Non-Invasive Tools in Perioperative Stroke Risk Assessment for Asymptomatic Carotid Artery Stenosis with a Focus on the Circle of Willis
Abstract
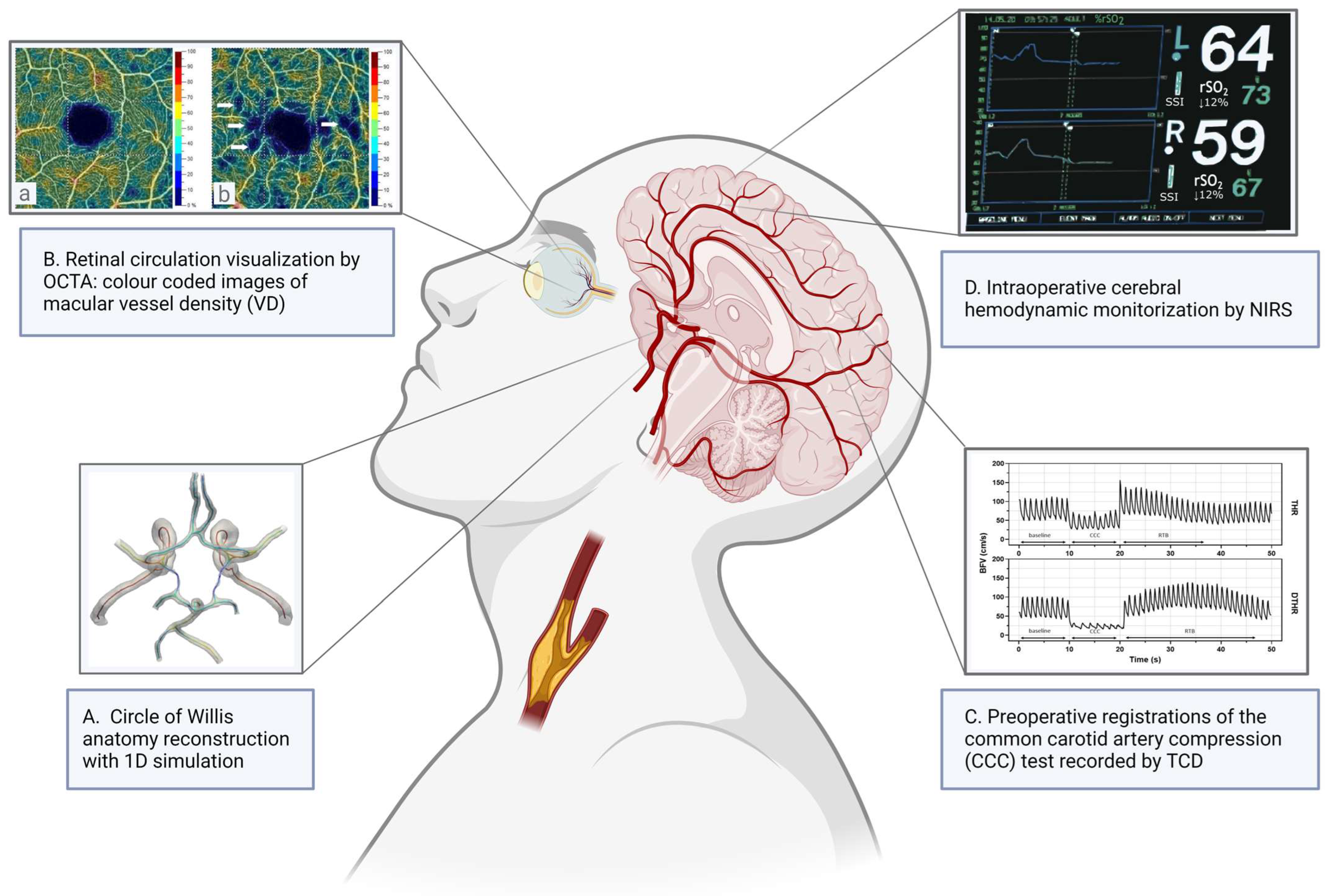
:1. Introduction
2. Pre- and Perioperative Risk Assessment of Impaired Cerebrovascular Reserve Capacity
2.1. Carotid-Related Cerebral Perfusion via the Collateral Vascular System
2.1.1. Circle of Willis
Epidemiology of CoW Variabilities
Effects of CoW Variations on Cerebrovascular Hemodynamics and Stroke
Effects of CoW Variations on Intraoperative Ischemic Stroke Risk
2.1.2. Role of the Secondary Cerebral Collaterals
2.2. Non-Invasive Diagnostic Tools to Measure and Evaluate Cerebrovascular Reactivity
2.2.1. Imaging of Retinal Circulation
Optical Coherence Tomography Angiography
2.2.2. Monitoring of Cerebrovascular Circulation
Cerebrovascular Reserve Capacity Estimation by Functional Transcranial Doppler
Non-Invasive Neuromonitoring by Near-Infrared Spectroscopy
2.3. Biomedical Modeling of Cerebrovascular Hemodynamics in Patients with CAS
2.3.1. Effect of CoW Variations on Hemodynamical Modeling
2.3.2. Hemodynamic Effects of Carotid Artery Stenosis
3. Cognitive Function Changes in Patients with Carotid Artery Stenosis
3.1. Prevention of Further Cognitive Decline with Carotid Reconstruction
3.2. The Role of Cerebrovascular Reserve Capacity in Patients with Carotid Stenosis and Cognitive Dysfunction
3.3. Effects of the CoW on Cognitive Dysfunction in Patients with Carotid Stenosis
4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An updated definition of stroke for the 21st century: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089. [Google Scholar] [CrossRef] [PubMed]
- Naylor, R.; Rantner, B.; Ancetti, S.; de Borst, G.J.; De Carlo, M.; Halliday, A.; Kakkos, S.K.; Markus, H.S.; McCabe, D.J.H.; Sillesen, H.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2023 Clinical Practice Guidelines on the Management of Atherosclerotic Carotid and Vertebral Artery Disease. Eur. J. Vasc. Endovasc. Surg. 2023, 65, 7–111. [Google Scholar] [CrossRef] [PubMed]
- Kleindorfer, D.O.; Towfighi, A.; Chaturvedi, S.; Cockroft, K.M.; Gutierrez, J.; Lombardi-Hill, D.; Kamel, H.; Kernan, W.N.; Kittner, S.J.; Leira, E.C.; et al. 2021 Guideline for the Prevention of Stroke in Patients with Stroke and Transient Ischemic Attack: A Guideline From the American Heart Association/American Stroke Association. Stroke 2021, 52, e364–e467. [Google Scholar] [CrossRef] [PubMed]
- Mechtouff, L.; Rascle, L.; Crespy, V.; Canet-Soulas, E.; Nighoghossian, N.; Millon, A. A narrative review of the pathophysiology of ischemic stroke in carotid plaques: A distinction versus a compromise between hemodynamic and embolic mechanism. Ann. Transl. Med. 2021, 9, 1208. [Google Scholar] [CrossRef] [PubMed]
- AbuRahma, A.F.; Avgerinos, E.D.; Chang, R.W.; Darling, R.C., 3rd; Duncan, A.A.; Forbes, T.L.; Malas, M.B.; Murad, M.H.; Perler, B.A.; Powell, R.J.; et al. Society for Vascular Surgery clinical practice guidelines for management of extracranial cerebrovascular disease. J. Vasc. Surg. 2022, 75, 4s–22s. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.R.; Ricco, J.B.; de Borst, G.J.; Debus, S.; de Haro, J.; Halliday, A.; Hamilton, G.; Kakisis, J.; Kakkos, S.; Lepidi, S.; et al. Editor’s Choice—Management of Atherosclerotic Carotid and Vertebral Artery Disease: 2017 Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur. J. Vasc. Endovasc. Surg. 2018, 55, 3–81. [Google Scholar] [CrossRef] [PubMed]
- Saam, T.; Ferguson, M.S.; Yarnykh, V.L.; Takaya, N.; Xu, D.; Polissar, N.L.; Hatsukami, T.S.; Yuan, C. Quantitative evaluation of carotid plaque composition by in vivo MRI. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 234–239. [Google Scholar] [CrossRef] [PubMed]
- den Hartog, A.G.; Bovens, S.M.; Koning, W.; Hendrikse, J.; Luijten, P.R.; Moll, F.L.; Pasterkamp, G.; de Borst, G.J. Current status of clinical magnetic resonance imaging for plaque characterisation in patients with carotid artery stenosis. Eur. J. Vasc. Endovasc. Surg. 2013, 45, 7–21. [Google Scholar] [CrossRef] [PubMed]
- de Weert, T.T.; Ouhlous, M.; Meijering, E.; Zondervan, P.E.; Hendriks, J.M.; van Sambeek, M.R.; Dippel, D.W.; van der Lugt, A. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Wintermark, M.; Jawadi, S.S.; Rapp, J.H.; Tihan, T.; Tong, E.; Glidden, D.V.; Abedin, S.; Schaeffer, S.; Acevedo-Bolton, G.; Boudignon, B.; et al. High-resolution CT imaging of carotid artery atherosclerotic plaques. AJNR Am. J. Neuroradiol. 2008, 29, 875–882. [Google Scholar] [CrossRef]
- Tawakol, A.; Migrino, R.Q.; Bashian, G.G.; Bedri, S.; Vermylen, D.; Cury, R.C.; Yates, D.; LaMuraglia, G.M.; Furie, K.; Houser, S.; et al. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J. Am. Coll. Cardiol. 2006, 48, 1818–1824. [Google Scholar] [CrossRef] [PubMed]
- Kerwin, W.S.; O’Brien, K.D.; Ferguson, M.S.; Polissar, N.; Hatsukami, T.S.; Yuan, C. Inflammation in carotid atherosclerotic plaque: A dynamic contrast-enhanced MR imaging study. Radiology 2006, 241, 459–468. [Google Scholar] [CrossRef]
- Hop, H.; de Boer, S.A.; Reijrink, M.; Kamphuisen, P.W.; de Borst, M.H.; Pol, R.A.; Zeebregts, C.J.; Hillebrands, J.L.; Slart, R.; Boersma, H.H.; et al. (18)F-sodium fluoride positron emission tomography assessed microcalcifications in culprit and non-culprit human carotid plaques. J. Nucl. Cardiol. 2019, 26, 1064–1075. [Google Scholar] [CrossRef] [PubMed]
