Correlation of Non-Invasive Transthoracic Doppler Echocardiography with Invasive Doppler Wire-Derived Coronary Flow Reserve and Their Impact on Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
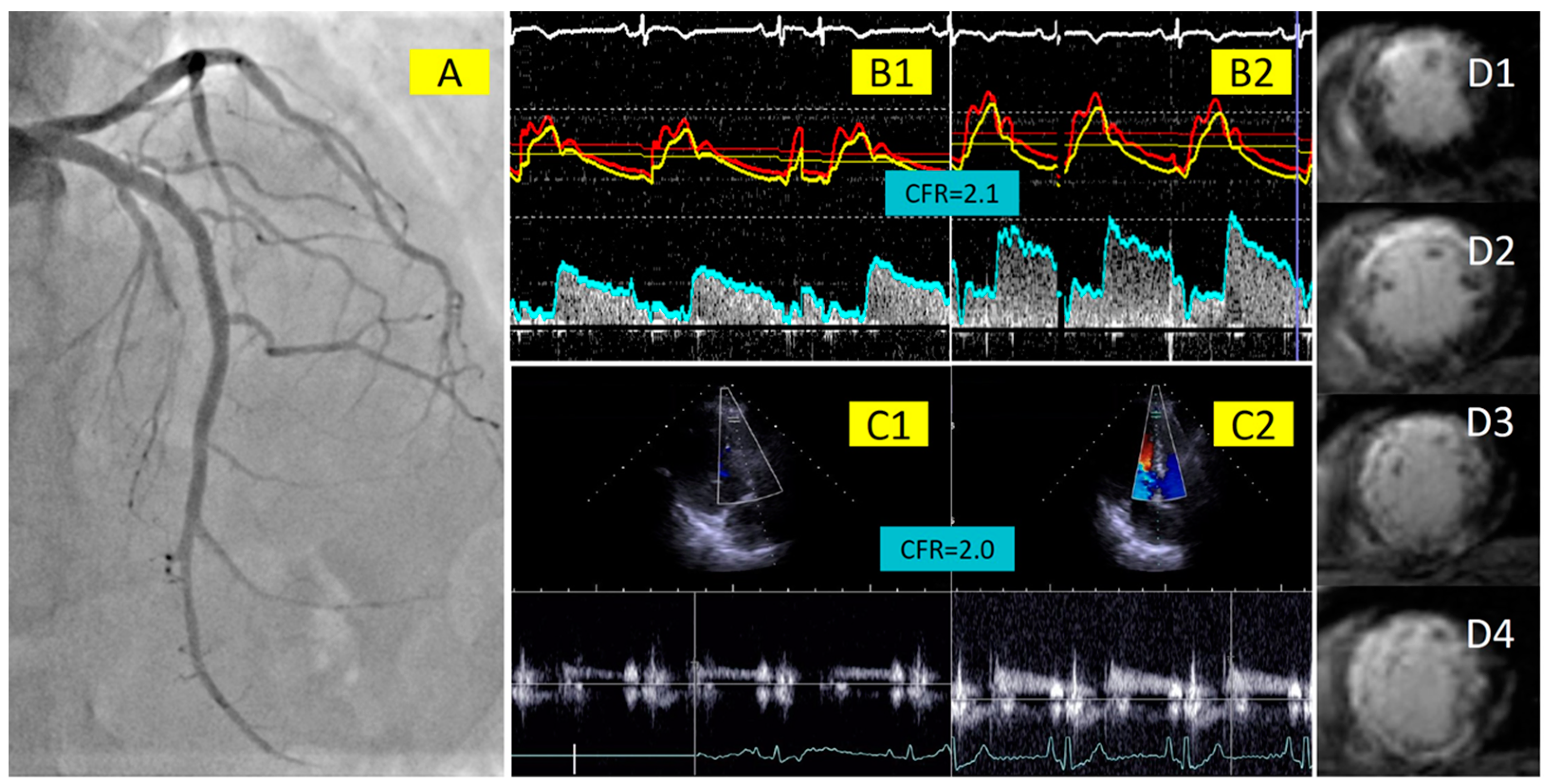
2.2. Invasive Measurements of Coronary Physiology Indices with Doppler Wire
2.3. Coronary Flow Velocity Measurement with Transthoracic Doppler Echocardiography
2.4. Cardiac Magnetic Resonance Imaging
2.5. Statistics
3. Results
3.1. Patient Cohort and Baseline Characteristics
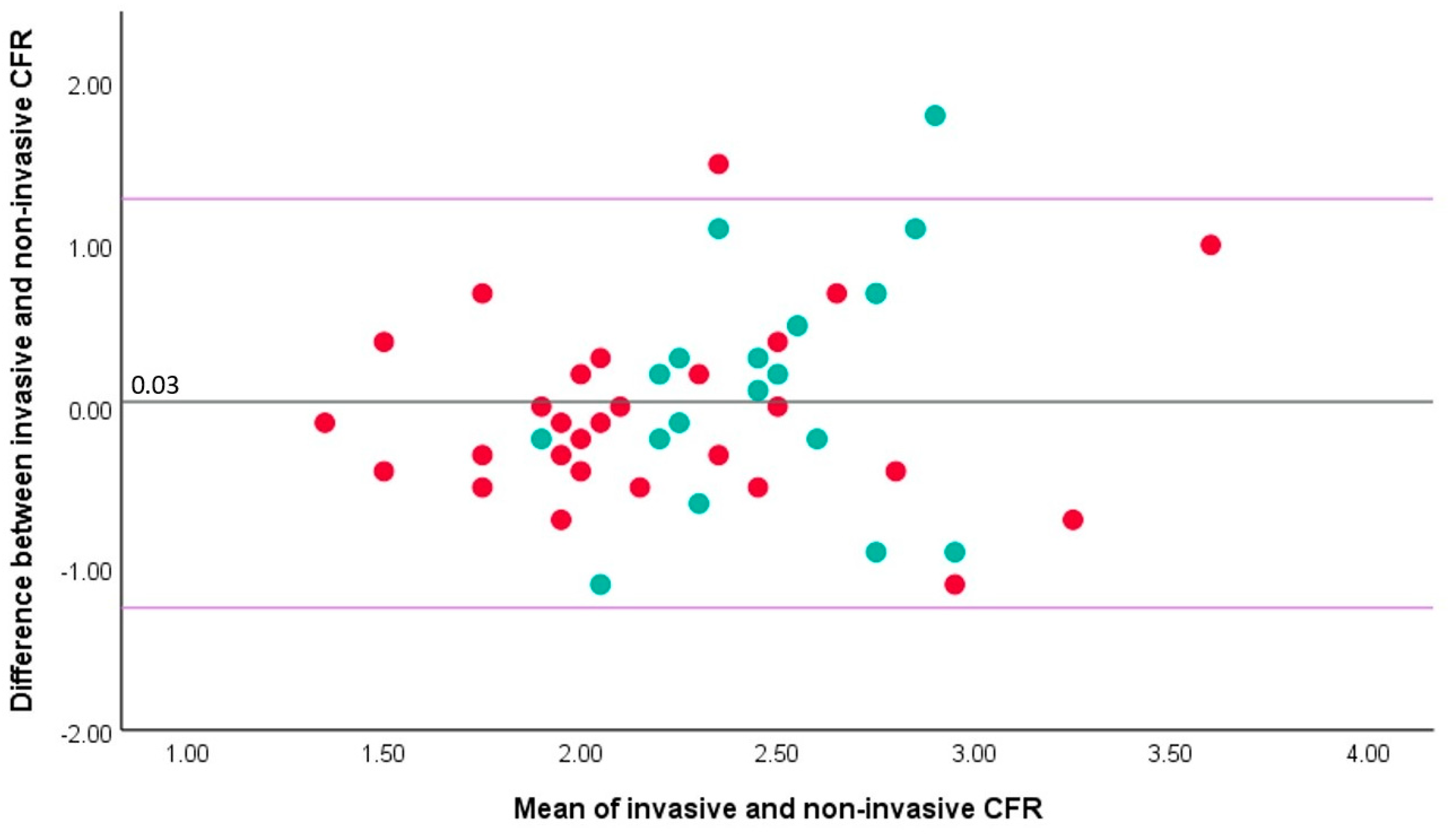
3.2. Correlation between Invasive and Non-Invasive CFR
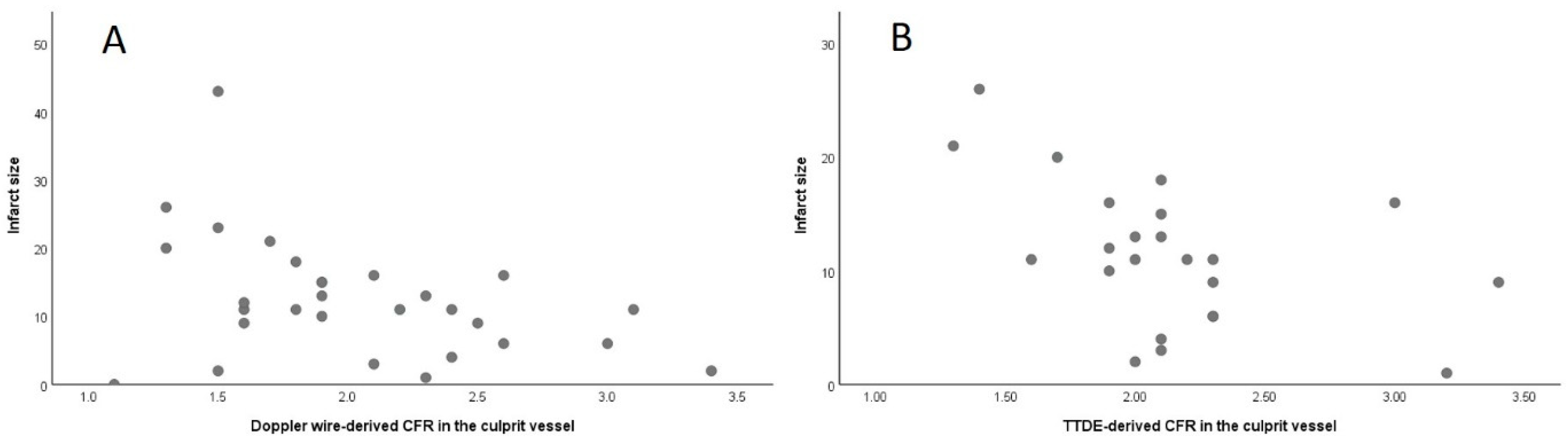
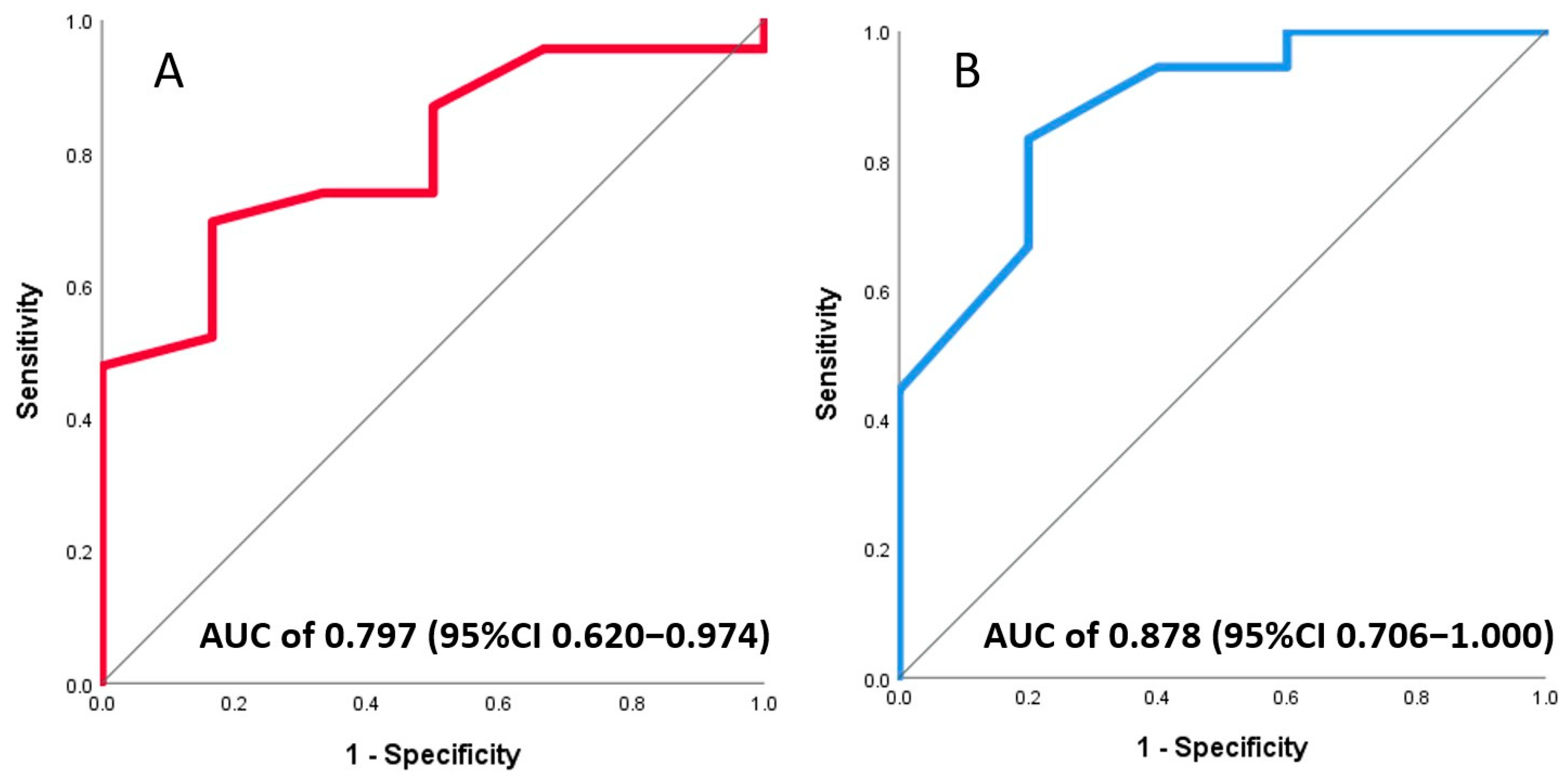
3.3. Association of Invasive and Non-Invasive CFR with Final Infarct Size
3.4. Association of Invasive and Non-Invasive CFR with LV Function
4. Discussion
4.1. Correlation between Intracoronary- and Echocardiography-Derived CFR
4.2. Echocardiography-Derived CFR Predicts Infarct Size and LV Function after STEMI
4.3. Clinical Utility of Echocardiography-Derived CFR
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction across the Spectrum of Cardiovascular Diseases: Jacc State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef] [PubMed]
- Gould, K.L.; Lipscomb, K. Effects of Coronary Stenoses on Coronary Flow Reserve and Resistance. Am. J. Cardiol. 1974, 34, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kelshiker, M.A.; Seligman, H.; Howard, J.P.; Rahman, H.; Foley, M.; Nowbar, A.N.; Rajkumar, C.A.; Shun-Shin, M.J.; Ahmad, Y.; Sen, S. Coronary Flow Reserve and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. Eur. Heart J. 2022, 43, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the Management of Acute Coronary Syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Garot, P.; Pascal, O.; Simon, M.; Monin, J.L.; Teiger, E.; Garot, J.; Guéret, P.; Dubois-Randé, J.L. Impact of Microvascular Integrity and Local Viability on Left Ventricular Remodelling after Reperfused Acute Myocardial Infarction. Heart 2003, 89, 393–397. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; de Winter, R.J.; Schotborgh, C.E.; Koch, K.T.; Meuwissen, M.; Voskuil, M.; Adams, R.; Mulder, K.J.; Tijssen, J.G.; Piek, J.J. Short- and Long-Term Recovery of Left Ventricular Function Predicted at the Time of Primary Percutaneous Coronary Intervention in Anterior Myocardial Infarction. J. Am. Coll. Cardiol. 2004, 43, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Hiasa, Y.; Ohara, Y.; Miyazaki, S.; Ogura, R.; Miyajima, H.; Yuba, K.; Suzuki, N.; Hosokawa, S.; Kishi, K.; et al. Usefulness of Coronary Flow Reserve Immediately after Primary Coronary Angioplasty for Acute Myocardial Infarction in Predicting Long-Term Adverse Cardiac Events. Am. J. Cardiol. 2007, 100, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, A.; Nijveldt, R.; Haeck, J.D.; Beek, A.M.; Koch, K.T.; Henriques, J.P.; van der Schaaf, R.J.; Vis, M.M.; Baan, J., Jr.; de Winter, R.J.; et al. Relation between the Assessment of Microvascular Injury by Cardiovascular Magnetic Resonance and Coronary Doppler Flow Velocity Measurements in Patients with Acute Anterior Wall Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 2230–2238. [Google Scholar] [CrossRef]
- Van de Hoef, T.P.; Bax, M.; Meuwissen, M.; Damman, P.; Delewi, R.; de Winter, R.J.; Koch, K.T.; Schotborgh, C.; Henriques, J.P.; Tijssen, J.G.; et al. Impact of Coronary Microvascular Function on Long-Term Cardiac Mortality in Patients with Acute St-Segment-Elevation Myocardial Infarction. Circ. Cardiovasc. Interv. 2013, 6, 207–215. [Google Scholar] [CrossRef]
- Gould, K.L.; Kirkeeide, R.L.; Buchi, M. Coronary Flow Reserve as a Physiologic Measure of Stenosis Severity. J. Am. Coll. Cardiol. 1990, 15, 459–474. [Google Scholar] [CrossRef]
- Fearon, W.F.; Balsam, L.B.; Farouque, H.M.; Caffarelli, A.D.; Robbins, R.C.; Fitzgerald, P.J.; Yock, P.G.; Yeung, A.C. Novel Index for Invasively Assessing the Coronary Microcirculation. Circulation 2003, 107, 3129–3132. [Google Scholar] [CrossRef] [PubMed]
