The Effect of Cell Culture Passage on the Efficacy of Mesenchymal Stromal Cells as a Cell Therapy Treatment
Abstract
:1. Introduction
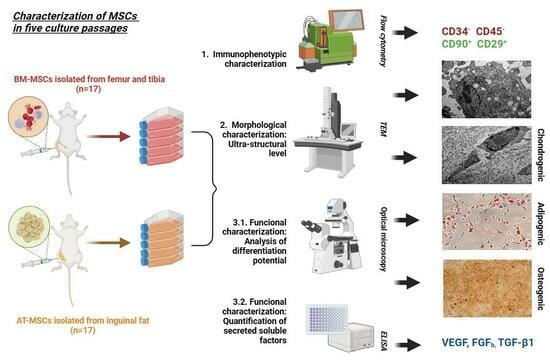
2. Materials and Methods
2.1. Isolation and Culture of BM-MSCs and AT-MSCs
2.2. Phenotypic Characterization
2.3. Functional Characterization
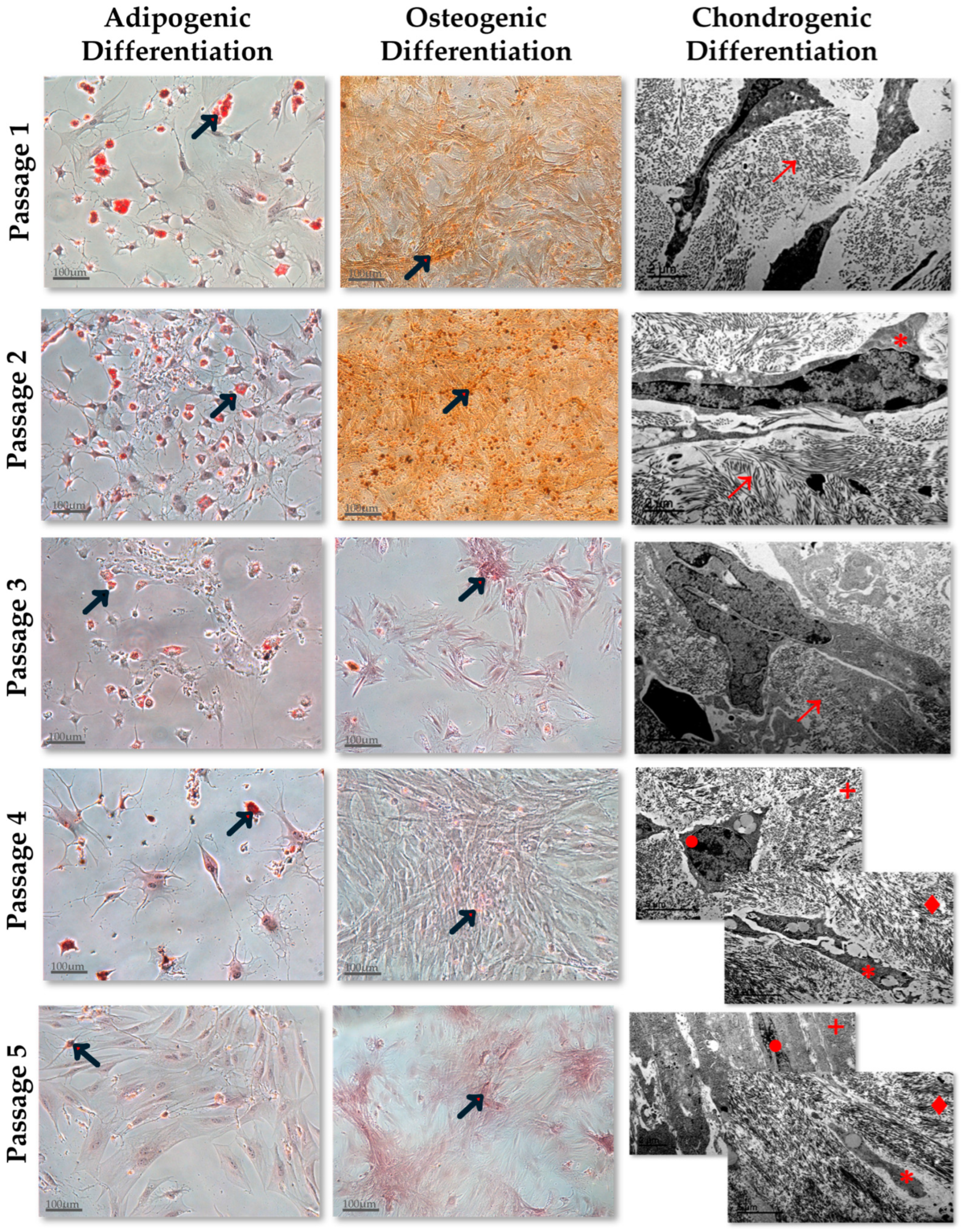
2.3.1. Adipogenic Differentiation Assay
2.3.2. Osteogenic Differentiation Assay
2.3.3. Chondrogenic Differentiation Assay
2.4. Cytokines Quantification in Cell Culture Supernatants
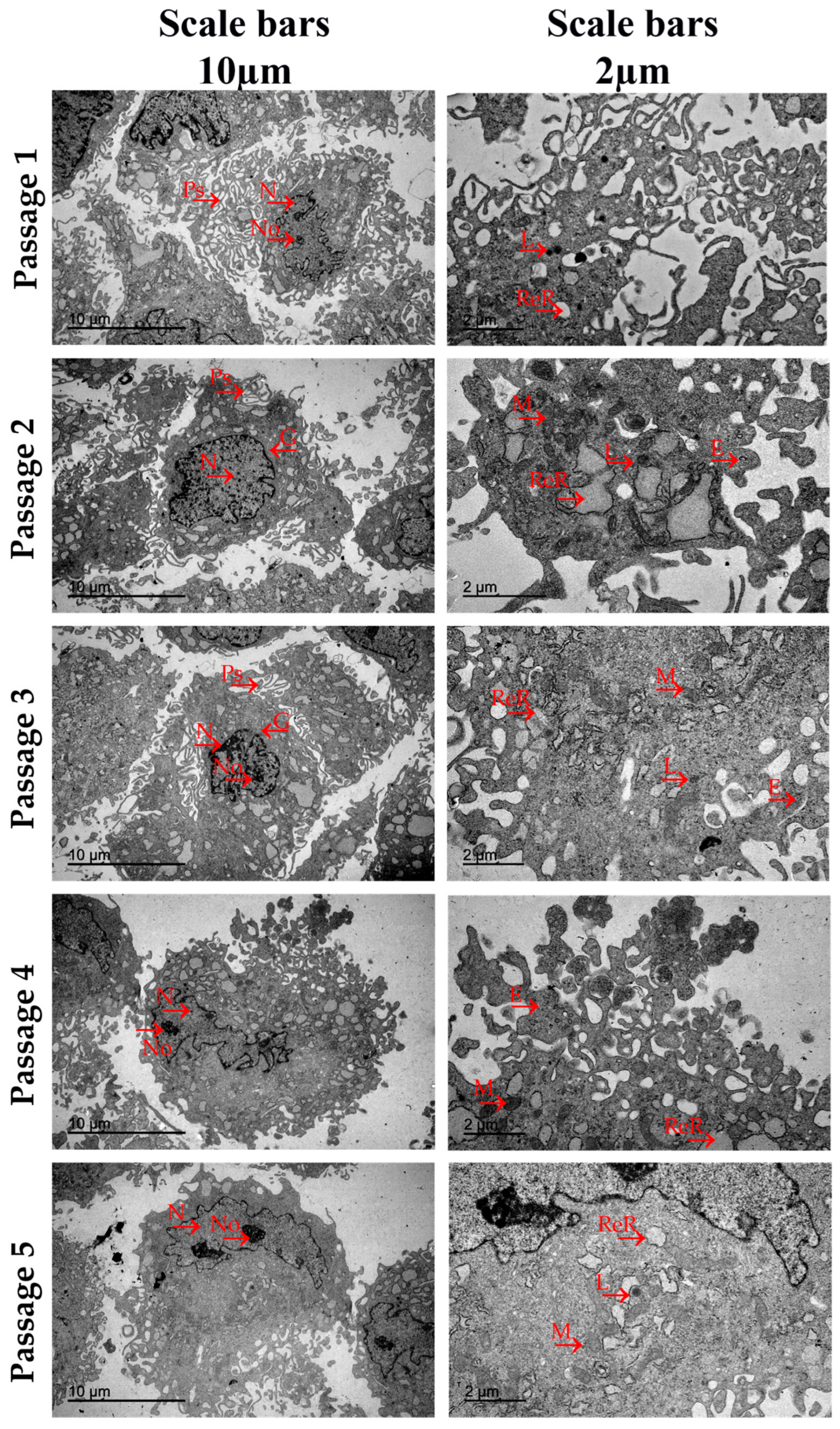
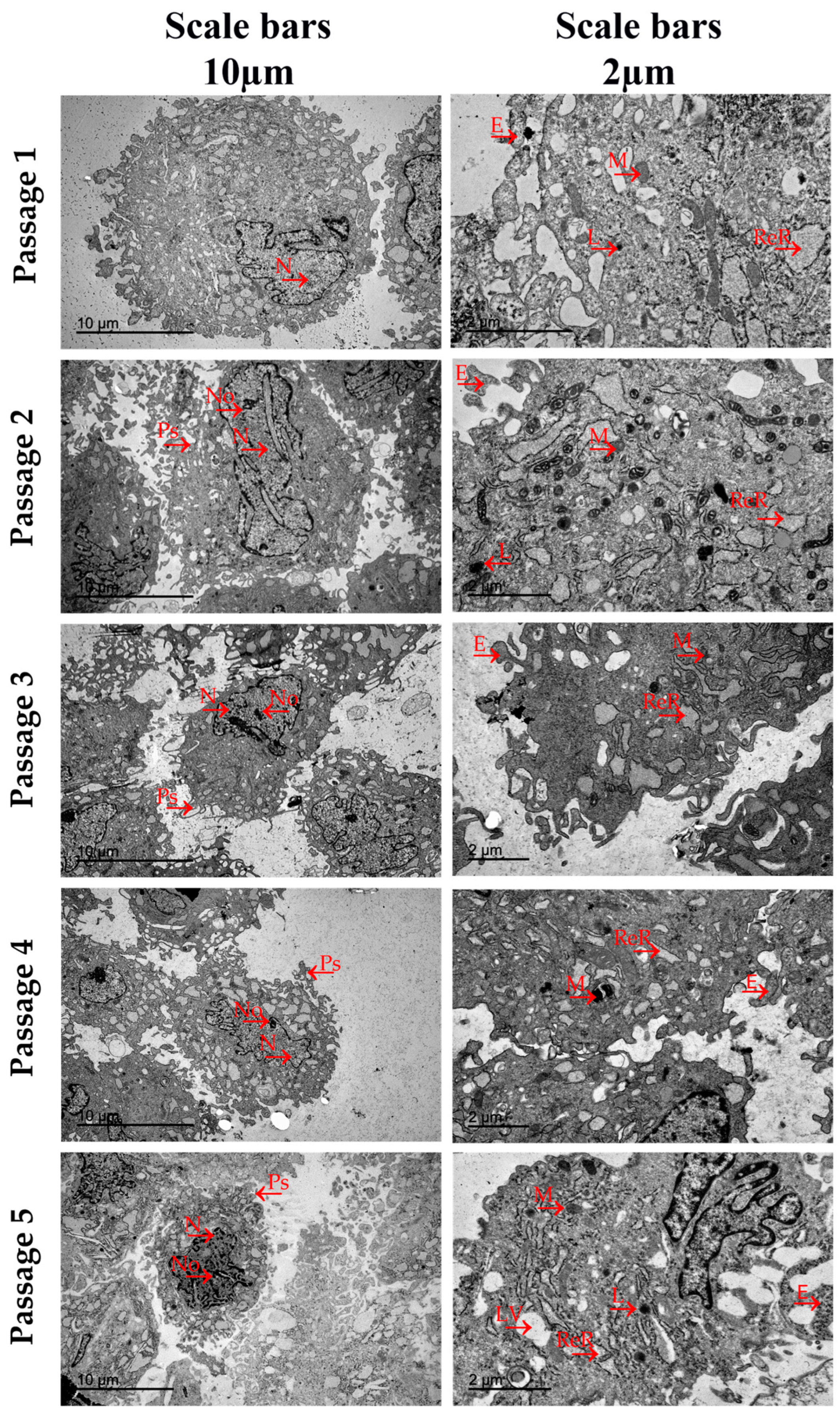
2.5. Ultrastructural Analysis by Transmission Electron Microscopy
2.6. Statistical Analysis
3. Results
3.1. Phenotypic Characterization
3.2. Functional Characterization
3.3. Cytokines Quantification in Cell Culture Supernatants
3.4. Ultrastructural Analysis by Transmission Electron Microscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Friedenstein, A.J.; Chailakhyan, R.K.; Latsinik, N.V.; Panasyuk, A.F.; Keiliss-Borok, I.V. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Cloning in vitro and retransplantation in vivo. Transplantation 1974, 17, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kandoi, S.; Misra, R.; Vijayalakshmi, S.; Rajagopal, K.; Verma, R.S. The mesenchymal stem cell secretome: A new paradigm towards cell-free therapeutic mode in regenerative medicine. Cytokine Growth Factor Rev. 2019, 46, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ma, O.K.; Chan, K.H. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J. Stem Cells 2016, 8, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Palomares Cabeza, V.; Hoogduijn, M.J.; Kraaijeveld, R.; Franquesa, M.; Witte-Bouma, J.; Wolvius, E.B.; Farrell, E.; Brama, P.A.J. Pediatric Mesenchymal Stem Cells Exhibit Immunomodulatory Properties toward Allogeneic T and B Cells Under Inflammatory Conditions. Front. Bioeng. Biotechnol. 2019, 7, 142. [Google Scholar] [CrossRef] [PubMed]