- Farkas, S.; Molnár, S.; Nagy, K.; Hortobagyi, T.; Csiba, L. Comparative in vivo and in vitro postmortem ultrasound assessment of intima-media thickness with additional histological analysis in human carotid arteries. Perspect. Med. 2012, 1, 170–176. [Google Scholar] [CrossRef]
- Huibers, A.; de Borst, G.J.; Wan, S.; Kennedy, F.; Giannopoulos, A.; Moll, F.L.; Richards, T. Non-invasive Carotid Artery Imaging to Identify the Vulnerable Plaque: Current Status and Future Goals. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Masuyama, T.; Mori, H.; Maeda, T.; Kitade, K.; Moriyasu, K.; Tsujimoto, M.; Fujimoto, K.; Koshimae, N.; Matsuura, N. Ultrasonic tissue characterization of the atherosclerotic carotid artery: Histological correlates or carotid integrated backscatter. Circ. J. 2003, 67, 1013–1016. [Google Scholar] [CrossRef] [PubMed]
- Saba, L.; Saam, T.; Jäger, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.L.; Chan, M.T.; Gelb, A.W. Perioperative stroke in noncardiac, nonneurosurgical surgery. Anesthesiology 2011, 115, 879–890. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Peixoto, J.; Canedo, A.; Kappelle, L.J.; Mansilha, A.; de Borst, G.J. Critical analysis of the literature and standards of reporting on stroke after carotid revascularization. J. Vasc. Surg. 2022, 75, 363–371.e362. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Mikhailidis, D.P.; Ringleb, P.A.; Brown, M.M.; Dardik, A.; Poredos, P.; Gray, W.A.; Nicolaides, A.N.; Lal, B.K.; Mansilha, A.; et al. An international, multispecialty, expert-based Delphi Consensus document on controversial issues in the management of patients with asymptomatic and symptomatic carotid stenosis. J. Vasc. Surg. 2024, 79, 420–435.e421. [Google Scholar] [CrossRef]
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J. Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Castanho, P.; Bazira, P.; Sanders, K. Anatomical variations of the circle of Willis and their prevalence, with a focus on the posterior communicating artery: A literature review and meta-analysis. Clin. Anat. 2021, 34, 978–990. [Google Scholar] [CrossRef] [PubMed]
- Alpers, B.J.; Berry, R.G. Circle of Willis in cerebral vascular disorders. The anatomical structure. Arch. Neurol. 1963, 8, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Alpers, B.J.; Berry, R.G.; Paddison, R.M. Anatomical studies of the circle of Willis in normal brain. AMA Arch. Neurol. Psychiatry 1959, 81, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Hartkamp, M.J.; van Der Grond, J.; van Everdingen, K.J.; Hillen, B.; Mali, W.P. Circle of Willis collateral flow investigated by magnetic resonance angiography. Stroke 1999, 30, 2671–2678. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S. A comprehensive study of the anatomical variations of the circle of willis in adult human brains. J. Clin. Diagn. Res. 2013, 7, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Krabbe-Hartkamp, M.J.; van der Grond, J.; de Leeuw, F.E.; de Groot, J.C.; Algra, A.; Hillen, B.; Breteler, M.M.; Mali, W.P. Circle of Willis: Morphologic variation on three-dimensional time-of-flight MR angiograms. Radiology 1998, 207, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, J.; Lv, F.; Li, K.; Luo, T.; Xie, P. A multidetector CT angiography study of variations in the circle of Willis in a Chinese population. J. Clin. Neurosci. 2011, 18, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Puchades-Orts, A.; Nombela-Gomez, M.; Ortuño-Pacheco, G. Variation in form of circle of Willis: Some anatomical and embryological considerations. Anat. Rec. 1976, 185, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Riggs, H.E.; Rupp, C. Variation in form of circle of Willis. The relation of the variations to collateral circulation: Anatomic analysis. Arch. Neurol. 1963, 8, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Hindenes, L.B.; Håberg, A.K.; Johnsen, L.H.; Mathiesen, E.B.; Robben, D.; Vangberg, T.R. Variations in the Circle of Willis in a large population sample using 3D TOF angiography: The Tromsø Study. PLoS ONE 2020, 15, e0241373. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Di Leo, G.; Banga, P.V.; Csobay-Novák, C.; Kolossváry, M.; Maurovich-Horvat, P.; Hüttl, K. Multidetector CT angiography of the Circle of Willis: Association of its variants with carotid artery disease and brain ischemia. Eur. Radiol. 2019, 29, 46–56. [Google Scholar] [CrossRef] [PubMed]
- De Caro, J.; Ciacciarelli, A.; Tessitore, A.; Buonomo, O.; Calzoni, A.; Francalanza, I.; Dell’Aera, C.; Cosenza, D.; Currò, C.T.; Granata, F.; et al. Variants of the circle of Willis in ischemic stroke patients. J. Neurol. 2021, 268, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Ophelders, M.E.H.; van Eldik, M.J.A.; Vos, I.N.; Beentjes, Y.S.; Velthuis, B.K.; Ruigrok, Y.M. Anatomical differences of intracranial arteries according to sex: A systematic review and meta-analysis. J. Neuroradiol. 2024, 51, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Zaninovich, O.A.; Ramey, W.L.; Walter, C.M.; Dumont, T.M. Completion of the Circle of Willis Varies by Gender, Age, and Indication for Computed Tomography Angiography. World Neurosurg. 2017, 106, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Gyöngyösi, Z.; Farkas, O.; Papp, L.; Bodnár, F.; Végh, T.; Fülesdi, B. The value of transcranial Doppler monitoring of cerebral blood flow changes during carotid endarterectomy performed under regional anesthesia—A case series. Transl. Neurosci. 2022, 13, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.; Kamel, H.; Gupta, A.; RoyChoudhury, A.; Girgis, P.; Glodzik, L. Incomplete circle of Willis variants and stroke outcome. Eur. J. Radiol. 2022, 153, 110383. [Google Scholar] [CrossRef]