- Jansen, T.P.J.; de Vos, A.; Paradies, V.; Dimitriu-Leen, A.; Crooijmans, C.; Elias-Smale, S.; Rodwell, L.; Maas, A.H.E.M.; Smits, P.C.; Pijls, N.; et al. Continuous Versus Bolus Thermodilution-Derived Coronary Flow Reserve and Microvascular Resistance Reserve and Their Association with Angina and Quality of Life in Patients with Angina and Nonobstructive Coronaries: A Head-to-Head Comparison. J. Am. Heart Assoc. 2023, 12, e030480. [Google Scholar] [CrossRef]
- Schindler, T.H.; Schelbert, H.R.; Quercioli, A.; Dilsizian, V. Cardiac Pet Imaging for the Detection and Monitoring of Coronary Artery Disease and Microvascular Health. JACC Cardiovasc. Imaging 2010, 3, 623–640. [Google Scholar] [CrossRef]
- Herzog, B.A.; Husmann, L.; Valenta, I.; Gaemperli, O.; Siegrist, P.T.; Tay, F.M.; Burkhard, N.; Wyss, C.A.; Kaufmann, P.A. Long-Term Prognostic Value of 13n-Ammonia Myocardial Perfusion Positron Emission Tomography Added Value of Coronary Flow Reserve. J. Am. Coll. Cardiol. 2009, 54, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Vegsundvåg, J.; Holte, E.; Wiseth, R.; Hegbom, K.; Hole, T. Coronary Flow Velocity Reserve in the Three Main Coronary Arteries Assessed with Transthoracic Doppler: A Comparative Study with Quantitative Coronary Angiography. J. Am. Soc. Echocardiogr. 2011, 24, 758–767. [Google Scholar] [CrossRef]
- Olsen, R.H.; Pedersen, L.R.; Snoer, M.; Christensen, T.E.; Ghotbi, A.A.; Hasbak, P.; Kjaer, A.; Haugaard, S.B.; Prescott, E. Coronary Flow Velocity Reserve by Echocardiography: Feasibility, Reproducibility and Agreement with Pet in Overweight and Obese Patients with Stable and Revascularized Coronary Artery Disease. Cardiovasc. Ultrasound 2016, 14, 22. [Google Scholar] [CrossRef] [PubMed]