- Berebichez-Fridman, R.; Montero-Olvera, P.R. Sources and Clinical Applications of Mesenchymal Stem Cells: State-of-the-art review. Sultan Qaboos Univ. Med. J. 2018, 18, e264–e277. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.S.; Choi, Y.; Kim, H.S.; Kim, H.O. Comparison of molecular profiles of human mesenchymal stem cells derived from bone marrow, umbilical cord blood, placenta and adipose tissue. Int. J. Mol. Med. 2016, 37, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Schulman, C.I.; Namias, N.; Pizano, L.; Rodriguez-Menocal, L.; Aickara, D.; Guzman, W.; Candanedo, A.; Maranda, E.; Beirn, A.; McBride, J.D.; et al. The effect of mesenchymal stem cells improves the healing of burn wounds: A phase 1 dose-escalation clinical trial. Scars Burn. Health 2022, 8, 20595131211070783, Erratum in Scars Burn. Health 2022, 8, 20595131221118066. [Google Scholar] [CrossRef]
- Wu, S.H.; Shirado, T.; Mashiko, T.; Feng, J.; Asahi, R.; Kanayama, K.; Mori, M.; Chi, D.; Sunaga, A.; Sarukawa, S.; et al. Therapeutic Effects of Human Adipose-Derived Products on Impaired Wound Healing in Irradiated Tissue. Plast. Reconstr. Surg. 2018, 142, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Canesi, M.; Giordano, R.; Lazzari, L.; Isalberti, M.; Isaias, I.U.; Benti, R.; Rampini, P.; Marotta, G.; Colombo, A.; Cereda, E.; et al. Finding a new therapeutic approach for no-option Parkinsonisms: Mesenchymal stromal cells for progressive supranuclear palsy. J. Transl. Med. 2016, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Park, K.S.; Jeong, B.C.; Lee, S.H. Regeneration of Cartilage in Human Knee Osteoarthritis with Autologous Adipose Tissue-Derived Stem Cells and Autologous Extracellular Matrix. BioRes. Open Access 2016, 5, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Song, D.; Ding, Y.; Sun, B.; Lu, C.; Mo, X.; Xu, H.; Liu, Y.; Wu, J. A comparative study on various cell sources for constructing tissue-engineered meniscus. Front. Bioeng. Biotechnol. 2023, 11, 1128762. [Google Scholar] [CrossRef] [PubMed]