- Wholey, M.W.A.; Nowak, I.; Wu, W.C.L. CTA and the Circle of Willis. Early use of multislice CTA to evaluate the distal internal carotid artery and the Circle of Willis and their correlation with stroke. Endovasc. Today 2009, 7, 33–44. [Google Scholar]
- Karatas, A.; Coban, G.; Cinar, C.; Oran, I.; Uz, A. Assessment of the Circle of Willis with Cranial Tomography Angiography. Med. Sci. Monit. 2015, 21, 2647–2652. [Google Scholar] [CrossRef]
- Waaijer, A.; van Leeuwen, M.S.; van der Worp, H.B.; Verhagen, H.J.; Mali, W.P.; Velthuis, B.K. Anatomic variations in the circle of Willis in patients with symptomatic carotid artery stenosis assessed with multidetector row CT angiography. Cerebrovasc. Dis. 2007, 23, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Alastruey, J.; Parker, K.H.; Peiró, J.; Byrd, S.M.; Sherwin, S.J. Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows. J. Biomech. 2007, 40, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.H.; Leung, A.; Lownie, S.P. Circle of Willis Collateral during Temporary Internal Carotid Artery Occlusion II: Observations from Computed Tomography Angiography. Can. J. Neurol. Sci. 2016, 43, 538–542. [Google Scholar] [CrossRef] [PubMed]
- Oumer, M.; Alemayehu, M.; Muche, A. Association between circle of Willis and ischemic stroke: A systematic review and meta-analysis. BMC Neurosci. 2021, 22, 3. [Google Scholar] [CrossRef] [PubMed]
- Banga, P.V.; Varga, A.; Csobay-Novák, C.; Kolossváry, M.; Szántó, E.; Oderich, G.S.; Entz, L.; Sótonyi, P. Incomplete circle of Willis is associated with a higher incidence of neurologic events during carotid eversion endarterectomy without shunting. J. Vasc. Surg. 2018, 68, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.P.; Kuzniec, S.; Molnar, L.J.; Cerri, G.G.; Puech-Leão, P.; Carvalho, C.A. Collateral blood supply through the ophthalmic artery: A steal phenomenon analyzed by color Doppler imaging. Ophthalmology 1998, 105, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.P.; Kuzniec, S.; Molnar, L.J.; Cerri, G.G.; Puech-Leão, P.; Carvalho, C.A. The effects of carotid endarterectomy on the retrobulbar circulation of patients with severe occlusive carotid artery disease. An investigation by color Doppler imaging. Ophthalmology 1999, 106, 306–310. [Google Scholar] [CrossRef]
- Arsava, E.M.; Vural, A.; Akpinar, E.; Gocmen, R.; Akcalar, S.; Oguz, K.K.; Topcuoglu, M.A. The detrimental effect of aging on leptomeningeal collaterals in ischemic stroke. J. Stroke Cerebrovasc. Dis. 2014, 23, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Akamatsu, Y.; Yang, S.Y.; Lee, C.C.; Baran, U.; Song, S.; Wang, R.K.; Tominaga, T.; Liu, J. Impaired Collateral Flow Compensation during Chronic Cerebral Hypoperfusion in the Type 2 Diabetic Mice. Stroke 2016, 47, 3014–3021. [Google Scholar] [CrossRef] [PubMed]
- Menon, B.K.; Smith, E.E.; Coutts, S.B.; Welsh, D.G.; Faber, J.E.; Goyal, M.; Hill, M.D.; Demchuk, A.M.; Damani, Z.; Cho, K.H.; et al. Leptomeningeal collaterals are associated with modifiable metabolic risk factors. Ann. Neurol. 2013, 74, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hung, S.H.; Kramer, S.; Werden, E.; Campbell, B.C.V.; Brodtmann, A. Pre-stroke Physical Activity and Cerebral Collateral Circulation in Ischemic Stroke: A Potential Therapeutic Relationship? Front. Neurol. 2022, 13, 804187. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, K.; Safouris, A.; Goyal, N.; Arthur, A.; Liebeskind, D.S.; Katsanos, A.H.; Sargento-Freitas, J.; Ribo, M.; Molina, C.; Chung, J.W.; et al. Association of statin pretreatment with collateral circulation and final infarct volume in acute ischemic stroke patients: A meta-analysis. Atherosclerosis 2019, 282, 75–79. [Google Scholar] [CrossRef] [PubMed]
- István, L.; Czakó, C.; Benyó, F.; Élő, Á.; Mihály, Z.; Sótonyi, P.; Varga, A.; Nagy, Z.Z.; Kovács, I. The effect of systemic factors on retinal blood flow in patients with carotid stenosis: An optical coherence tomography angiography study. Geroscience 2022, 44, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Chazen, J.L.; Hartman, M.; Delgado, D.; Anumula, N.; Shao, H.; Mazumdar, M.; Segal, A.Z.; Kamel, H.; Leifer, D.; et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: A systematic review and meta-analysis. Stroke 2012, 43, 2884–2891. [Google Scholar] [CrossRef]
- Sainbhi, A.S.; Gomez, A.; Froese, L.; Slack, T.; Batson, C.; Stein, K.Y.; Cordingley, D.M.; Alizadeh, A.; Zeiler, F.A. Non-Invasive and Minimally-Invasive Cerebral Autoregulation Assessment: A Narrative Review of Techniques and Implications for Clinical Research. Front. Neurol. 2022, 13, 872731. [Google Scholar] [CrossRef] [PubMed]