- Cortigiani, L.; Rigo, F.; Gherardi, S.; Bovenzi, F.; Molinaro, S.; Picano, E.; Sicari, R. Coronary Flow Reserve During Dipyridamole Stress Echocardiography Predicts Mortality. JACC Cardiovasc. Imaging 2012, 5, 1079–1085. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, I.; Gan, L.-M.; Svedlund, S.; Wittfeldt, A.; Råmunddal, T.; Angerås, O.; Albertsson, P.; Matejka, G.; Omerovic, E. Non-Invasive Evaluation of Coronary Flow Reserve with Transthoracic Doppler Echocardiography Predicts the Presence of Significant Stenosis in Coronary Arteries. Int. J. Cardiol. 2014, 176, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Tesic, M.; Djordjevic-Dikic, A.; Giga, V.; Stepanovic, J.; Dobric, M.; Jovanovic, I.; Petrovic, M.; Mehmedbegovic, Z.; Milasinovic, D.; Dedovic, V.; et al. Prognostic Value of Transthoracic Doppler Echocardiography Coronary Flow Velocity Reserve in Patients with Nonculprit Stenosis of Intermediate Severity Early after Primary Percutaneous Coronary Intervention. J. Am. Soc. Echocardiogr. 2018, 31, 880–887. [Google Scholar] [CrossRef]
- Nohtomi, Y.; Takeuchi, M.; Nagasawa, K.; Arimura, K.; Miyata, K.; Kuwata, K.; Yamawaki, T.; Kondo, S.; Yamada, A.; Okamatsu, S. Persistence of Systolic Coronary Flow Reversal Predicts Irreversible Dysfunction after Reperfused Anterior Myocardial Infarction. Heart 2003, 89, 382–388. [Google Scholar] [CrossRef]
- Shintani, Y.; Ito, H.; Iwakura, K.; Kawano, S.; Tanaka, K.; Masuyama, T.; Hori, M.; Fujii, K. Usefulness of Impairment of Coronary Microcirculation in Predicting Left Ventricular Dilation after Acute Myocardial Infarction. Am. J. Cardiol. 2004, 93, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Trifunovic, D.; Sobic-Saranovic, D.; Beleslin, B.; Stankovic, S.; Marinkovic, J.; Orlic, D.; Vujisic-Tesic, B.; Petrovic, M.; Nedeljkovic, I.; Banovic, M.; et al. Coronary Flow of the Infarct Artery Assessed by Transthoracic Doppler after Primary Percutaneous Coronary Intervention Predicts Final Infarct Size. Int. J. Cardiovasc. Imaging 2014, 30, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- De Waard, G.A.; Fahrni, G.; de Wit, D.; Kitabata, H.; Williams, R.; Patel, N.; Teunissen, P.F.; van de Ven, P.M.; Umman, S.; Knaapen, P.; et al. Hyperaemic Microvascular Resistance Predicts Clinical Outcome and Microvascular Injury after Myocardial Infarction. Heart 2018, 104, 127–134. [Google Scholar] [CrossRef] [PubMed]