- Maziarz, R.T. Mesenchymal stromal cells: Potential roles in graft-versus-host disease prophylaxis and treatment. Transfusion 2016, 56, 9S–14S. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Li, G. Tissue source determines the differentiation potentials of mesenchymal stem cells: A comparative study of human mesenchymal stem cells from bone marrow and adipose tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Im, G.I. Bone marrow-derived stem/stromal cells and adipose tissue-derived stem/stromal cells: Their comparative efficacies and synergistic effects. J. Biomed. Mater. Res. A 2017, 105, 2640–2648. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Dmitrieva, R.I.; Minullina, I.R.; Bilibina, A.A.; Tarasova, O.V.; Anisimov, S.V.; Zaritskey, A.Y. Bone marrow- and subcutaneous adipose tissue-derived mesenchymal stem cells: Differences and similarities. Cell Cycle 2012, 11, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Mantovani, C.; Raimondo, S.; Haneef, M.S.; Geuna, S.; Terenghi, G.; Shawcross, S.G.; Wiberg, M. Morphological, molecular and functional differences of adult bone marrow- and adipose-derived stem cells isolated from rats of different ages. Exp. Cell Res. 2012, 318, 2034–2048. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, F.A.; van de Vyver, M.; Ferris, W.F. Isolation and Characterization of Different Mesenchymal Stem Cell Populations from Rat Femur. Methods Mol. Biol. 2019, 1916, 133–147. [Google Scholar] [CrossRef]
- Jakl, V.; Popp, T.; Haupt, J.; Port, M.; Roesler, R.; Wiese, S.; Friemert, B.; Rojewski, M.T.; Schrezenmeier, H. Effect of Expansion Media on Functional Characteristics of Bone Marrow-Derived Mesenchymal Stromal Cells. Cells 2023, 12, 2105. [Google Scholar] [CrossRef]