- István, L.; Czakó, C.; Élő, Á.; Mihály, Z.; Sótonyi, P.; Varga, A.; Ungvári, Z.; Csiszár, A.; Yabluchanskiy, A.; Conley, S.; et al. Imaging retinal microvascular manifestations of carotid artery disease in older adults: From diagnosis of ocular complications to understanding microvascular contributions to cognitive impairment. Geroscience 2021, 43, 1703–1723. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; Macgillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Czakó, C.; István, L.; Ecsedy, M.; Récsán, Z.; Sándor, G.; Benyó, F.; Horváth, H.; Papp, A.; Resch, M.; Borbándy, Á.; et al. The effect of image quality on the reliability of OCT angiography measurements in patients with diabetes. Int. J. Retin. Vitr. 2019, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Czakó, C.; István, L.; Benyó, F.; Élő, Á.; Erdei, G.; Horváth, H.; Nagy, Z.Z.; Kovács, I. The Impact of Deterministic Signal Loss on OCT Angiography Measurements. Transl. Vis. Sci. Technol. 2020, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.J.; Camino, A.; Liu, L.; Zhang, X.; Wang, J.; Gao, S.S.; Jia, Y.; Huang, D. Signal Strength Reduction Effects in OCT Angiography. Ophthalmol. Retin. 2019, 3, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Al-Sheikh, M.; Ghasemi Falavarjani, K.; Akil, H.; Sadda, S.R. Impact of image quality on OCT angiography based quantitative measurements. Int. J. Retin. Vitr. 2017, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Frueh, B.E.; Steinmair, D.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S.; Munk, M.R. Cataract significantly influences quantitative measurements on swept-source optical coherence tomography angiography imaging. PLoS ONE 2018, 13, e0204501. [Google Scholar] [CrossRef] [PubMed]
- Holló, G. Influence of Posterior Subcapsular Cataract on Structural OCT and OCT Angiography Vessel Density Measurements in the Peripapillary Retina. J. Glaucoma 2019, 28, e61–e63. [Google Scholar] [CrossRef] [PubMed]
- Monteiro-Henriques, I.; Rocha-Sousa, A.; Barbosa-Breda, J. Optical coherence tomography angiography changes in cardiovascular systemic diseases and risk factors: A Review. Acta Ophthalmol. 2022, 100, e1–e15. [Google Scholar] [CrossRef] [PubMed]
- Lahme, L.; Marchiori, E.; Panuccio, G.; Nelis, P.; Schubert, F.; Mihailovic, N.; Torsello, G.; Eter, N.; Alnawaiseh, M. Changes in retinal flow density measured by optical coherence tomography angiography in patients with carotid artery stenosis after carotid endarterectomy. Sci. Rep. 2018, 8, 17161. [Google Scholar] [CrossRef] [PubMed]
- Machalińska, A.; Kawa, M.P.; Babiak, K.; Sobuś, A.; Grabowicz, A.; Lejkowska, R.; Kazimierczak, A.; Rynio, P.; Safranow, K.; Wilk, G.; et al. Retinal vessel dynamic functionality in the eyes of asymptomatic patients with significant internal carotid artery stenosis. Int. Angiol. A J. Int. Union. Angiol. 2019, 38, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Batu Oto, B.; Kılıçarslan, O.; Kayadibi, Y.; Yılmaz Çebi, A.; Adaletli, İ.; Yıldırım, S.R. Retinal Microvascular Changes in Internal Carotid Artery Stenosis. J. Clin. Med. 2023, 12, 6014. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.W.; Cheng, H.C.; Chang, F.C.; Wang, A.G. Optical Coherence Tomography Angiography Evaluation of Retinal Microvasculature before and after Carotid Angioplasty and Stenting. Sci. Rep. 2019, 9, 14755. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Schmidt-Kastner, R.; Hamasaki, D.I.; Yamamoto, H.; Parel, J.M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp. Eye Res. 2006, 82, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Jeong, H.; Miwa, Y.; Shinojima, A.; Katada, Y.; Tsubota, K.; Kurihara, T. Retinal dysfunction induced in a mouse model of unilateral common carotid artery occlusion. PeerJ 2021, 9, e11665. [Google Scholar] [CrossRef] [PubMed]
- Orihashi, K.; Matsuura, Y.; Sueda, T.; Shikata, H.; Morita, S.; Hirai, S.; Sueshiro, M.; Okada, K. Flow velocity of central retinal artery and retrobulbar vessels during cardiovascular operations. J. Thorac. Cardiovasc. Surg. 1997, 114, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Mihály, Z.; István, L.; Czakó, C.; Benyó, F.; Borzsák, S.; Varga, A.; Magyar-Stang, R.; Banga, P.V.; Élő, Á.; Debreczeni, R.; et al. The Effect of Circle of Willis Morphology on Retinal Blood Flow in Patients with Carotid Stenosis Measured by Optical Coherence Tomography Angiography. J. Clin. Med. 2023, 12, 5335. [Google Scholar] [CrossRef] [PubMed]
- Aaslid, R.; Markwalder, T.M.; Nornes, H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. J. Neurosurg. 1982, 57, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, J.; Yap, K.H.; Ahmad, G.; Ghosh, J. Transcranial Doppler ultrasound: A review of the physical principles and major applications in critical care. Int. J. Vasc. Med. 2013, 2013, 629378. [Google Scholar] [CrossRef] [PubMed]
- Markus, H.; Cullinane, M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001, 124, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.C.; Huang, Y.W.; Shieh, J.S.; Huang, S.J.; Yip, P.K.; Jeng, J.S. Dynamic cerebral autoregulation in carotid stenosis before and after carotid stenting. J. Vasc. Surg. 2008, 48, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Panerai, R.B.; Haunton, V.; Katsogridakis, E.; Saeed, N.P.; Salinet, A.; Brodie, F.; Syed, N.; D’Sa, S.; Robinson, T.G. The Leicester cerebral haemodynamics database: Normative values and the influence of age and sex. Physiol. Meas. 2016, 37, 1485–1498. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, M.; Schwarzer, G.; Briel, M.; Altamura, C.; Palazzo, P.; King, A.; Bornstein, N.M.; Petersen, N.; Motschall, E.; Hetzel, A.; et al. Cerebrovascular reactivity predicts stroke in high-grade carotid artery disease. Neurology 2014, 83, 1424–1431. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.O.; Lee, S.J.; Lee, T.K. A Breath-Holding Index Applied to the Internal Carotid Artery Siphon in Transcranial Doppler Studies. J. Neuroimaging 2020, 30, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Pstras, L.; Thomaseth, K.; Waniewski, J.; Balzani, I.; Bellavere, F. The Valsalva manoeuvre: Physiology and clinical examples. Acta Physiol. 