- El Farissi, M.; Zimmermann, F.M.; De Maria, G.L.; van Royen, N.; van Leeuwen, M.A.H.; Carrick, D.; Carberry, J.; Wijnbergen, I.F.; Konijnenberg, L.S.F.; Hoole, S.P.; et al. The Index of Microcirculatory Resistance after Primary Pci: A Pooled Analysis of Individual Patient Data. JACC Cardiovasc. Interv. 2023, 16, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, K.; Hoshino, M.; Usui, E.; Hanyu, Y.; Sugiyama, T.; Kanaji, Y.; Hada, M.; Nagamine, T.; Nogami, K.; Ueno, H.; et al. Noninvasive Transthoracic Doppler Flow Velocity and Invasive Thermodilution to Assess Coronary Flow Reserve. Quant. Imaging Med. Surg. 2024, 14, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Dikic, A.D.; Tesic, M.; Boskovic, N.; Giga, V.; Stepanovic, J.; Petrovic, M.; Dobric, M.; Aleksandric, S.; Juricic, S.; Dikic, M.; et al. Prognostic Value of Preserved Coronary Flow Velocity Reserve by Noninvasive Transthoracic Doppler Echocardiography in Patients with Angiographically Intermediate Left Main Stenosis. J. Am. Soc. Echocardiogr. 2019, 32, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Montaldo, C.; Zedda, N.; Montisci, R.; Ruscazio, M.; Lai, G.; Cadeddu, M.; Meloni, L.; Iliceto, S. Validation of a New Noninvasive Method (Contrast-Enhanced Transthoracic Second Harmonic Echo Doppler) for the Evaluation of Coronary Flow Reserve: Comparison with Intracoronary Doppler Flow Wire. J. Am. Coll. Cardiol. 1999, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Yoshida, K.; Akasaka, T.; Asami, Y.; Ogata, Y.; Takagi, T.; Kaji, S.; Kawamoto, T.; Ueda, Y.; Morioka, S. Noninvasive Assessment of Coronary Flow Velocity and Coronary Flow Velocity Reserve in the Left Anterior Descending Coronary Artery by Doppler Echocardiography: Comparison with Invasive Technique. J. Am. Coll. Cardiol. 1998, 32, 1251–1259. [Google Scholar] [CrossRef] [PubMed]