- Reich, C.M.; Raabe, O.; Wenisch, S.; Bridger, P.S.; Kramer, M.; Arnhold, S. Isolation, culture and chondrogenic differentiation of canine adipose tissue- and bone marrow-derived mesenchymal stem cells--a comparative study. Vet. Res. Commun. 2012, 36, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Humenik, F.; Maloveska, M.; Hudakova, N.; Petrouskova, P.; Hornakova, L.; Domaniza, M.; Mudronova, D.; Bodnarova, S.; Cizkova, D. A Comparative Study of Canine Mesenchymal Stem Cells Isolated from Different Sources. Animals 2022, 12, 1502. [Google Scholar] [CrossRef]
- Lotfy, A.; Salama, M.; Zahran, F.; Jones, E.; Badawy, A.; Sobh, M. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: A comparative study. Int. J. Stem Cells 2014, 7, 135–142. [Google Scholar] [CrossRef]
- Nammian, P.; Asadi-Yousefabad, S.L.; Daneshi, S.; Sheikhha, M.H.; Tabei, S.M.B.; Razban, V. Comparative analysis of mouse bone marrow and adipose tissue mesenchymal stem cells for critical limb ischemia cell therapy. Stem Cell Res. Ther. 2021, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Carmona, M.D.; Cañadillas, S.; Romero, M.; Blanco, A.; Nogueras, S.; Herrera, C. Intramyocardial bone marrow mononuclear cells versus bone marrow-derived and adipose mesenchymal cells in a rat model of dilated cardiomyopathy. Cytotherapy 2017, 19, 947–961. [Google Scholar] [CrossRef]
- Kwon, H.M.; Hur, S.M.; Park, K.Y.; Kim, C.K.; Kim, Y.M.; Kim, H.S.; Shin, H.C.; Won, M.H.; Ha, K.S.; Kwon, Y.G.; et al. Multiple paracrine factors secreted by mesenchymal stem cells contribute to angiogenesis. Vascul. Pharmacol. 2014, 63, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Kehl, D.; Generali, M.; Mallone, A.; Heller, M.; Uldry, A.-C.; Cheng, P.; Gantenbein, B.; Hoerstrup, S.P.; Weber, B. Proteomic analysis of human mesenchymal stromal cell secretomes: A systematic comparison of the angiogenic potential. NPJ Regen. Med. 2019, 4, 8. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.T.; Asgari, A.; Lokmic, Z.; Sinclair, R.; Dusting, G.J.; Lim, S.Y.; Dilley, R.J. Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev. 2012, 21, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Du, W.J.; Chi, Y.; Yang, Z.X.; Li, Z.J.; Cui, J.J.; Song, B.Q.; Li, X.; Yang, S.G.; Han, Z.B.; Han, Z.C. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res. Ther. 2016, 7, 163. [Google Scholar] [CrossRef] [PubMed]
- Wegmeyer, H.; Bröske, A.M.; Leddin, M.; Kuentzer, K.; Nisslbeck, A.K.; Hupfeld, J.; Wiechmann, K.; Kuhlen, J.; von Schwerin, C.; Stein, C.; et al. Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 2013, 22, 2606–2618. [Google Scholar] [CrossRef] [PubMed]
- Ntege, E.H.; Sunami, H.; Shimizu, Y. Advances in regenerative therapy: A review of the literature and future directions. Regen. Ther. 2020, 14, 136–153. [Google Scholar] [CrossRef] [PubMed]
- Göran Ronquist, K. Extracellular vesicles and energy metabolism. Clin. Chim. Acta 2019, 488, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.F.; Zhong, Z.N.; Fu, X.F.; Peng, D.X.; Lu, G.H.; Li, W.H.; Xu, H.Y.; Hu, H.B.; He, J.M.; Su, W.Y.; et al. Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neurosci. Lett. 2013, 541, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Manea, C.M.; Rusu, M.C.; Constantin, D.; Mănoiu, V.M.; Moldovan, L.; Jianu, A.M. Ultrastructural features of human adipose-derived multipotent mesenchymal stromal cells. Rom. J. Morphol. Embryol. 2014, 55, 1363–1369. [Google Scholar]
- Miko, M.; Danisovic, L.; Majidi, A.; Varga, I. Ultrastructural analysis of different human mesenchymal stem cells after in vitro expansion: A technical review. Eur. J. Histochem. 2015, 59, 2528. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Pascucci, L.; Rossi, B.; Bazzucchi, C.; Lanci, A.; Ceccoli, M.; Merlo, B. Ultrastructural characteristics and immune profile of equine MSCs from fetal adnexa. Reproduction 2017, 154, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Wanet, A.; Arnould, T.; Najimi, M.; Renard, P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015, 24, 1957–1971. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, H.Y.; Hong, I.S. Metabolic Regulation and Related Molecular Mechanisms in Various Stem Cell Functions. Curr. Stem Cell Res. Ther. 2020, 15, 531–546. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.; Earnshaw, W.; Lippincott-Schwartz, J.; Johnson, G. Cell Biology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2016; p. 908. ISBN 9780323399944. [Google Scholar]
- Eleuteri, S.; Fierabracci, A. Insights into the Secretome of Mesenchymal Stem Cells and Its Potential Applications. Int. J. Mol. Sci. 2019, 20, 4597. [Google Scholar] [CrossRef] [PubMed]
- Kozlowska, U.; Krawczenko, A.; Futoma, K.; Jurek, T.; Rorat, M.; Patrzalek, D.; Klimczak, A. Similarities and differences between mesenchymal stem/progenitor cells derived from various human tissues. World J. Stem Cells 2019, 11, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Maacha, S.; Sidahmed, H.; Jacob, S.; Gentilcore, G.; Calzone, R.; Grivel, J.-C.; Cugno, C. Paracrine Mechanisms of Mesenchymal Stromal Cells in Angiogenesis. Stem Cells Int. 2020, 2020, 4356359. [Google Scholar] [CrossRef] [PubMed]

| Culture Passage | Cell Type | %CD29 | %CD90 | %CD34 | %CD45 | Side Scatter | Forward Scatter |
|---|---|---|---|---|---|---|---|
| 1 | AT-MSCs | 98.91 ± 1.23 | 93.27 ± 6.15 | 0.05 ± 0.04 | 0.08 ± 0.09 | 455.1 ± 36.8 | 476.4 ± 58.2 |
| BM-MSCs | 92.82 ± 8.17 | 87.81 ± 12.31 | 0.32 ± 0.35 | 0.37 ± 0.34 | 418.99 ± 69.79 | 459.0 ± 110.0 | |
| 2 | AT-MSCs | 99.06 ± 0.85 | 95.94 ± 3.03 | 0.04 ± 0.03 | 0.07 ± 0.06 | 433.3 ± 213.9 | 514.2 ± 79.1 |
| BM-MSCs | 98.04 ± 1.58 | 97.44 ± 2.46 | 0.018 ± 0.04 | 0.31 ± 0.09 | 596.1 ± 153.5 | 521.1 ± 45.3 | |
| 3 | AT-MSCs | 98.71 ± 1.29 | 96.79 ± 6.09 | 0.03 ± 0.03 | 0.06 ± 0.06 | 461.3 ± 57.5 | 541.5 ± 35.1 |
| BM-MSCs | 94.38 ± 5.01 | 80.62 ± 15.79 | 0.09 ± 0.09 | 0.37 ± 0.21 | 628.9 ± 229.5 | 367.7 ± 17.1 | |