2016, 217, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Tiecks, F.P.; Lam, A.M.; Matta, B.F.; Strebel, S.; Douville, C.; Newell, D.W. Effects of the valsalva maneuver on cerebral circulation in healthy adults. A transcranial Doppler Study. Stroke 1995, 26, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.M.; Santos, R.; Freitas, J.; Panerai, R.B.; Azevedo, E. Autonomic dysfunction affects dynamic cerebral autoregulation during Valsalva maneuver: Comparison between healthy and autonomic dysfunction subjects. J. Appl. Physiol. 2014, 117, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Czosnyka, M.; Brady, K.; Reinhard, M.; Smielewski, P.; Steiner, L.A. Monitoring of cerebrovascular autoregulation: Facts, myths, and missing links. Neurocrit. Care 2009, 10, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: Bedside assessment and clinical implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Wallasch, T.M.; Kropp, P. Cerebrovascular response to valsalva maneuver: Methodology, normal values, and retest reliability. J. Clin. Ultrasound 2012, 40, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.L.; Panerai, R.B.; Potter, J.F. Critical closing pressure explains cerebral hemodynamics during the Valsalva maneuver. J. Appl. Physiol. 1999, 86, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Tiecks, F.P.; Douville, C.; Byrd, S.; Lam, A.M.; Newell, D.W. Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke 1996, 27, 1177–1182. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Leung, H.W.; Chen, X.Y.; Han, J.H.; Leung, W.H.; Soo, O.Y.; Lau, Y.L.; Wong, K.S. Autonomic dysfunction in ischemic stroke with carotid stenosis. Acta Neurol. Scand. 2012, 126, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Cavill, G.; Simpson, E.J.; Mahajan, R.P. Factors affecting assessment of cerebral autoregulation using the transient hyperaemic response test. Br. J. Anaesth. 1998, 81, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Magyar-Stang, R.; István, L.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Sótonyi, P.; Tamás, H.; Koller, A.; et al. Impaired cerebrovascular reactivity correlates with reduced retinal vessel density in patients with carotid artery stenosis: Cross-sectional, single center study. PLoS ONE 2023, 18, e0291521. [Google Scholar] [CrossRef] [PubMed]
- Magyar-Stang, R.; Pál, H.; Csányi, B.; Gaál, A.; Mihály, Z.; Czinege, Z.; Csipo, T.; Ungvari, Z.; Sótonyi, P.; Varga, A.; et al. Assessment of cerebral autoregulatory function and inter-hemispheric blood flow in older adults with internal carotid artery stenosis using transcranial Doppler sonography-based measurement of transient hyperemic response after carotid artery compression. GeroScience 2023, 45, 3333–3357. [Google Scholar] [CrossRef] [PubMed]
- Naraynsingh, V.; Harnarayan, P.; Maharaj, R.; Dan, D.; Hariharan, S. Preoperative digital carotid compression as a predictor of the need for shunting during carotid endarterectomy. Open Cardiovasc. Med. J. 2013, 7, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Visser, G.H.; Wieneke, G.H.; van Huffelen, A.C.; Eikelboom, B.C. The use of preoperative transcranial Doppler variables to predict which patients do not need a shunt during carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2000, 19, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Highton, D.; Elwell, C.; Smith, M. Noninvasive cerebral oximetry: Is there light at the end of the tunnel? Curr. Opin. Anaesthesiol. 2010, 23, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, A.; Scandroglio, M.; Minicucci, F.; Magrin, S.; Carozzo, A.; Casati, A. A clinical evaluation of near-infrared cerebral oximetry in the awake patient to monitor cerebral perfusion during carotid endarterectomy. J. Clin. Anesth. 2005, 17, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Pedrini, L.; Magnoni, F.; Sensi, L.; Pisano, E.; Ballestrazzi, M.S.; Cirelli, M.R.; Pilato, A. Is Near-Infrared Spectroscopy a Reliable Method to Evaluate Clamping Ischemia during Carotid Surgery? Stroke Res. Treat. 2012, 2012, 156975. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Cheng, R.; Dong, L.; Ryan, S.J.; Saha, S.P.; Yu, G. Cerebral monitoring during carotid endarterectomy using near-infrared diffuse optical spectroscopies and electroencephalogram. Phys. Med. Biol. 2011, 56, 3015–3032. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, F.; Ruberto, F.; Tosi, A.; Martelli, S.; Bruno, K.; Summonti, D.; D’Alio, A.; Diana, B.; Anile, M.; Panico, A.; et al. Regional cerebral saturation versus transcranial Doppler during carotid endarterectomy under regional anaesthesia. Eur. J. Anaesthesiol. 2009, 26, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Samra, S.K.; Dy, E.A.; Welch, K.; Dorje, P.; Zelenock, G.B.; Stanley, J.C. Evaluation of a cerebral oximeter as a monitor of cerebral ischemia during carotid endarterectomy. Anesthesiology 2000, 93, 964–970. [Google Scholar] [CrossRef]
- Ritter, J.C.; Green, D.; Slim, H.; Tiwari, A.; Brown, J.; Rashid, H. The role of cerebral oximetry in combination with awake testing in patients undergoing carotid endarterectomy under local anaesthesia. Eur. J. Vasc. Endovasc. Surg. 2011, 41, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, M.; Lindström, D.; Wanhainen, A.; Djavani Gidlund, K.; Gillgren, P. Near Infrared Spectroscopy as a Predictor for Shunt Requirement during Carotid Endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Mille, T.; Tachimiri, M.E.; Klersy, C.; Ticozzelli, G.; Bellinzona, G.; Blangetti, I.; Pirrelli, S.; Lovotti, M.; Odero, A. Near infrared spectroscopy monitoring during carotid endarterectomy: Which threshold value is critical? Eur. J. Vasc. Endovasc. Surg. 2004, 27, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; McInnis, C.L.; Ross-White, A.; Day, A.G.; Norman, P.A.; Boyd, J.G. Overview and Diagnostic Accuracy of Near Infrared Spectroscopy in Carotid Endarterectomy: A Systematic Review and Meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 695–704. [Google Scholar] [CrossRef]