- Demir, O.M.; Boerhout, C.K.; de Waard, G.A.; van de Hoef, T.P.; Patel, N.; Beijk, M.A.; Williams, R.; Rahman, H.; Everaars, H.; Kharbanda, R.K.; et al. Comparison of Doppler Flow Velocity and Thermodilution Derived Indexes of Coronary Physiology. JACC Cardiovasc. Interv. 2022, 15, 1060–1070. [Google Scholar] [CrossRef]
- Meimoun, P.; Boulanger, J.; Luycx-Bore, A.; Zemir, H.; Elmkies, F.; Malaquin, D.; Doutrelan, L.; Tribouilloy, C. Non-Invasive Coronary Flow Reserve after Successful Primary Angioplasty for Acute Anterior Myocardial Infarction Is an Independent Predictor of Left Ventricular Adverse Remodelling. Eur. J. Echocardiogr. 2010, 11, 711–718. [Google Scholar] [CrossRef]
- Rigo, F.; Varga, Z.; Di Pede, F.; Grassi, G.; Turiano, G.; Zuin, G.; Coli, U.; Raviele, A.; Picano, E. Early Assessment of Coronary Flow Reserve by Transthoracic Doppler Echocardiography Predicts Late Remodeling in Reperfused Anterior Myocardial Infarction. J. Am. Soc. Echocardiogr. 2004, 17, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Bulluck, H. Risk Stratification by Cardiovascular Magnetic Resonance after Reperfused St-Segment Elevation Myocardial Infarction: Ready for Prime Time? JACC Cardiovasc. Imaging 2018, 11, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship between Infarct Size and Outcomes Following Primary Pci: Patient-Level Analysis from 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef] [PubMed]
- Malgie, J.; Clephas, P.R.D.; Rocca, H.-P.B.-L.; de Boer, R.A.; Brugts, J.J. Guideline-Directed Medical Therapy for Hfref: Sequencing Strategies and Barriers for Life-Saving Drug Therapy. Heart Fail. Rev. 2023, 28, 1221–1234. [Google Scholar] [CrossRef] [PubMed]
- Schroder, J.; Michelsen, M.M.; Mygind, N.D.; E Suhrs, H.; Bove, K.B.; Bechsgaard, D.F.; Aziz, A.; Gustafsson, I.; Kastrup, J.; Prescott, E. Coronary Flow Velocity Reserve Predicts Adverse Prognosis in Women with Angina and No Obstructive Coronary Artery Disease: Results from the Ipower Study. Eur. Heart J. 2021, 42, 228–239. [Google Scholar] [CrossRef]