| 4 | AT-MSCs | 99.24 ± 0.60 | 98.87 ± 0.71 | 0.03 ± 0.02 | 0.05 ± 0.04 | 456.3 ± 35.5 | 589.09 ± 33.96 |
| BM-MSCs | 96.57 ± 2.24 | 90.14 ± 7.99 | 0.26 ± 0.03 | 0.39 ± 0.09 | 698.7 ± 3.5 | 412.3 ± 18.1 | |
| 5 | AT-MSCs | 98.46 ± 1.11 | 97.56 ± 1.96 | 0.02 ± 0.02 | 0.05 ± 0.04 | 558.0 ± 54.8 | 622.7 ± 72.9 |
| BM-MSCs | 97.37 ± 2.50 | 95.40 ± 4.38 | 0.12 ± 0.09 | 0.41 ± 0.02 | 773.0 ± 44.9 | 548.1 ± 37.4 |
| VEGF (pg/mL) | TGF-β1 (pg/mL) | FGF-2 (pg/mL) | ||||
|---|---|---|---|---|---|---|
| BM-MSCs | AT-MSCs | BM-MSCs | AT-MSCs | BM-MSCs | AT-MSCs | |
| P1 | 7.32 ± 0.25 | 7.49 ± 0.04 | 33.89 ± 1.49 | 18.69 ± 0.07 | 2.78 ± 0.17 | 1.32 ± 0.01 |
| P2 | 6.76 ± 0.01 | 9.12 ± 0.01 | 29.98 ± 0.58 | 26.39 ± 0.13 | 3.01 ± 0.13 | 2.81 ± 0.01 |
| P3 | 13.61 ± 0.001 | 17.08 ± 0.03 | 49.74 ± 0.07 | 33.69 ± 0.24 | 6.21 ± 0.01 | 5.71 ± 0.02 |
| P4 | 10.22 ± 0.32 | 20.06 ± 0.22 | 47.23 ± 0.60 | 49.74 ± 1.02 | 6.49 ± 0.21 | 8.17 ± 0.04 |
| P5 | 7.38 ± 0.61 | 24.72 ± 0.04 | 41.38 ± 3.30 | 71.94 ± 0.20 | 7.61 ± 0.84 | 15.42 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carmona-Luque, M.; Ballesteros-Ribelles, A.; Millán-López, A.; Blanco, A.; Nogueras, S.; Herrera, C. The Effect of Cell Culture Passage on the Efficacy of Mesenchymal Stromal Cells as a Cell Therapy Treatment. J. Clin. Med. 2024, 13, 2480. https://doi.org/10.3390/jcm13092480
Carmona-Luque M, Ballesteros-Ribelles A, Millán-López A, Blanco A, Nogueras S, Herrera C. The Effect of Cell Culture Passage on the Efficacy of Mesenchymal Stromal Cells as a Cell Therapy Treatment. Journal of Clinical Medicine. 2024; 13(9):2480. https://doi.org/10.3390/jcm13092480
Chicago/Turabian StyleCarmona-Luque, MDolores, Antonio Ballesteros-Ribelles, Alejandro Millán-López, Alfonso Blanco, Sonia Nogueras, and Concha Herrera. 2024. "The Effect of Cell Culture Passage on the Efficacy of Mesenchymal Stromal Cells as a Cell Therapy Treatment" Journal of Clinical Medicine 13, no. 9: 2480. https://doi.org/10.3390/jcm13092480