- Pennekamp, C.W.; Immink, R.V.; den Ruijter, H.M.; Kappelle, L.J.; Bots, M.L.; Buhre, W.F.; Moll, F.L.; de Borst, G.J. Near-infrared spectroscopy to indicate selective shunt use during carotid endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2013, 46, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Avolio, A.P. Multi-branched model of the human arterial system. Med. Biol. Eng. Comput. 1980, 18, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Mynard, J.P.; Nithiarasu, P. A 1D arterial blood flow model incorporating ventricular pressure, aortic valve and regional coronary flow using the locally conservative Galerkin (LCG) method. Commun. Numer. Methods Eng. 2008, 24, 367–417. [Google Scholar] [CrossRef]
- Bárdossy, G.; Halász, G. Modeling blood flow in the arterial system. Period. Polytech. Mech. Eng. 2011, 55, 49–55. [Google Scholar] [CrossRef]
- Wéber, R.; Gyürki, D.; Paál, G. First blood: An efficient, hybrid one- and zero-dimensional, modular hemodynamic solver. Int. J. Numer. Method. Biomed. Eng. 2023, 39, e3701. [Google Scholar] [CrossRef] [PubMed]
- Gyürki, D.; Sótonyi, P.; Paál, G. Central arterial pressure estimation based on two peripheral pressure measurements using one-dimensional blood flow simulation. Comput. Methods Biomech. Biomed. Eng. 2023, 27, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Hillen, B.; Hoogstraten, H.W.; Post, L. A mathematical model of the flow in the circle of Willis. J. Biomech. 1986, 19, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Helthuis, J.H.G.; van Doormaal, T.P.C.; Amin-Hanjani, S.; Du, X.; Charbel, F.T.; Hillen, B.; van der Zwan, A. A patient-specific cerebral blood flow model. J. Biomech. 2020, 98, 109445. [Google Scholar] [CrossRef] [PubMed]
- Cassot, F.; Zagzoule, M.; Marc-Vergnes, J.P. Hemodynamic role of the circle of Willis in stenoses of internal carotid arteries. An analytical solution of a linear model. J. Biomech. 2000, 33, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Kennedy McConnell, F.; Payne, S. The Dual Role of Cerebral Autoregulation and Collateral Flow in the Circle of Willis after Major Vessel Occlusion. IEEE Trans. Biomed. Eng. 2017, 64, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.P.; Yu, H.; Yang, Z.; Schwieterman, R.; Ludwig, B. 1D simulation of blood flow characteristics in the circle of Willis using THINkS. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Luppi, L.; König, C.S.; Rinaldo, V.; Das, S.K. Study of the collateral capacity of the circle of Willis of patients with severe carotid artery stenosis by 3D computational modeling. J. Biomech. 2008, 41, 2735–2742. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Jani, N.D.; Narvid, J.; Shadden, S.C. The Role of Circle of Willis Anatomy Variations in Cardio-embolic Stroke: A Patient-Specific Simulation Based Study. Ann. Biomed. Eng. 2018, 46, 1128–1145. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Musio, F.; Ma, Y.; Juchler, N.; Paetzold, J.; Al-Maskari, R.; Höher, L.; Li, H.; Hamamci, I.E.; Sekuboyina, A.; et al. Benchmarking the CoW with the TopCoW Challenge: Topology-Aware Anatomical Segmentation of the Circle of Willis for CTA and MRA. arXiv 2023, arXiv:2312.17670. [Google Scholar]
- Csippa, B.; Mihály, Z.; Czinege, Z.; Németh, M.B.; Halász, G.; Paál, G.; Sótonyi, P. Comparison of Manual versus Semi-Automatic Segmentations of the Stenotic Carotid Artery Bifurcation. Appl. Sci. 2021, 11, 8192. [Google Scholar] [CrossRef]
- Xu, P.; Liu, X.; Zhang, H.; Ghista, D.; Zhang, D.; Shi, C.; Huang, W. Assessment of boundary conditions for CFD simulation in human carotid artery. Biomech. Model. Mechanobiol. 2018, 17, 1581–1597. [Google Scholar] [CrossRef] [PubMed]
- Groen, H.C.; Simons, L.; van den Bouwhuijsen, Q.J.; Bosboom, E.M.; Gijsen, F.J.; van der Giessen, A.G.; van de Vosse, F.N.; Hofman, A.; van der Steen, A.F.; Witteman, J.C.; et al. MRI-based quantification of outflow boundary conditions for computational fluid dynamics of stenosed human carotid arteries. J. Biomech. 2010, 43, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Lopes, D.; Puga, H.; Teixeira, J.; Lima, R. Blood flow simulations in patient-specific geometries of the carotid artery: A systematic review. J. Biomech. 2020, 111, 110019. [Google Scholar] [CrossRef]
- Lee, S.W.; Antiga, L.; Spence, J.D.; Steinman, D.A. Geometry of the carotid bifurcation predicts its exposure to disturbed flow. Stroke 2008, 39, 2341–2347. [Google Scholar] [CrossRef] [PubMed]
- Hoi, Y.; Wasserman, B.A.; Lakatta, E.G.; Steinman, D.A. Effect of common carotid artery inlet length on normal carotid bifurcation hemodynamics. J. Biomech. Eng. 2010, 132, 121008. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Lee, S.W.; Fischer, P.F.; Bassiouny, H.S.; Loth, F. Direct numerical simulation of transitional flow in a stenosed carotid bifurcation. J. Biomech. 2008, 41, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Mancini, V.; Bergersen, A.W.; Vierendeels, J.; Segers, P.; Valen-Sendstad, K. High-Frequency Fluctuations in Post-stenotic Patient Specific Carotid Stenosis Fluid Dynamics: A Computational Fluid Dynamics Strategy Study. Cardiovasc. Eng. Technol. 2019, 10, 277–298. [Google Scholar] [CrossRef] [PubMed]