- Perone, F.; Bernardi, M.; Redheuil, A.; Mafrica, D.; Conte, E.; Spadafora, L.; Ecarnot, F.; Tokgozoglu, L.; Santos-Gallego, C.G.; Kaiser, S.E.; et al. Role of Cardiovascular Imaging in Risk Assessment: Recent Advances, Gaps in Evidence, and Future Directions. J. Clin. Med. 2023, 12, 5563. [Google Scholar] [CrossRef]


| Age, Years | 59 (53–62) | |
|---|---|---|
| Clinical presentation and demographics | Male, % | 86 |
| Time to reperfusion, h | 180 (120–252) | |
| Systolic blood pressure, mmHg | 125 ±15 | |
| Diastolic blood pressure, mmHg | 80 ± 11 | |
| Heart rate, beats/min | 75 ± 14 | |
| Hypertension, % | 62 | |
| Dyslipidemia, % | 80 | |
| Diabetes, % | 17 | |
| Smoking, % | 65 | |
| BMI, kg/m2 | 28 ± 4 | |
| Maximum CK, U/L | 2718 ± 1848 | |
| Maximum high-sensitivity Troponin T, ng/L | 6119 ± 3450 | |
| Procedural characteristics | Infarct-related artery, % | 69 (LAD) |
| 31 (RCA) | ||
| Transradial access, % | 100 | |
| Manual thrombectomy, % | 17 | |
| Glycoprotein inhibitor use, % | 13 | |
| Direct stenting, % | 10 | |
| Total stented length, mm | 26 (21–47) | |
| Post-dilatation, % | 58 | |
| Echocardiography during index hospitalization | Left ventricular ejection fraction, % | 45 ± 11 |
| End-diastolic diameter, mm | 54 ± 5 | |
| End-systolic diameter, mm | 38 ± 5 | |
| Incomplete (≤70%) ST-segment resolution on ECG | 48 |
| CMR-Based Infarct Size <18% (n = 23) | CMR-Based Infarct Size ≥18% (n = 6) | p-Value | ||
|---|---|---|---|---|
| Invasive Doppler wire-based coronary physiology | APV resting, cm/s | 21.17 ± 6.97 | 22.17 ± 8.08 | 0.77 |
| APV hyperemia, cm/s | 43.78 ± 11.10 | 32.17 ± 8.63 | 0.02 | |
| CFR | 2.16 ± 0.55 | 1.52 ± 0.20 | 0.01 | |
| Pa resting, mmHg | 93.13 ± 10.84 | 85.50 ± 5.54 | 0.11 | |
| Pd resting, mmHg | 89.78 ± 12.20 | 77.83 ± 6.97 | 0.03 | |
| Pa hyperemia, mmHg | 94.43 ± 16.96 | 84.00 ± 7.77 | 0.16 | |
| Pd hyperemia, mmHg | 87.52 ± 18.63 | 76.83 ± 12.71 | 0.20 | |
| FFR | 0.93 ± 0.05 | 0.89 ± 0.04 | 0.11 | |
| HMR | 2.16 ± 0.68 | 2.60 ± 0.46 | 0.12 | |
| Non-invasive transthoracic Doppler echocardiography-based coronary physiology * | APV resting, m/s | 0.26 ± 0.04 | 0.30 ± 0.13 | 0.54 |
