27, 1031–1037. [Google Scholar] [CrossRef] [PubMed]
- Suzuma, K.; Murakami, T.; Watanabe, D.; Miyamoto, K.; Kita, M.; Takagi, H.; Yoshimura, N. Intravitreal tissue plasminogen activator for treatment of central retinal vein occlusion associated with diabetic retinopathy. Nippon Ganka Gakkai Zasshi 2009, 113, 492–497. [Google Scholar] [PubMed]
- Inoue, N.; Kato, A.; Araki, T.; Kimura, T.; Kinoshita, T.; Okamoto, F.; Murakami, T.; Mitamura, Y.; Sakamoto, T.; Miki, A.; et al. Visual prognosis of submacular hemorrhage secondary to age-related macular degeneration: A retrospective multicenter survey. PLoS ONE 2022, 17, e0271447. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.I.; Yasukawa, T.; Kato, A.; Kubota, A.; Usui, H.; Takase, N.; Kuwayama, S.; Ogura, Y. Tissue Plasminogen Activator as an Antiangiogenic Agent in Experimental Corneal Neovascularization in Rabbits. Ophthalmic Res. 2018, 59, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Ozone, D.; Mizutani, T.; Nozaki, M.; Ohbayashi, M.; Hasegawa, N.; Kato, A.; Yasukawa, T.; Ogura, Y. Tissue Plasminogen Activator as an Antiangiogenic Agent in Experimental Laser-Induced Choroidal Neovascularization in Mice. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5348–5354. [Google Scholar] [CrossRef]
- Klettner, A.; Groteluschen, S.; Treumer, F.; Roider, J.; Hillenkamp, J. Compatibility of recombinant tissue plasminogen activator (rtPA) and aflibercept or ranibizumab coapplied for neovascular age-related macular degeneration with submacular haemorrhage. Br. J. Ophthalmol. 2015, 99, 864–869. [Google Scholar] [CrossRef]

| Cases | 25 Eyes of 25 Patients | |
|---|---|---|
| Gender | 16 men and 9 women | |
| Mean age | 76.7 ± 9.8 years old | |
| Subtype | Type 2 MNV | 16 eyes |
| Type 1 MNV with fibrovascular PED | 5 eyes | |
| PCV | 4 eyes | |
| Previous treatment history | Treatment-naïve | 15 eyes |
| Anti-VEGF therapy and/or PDT | 10 eyes |
| Cases | 25 Eyes of 25 Patients | |
|---|---|---|
| None additional treatment | 1 eye | |
| Type of anti-VEGF | IVR mono therapy | 14 eyes |
| IVA mono therapy | 5 eyes | |
| Switch from IVR to IVA | 5 eyes | |
| Mean number of injections | 3.6 ± 2.2 times |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ando, M.; Kato, A.; Kimura, M.; Ogura, S.; Kuwayama, S.; Kominami, A.; Kuwayama, S.; Obayashi, T.; Ando, R.; Monoe, T.; et al. Effects of Combination Therapy with Intravitreal Ranibizumab and Tissue Plasminogen Activator for Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2024, 13, 2417. https://doi.org/10.3390/jcm13082417
Ando M, Kato A, Kimura M, Ogura S, Kuwayama S, Kominami A, Kuwayama S, Obayashi T, Ando R, Monoe T, et al. Effects of Combination Therapy with Intravitreal Ranibizumab and Tissue Plasminogen Activator for Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2024; 13(8):2417. https://doi.org/10.3390/jcm13082417
Chicago/Turabian StyleAndo, Michiko, Aki Kato, Masayo Kimura, Shuntaro Ogura, Soichiro Kuwayama, Aoi Kominami, Satoshi Kuwayama, Tomohiro Obayashi, Ryota Ando, Takafumi Monoe, and et al. 2024. "Effects of Combination Therapy with Intravitreal Ranibizumab and Tissue Plasminogen Activator for Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 13, no. 8: 2417. https://doi.org/10.3390/jcm13082417