- Schirmer, C.M.; Malek, A.M. Computational fluid dynamic characterization of carotid bifurcation stenosis in patient-based geometries. Brain Behav. 2012, 2, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Huang, X.; Benitez Mendieta, J.; Wang, J.; Paritala, P.K.; Lloyd, T.; Li, Z. The Need to Shift from Morphological to Structural Assessment for Carotid Plaque Vulnerability. Biomedicines 2022, 10, 3038. [Google Scholar] [CrossRef] [PubMed]
- Kane, T.; Pugh, M.A. Usefulness of Cerebral Oximetry in Preventing Postoperative Cognitive Dysfunction in Patients Undergoing Coronary Artery Bypass Grafting. Aana J. 2017, 85, 49–54. [Google Scholar] [PubMed]
- Suraarunsumrit, P.; Pathonsmith, C.; Srinonprasert, V.; Sangarunakul, N.; Jiraphorncharas, C.; Siriussawakul, A. Postoperative cognitive dysfunction in older surgical patients associated with increased healthcare utilization: A prospective study from an upper-middle-income country. BMC Geriatr. 2022, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Relander, K.; Hietanen, M.; Nuotio, K.; Ijäs, P.; Tikkala, I.; Saimanen, E.; Lindsberg, P.J.; Soinne, L. Cognitive Dysfunction and Mortality after Carotid Endarterectomy. Front. Neurol. 2020, 11, 593719. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, J.; Christensen, K.B.; Lund, T.; Lohse, N.; Rasmussen, L.S. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 2009, 110, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Ton, T.G.N.; DeLeire, T.; May, S.G.; Hou, N.; Tebeka, M.G.; Chen, E.; Chodosh, J. The financial burden and health care utilization patterns associated with amnestic mild cognitive impairment. Alzheimers Dement. 2017, 13, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Zissimopoulos, J.; Crimmins, E.; St Clair, P. The Value of Delaying Alzheimer’s Disease Onset. Forum Health Econ. Policy 2014, 18, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Lazar, R.M.; Wadley, V.G.; Myers, T.; Jones, M.R.; Heck, D.V.; Clark, W.M.; Marshall, R.S.; Howard, V.J.; Voeks, J.H.; Manly, J.J.; et al. Baseline Cognitive Impairment in Patients with Asymptomatic Carotid Stenosis in the CREST-2 Trial. Stroke 2021, 52, 3855–3863. [Google Scholar] [CrossRef] [PubMed]
- Succar, B.; Zhou, W. Does Carotid Intervention Improve Cognitive Function? Adv. Surg. 2023, 57, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Faggioli, G.; Ancetti, S.; Naylor, A.R. Editor’s Choice—Asymptomatic Carotid Stenosis and Cognitive Impairment: A Systematic Review. Eur. J. Vasc. Endovasc. Surg. 2021, 61, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Paraskevas, K.I.; Mikhailidis, D.P.; Spinelli, F.; Faggioli, G.; Saba, L.; Silvestrini, M.; Svetlikov, A.; Stilo, F.; Pini, R.; Myrcha, P.; et al. Asymptomatic carotid stenosis and cognitive impairment. J. Cardiovasc. Surg. 2023, 64, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, N.D.; Asaadi, S.; Thirumala, P.D.; Avgerinos, E.D. A systematic review of cognitive function after carotid endarterectomy in asymptomatic patients. J. Vasc. Surg. 2022, 75, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Foret, T.; Guillaumin, M.; Desmarets, M.; Costa, P.; Rinckenbach, S.; du Mont, L.S. Association between carotid revascularization for asymptomatic stenosis and cognitive functions. Vasa 2022, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Aceto, P.; Lai, C.; De Crescenzo, F.; Crea, M.A.; Di Franco, V.; Pellicano, G.R.; Perilli, V.; Lai, S.; Papanice, D.; Sollazzi, L. Cognitive decline after carotid endarterectomy: Systematic review and meta-analysis. Eur. J. Anaesthesiol. 2020, 37, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B. Systematic review of near-infrared spectroscopy determined cerebral oxygenation during non-cardiac surgery. Front. Physiol. 2014, 5, 93. [Google Scholar] [CrossRef] [PubMed]
- Mohandas, B.S.; Jagadeesh, A.M.; Vikram, S.B. Impact of monitoring cerebral oxygen saturation on the outcome of patients undergoing open heart surgery. Ann. Card. Anaesth. 2013, 16, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Colak, Z.; Borojevic, M.; Bogovic, A.; Ivancan, V.; Biocina, B.; Majeric-Kogler, V. Influence of intraoperative cerebral oximetry monitoring on neurocognitive function after coronary artery bypass surgery: A randomized, prospective study. Eur. J. Cardiothorac. Surg. 2015, 47, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, W.; Shi, H. The Association between Postoperative Cognitive Dysfunction and Cerebral Oximetry during Geriatric Orthopedic Surgery: A Randomized Controlled Study. BioMed Res. Int. 2021, 2021, 5733139. [Google Scholar] [CrossRef] [PubMed]
- Kamenskaya, O.V.; Loginova, I.Y.; Lomivorotov, V.V. Brain Oxygen Supply Parameters in the Risk Assessment of Cerebral Complications during Carotid Endarterectomy. J. Cardiothorac. Vasc. Anesth. 2017, 31, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Holmgaard, F.; Vedel, A.G.; Rasmussen, L.S.; Paulson, O.B.; Nilsson, J.C.; Ravn, H.B. The association between postoperative cognitive dysfunction and cerebral oximetry during cardiac surgery: A secondary analysis of a randomised trial. Br. J. Anaesth. 