| APV hyperemia, m/s | 0.59 ± 0.14 | 0.49 ± 0.18 | 0.37 | |
| CFR | 2.25 ± 0.46 | 1.62 ± 0.36 | 0.02 | |
| Cardiac magnetic resonance † | LVEDV, mL ** | 144.00 ± 58.00 | 222.71 ± 98.00 | <0.01 |
| LVEDV indexed, mL/m2 ** | 76 ± 35 | 96 ± 52 | <0.01 | |
| LVESV, mL ** | 77 ± 43 | 131 ± 77 | 0.01 | |
| LVESV indexed, mL/2 ** | 33 ± 20 | 65 ± 40 | <0.01 | |
| LVEF, % | 54 ± 7 | 36 ± 8 | <0.01 | |
| Infarct size, % of the LV ** | 11.00 ± 9.00 | 21.00 ± 7.00 | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milasinovic, D.; Tesic, M.; Nedeljkovic Arsenovic, O.; Maksimovic, R.; Sobic Saranovic, D.; Jelic, D.; Zivkovic, M.; Dedovic, V.; Juricic, S.; Mehmedbegovic, Z.; et al. Correlation of Non-Invasive Transthoracic Doppler Echocardiography with Invasive Doppler Wire-Derived Coronary Flow Reserve and Their Impact on Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. J. Clin. Med. 2024, 13, 2484. https://doi.org/10.3390/jcm13092484
Milasinovic D, Tesic M, Nedeljkovic Arsenovic O, Maksimovic R, Sobic Saranovic D, Jelic D, Zivkovic M, Dedovic V, Juricic S, Mehmedbegovic Z, et al. Correlation of Non-Invasive Transthoracic Doppler Echocardiography with Invasive Doppler Wire-Derived Coronary Flow Reserve and Their Impact on Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention. Journal of Clinical Medicine. 2024; 13(9):2484. https://doi.org/10.3390/jcm13092484
Chicago/Turabian StyleMilasinovic, Dejan, Milorad Tesic, Olga Nedeljkovic Arsenovic, Ruzica Maksimovic, Dragana Sobic Saranovic, Dario Jelic, Milorad Zivkovic, Vladimir Dedovic, Stefan Juricic, Zlatko Mehmedbegovic, and et al. 2024. "Correlation of Non-Invasive Transthoracic Doppler Echocardiography with Invasive Doppler Wire-Derived Coronary Flow Reserve and Their Impact on Infarct Size in Patients with ST-Segment Elevation Myocardial Infarction Treated with Primary Percutaneous Coronary Intervention" Journal of Clinical Medicine 13, no. 9: 2484. https://doi.org/10.3390/jcm13092484