2019, 123, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Fudickar, A.; Peters, S.; Stapelfeldt, C.; Serocki, G.; Leiendecker, J.; Meybohm, P.; Steinfath, M.; Bein, B. Postoperative cognitive deficit after cardiopulmonary bypass with preserved cerebral oxygenation: A prospective observational pilot study. BMC Anesthesiol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Inčiūra, D.; Antuševas, A.; Aladaitis, A.; Gimžauskaitė, A.; Velička, L.; Kavaliauskienė, Ž. Near-infrared spectroscopy as a predictor of cerebral ischaemia during carotid endarterectomy in awake patients. Vascular 2020, 28, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Silvestrini, M.; Paolino, I.; Vernieri, F.; Pedone, C.; Baruffaldi, R.; Gobbi, B.; Cagnetti, C.; Provinciali, L.; Bartolini, M. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology 2009, 72, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, S.; Perozzi, C.; Altamura, C.; Vernieri, F.; Luzzi, S.; Bartolini, M.; Provinciali, L.; Silvestrini, M. Severe carotid stenosis and impaired cerebral hemodynamics can influence cognitive deterioration. Neurology 2013, 80, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Buratti, L.; Balucani, C.; Viticchi, G.; Falsetti, L.; Altamura, C.; Avitabile, E.; Provinciali, L.; Vernieri, F.; Silvestrini, M. Cognitive deterioration in bilateral asymptomatic severe carotid stenosis. Stroke 2014, 45, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, S.; Carbonari, L.; Pagliariccio, G.; Bartolini, M.; Cagnetti, C.; Viticchi, G.; Buratti, L.; Provinciali, L.; Silvestrini, M. Neurocognitive functioning and cerebrovascular reactivity after carotid endarterectomy. Neurology 2018, 90, e307–e315. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Feridooni, T.; Pikula, A.; Styra, R.; Mikulis, D.J.; Howe, K.L. Evaluating the influence of altered cerebral hemodynamics on cognitive performance in asymptomatic carotid artery stenosis: A systematic review. J. Vasc. Surg. 2024, 79, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Abraham, A.G.; Swenor, B.K.; Sharrett, A.R.; Ramulu, P.Y. Application of Optical Coherence Tomography in the Detection and Classification of Cognitive Decline. J. Curr. Glaucoma Pract. 2018, 12, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yu, K.; Zhang, L.; Liu, Y. Cognitive Decline in Asymptomatic Middle Cerebral Artery Stenosis Patients with Moderate and Poor Collaterals: A 2-Year Follow-Up Study. Med. Sci. Monit. 2019, 25, 4051–4058. [Google Scholar] [CrossRef]
- Zhang, N.; Xie, B.; Feng, Y.; Li, Q.; Li, X. Effects of asymptomatic cerebral artery stenosis of anterior circulation and establishment of collateral circulation on cognition. Clin. Neurol. Neurosurg. 2023, 233, 107889. [Google Scholar] [CrossRef] [PubMed]
- Sussman, E.S.; Kellner, C.P.; Mergeche, J.L.; Bruce, S.S.; McDowell, M.M.; Heyer, E.J.; Connolly, E.S. Radiographic absence of the posterior communicating arteries and the prediction of cognitive dysfunction after carotid endarterectomy. J. Neurosurg. 2014, 121, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Lai, Z.; Lv, Y.; Qu, J.; Zuo, Z.; You, H.; Wu, B.; Hou, B.; Liu, C.; Feng, F. Effective collateral circulation may indicate improved perfusion territory restoration after carotid endarterectomy. Eur. Radiol. 2018, 28, 727–735. [Google Scholar] [CrossRef] [PubMed]
| Author | Year | Study Type | Criteria for Aplastic or Missing CoW Segment | Criteria for Hypoplastic or Stenotic CoW Segment | Complete CoW (%) |
|---|---|---|---|---|---|
| Gyöngyösi et al. [36] | 2023 | Imaging (CTA) | Non-visible | <0.5 mm | 71.2% |
| Hindenes et al. [31] | 2020 | Imaging (MR-3D TOF) | <1 mm | Not evaluated | 11.9% |
| Lin et al. [37] | 2023 | Imaging (MR-3D TOF) | Vessel diameter less than 50% of the contralateral or ipsilateral side (other segments) | Not evaluated | 66.0% |
| Wholey et al. [38] | 2009 | Imaging (CTA) | Non-visible | Not evaluated | 18.0% |
| Hartkamp et al. [25] | 1999 | Imaging (MR-3D TOF) | Non-visible | <0.8 mm | 63% patients, 47% controls |
| Karatas et al. [39] | 2015 | Imaging (CTA) | Non-visible | <1 mm | 71.0% |
| Li et al. [28] | 2010 | Imaging (CTA) | Non-visible | <1 mm | 27.0% |
| Krabbe-Hartkamp et al. [27] | 1998 | Imaging (MR-3D TOF) | Non-visible | <0.8 mm | 42.0% |
| Waaijer et al. [40] | 2007 | Imaging (CTA) | Non-visible | <1 mm | 22.0% |
| Puchades-Orts et al. [29] | 1975 | Autopsy study | Non-visible | Not defined | 13.0% |
| Riggs and Rupp [30] | 1962 | Autopsy study | Non-visible | Not defined | 21.0% |
| Alpers et al. [24] | 1958 | Autopsy study | Non-visible | <1 mm | 52.3% |
| Alpers and Berry [23] | 1963 | Autopsy study | Non-visible | Not defined | 52% in normal brains, 33% in pathologic brains |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lengyel, B.; Magyar-Stang, R.; Pál, H.; Debreczeni, R.; Sándor, Á.D.; Székely, A.; Gyürki, D.; Csippa, B.; István, L.; Kovács, I.; et al. Non-Invasive Tools in Perioperative Stroke Risk Assessment for Asymptomatic Carotid Artery Stenosis with a Focus on the Circle of Willis. J. Clin. Med. 2024, 13, 2487. https://doi.org/10.3390/jcm13092487
Lengyel B, Magyar-Stang R, Pál H, Debreczeni R, Sándor ÁD, Székely A, Gyürki D, Csippa B, István L, Kovács I, et al. Non-Invasive Tools in Perioperative Stroke Risk Assessment for Asymptomatic Carotid Artery Stenosis with a Focus on the Circle of Willis. Journal of Clinical Medicine. 2024; 13(9):2487. https://doi.org/10.3390/jcm13092487
Chicago/Turabian StyleLengyel, Balázs, Rita Magyar-Stang, Hanga Pál, Róbert Debreczeni, Ágnes Dóra Sándor, Andrea Székely, Dániel Gyürki, Benjamin Csippa, Lilla István, Illés Kovács, and et al. 2024. "Non-Invasive Tools in Perioperative Stroke Risk Assessment for Asymptomatic Carotid Artery Stenosis with a Focus on the Circle of Willis" Journal of Clinical Medicine 13, no. 9: 2487. https://doi.org/10.3390/jcm13092487





