The Impact of the Brain-Derived Neurotrophic Factor Gene on Trauma and Spatial Processing
Abstract
:1. Introduction
2. The Neural Basis of Post-Traumatic Stress Disorder (PTSD)
3. Brain-Derived Neurotrophic Factor (BDNF)
4. Hippocampal Function, Navigation and BDNF
- (i)
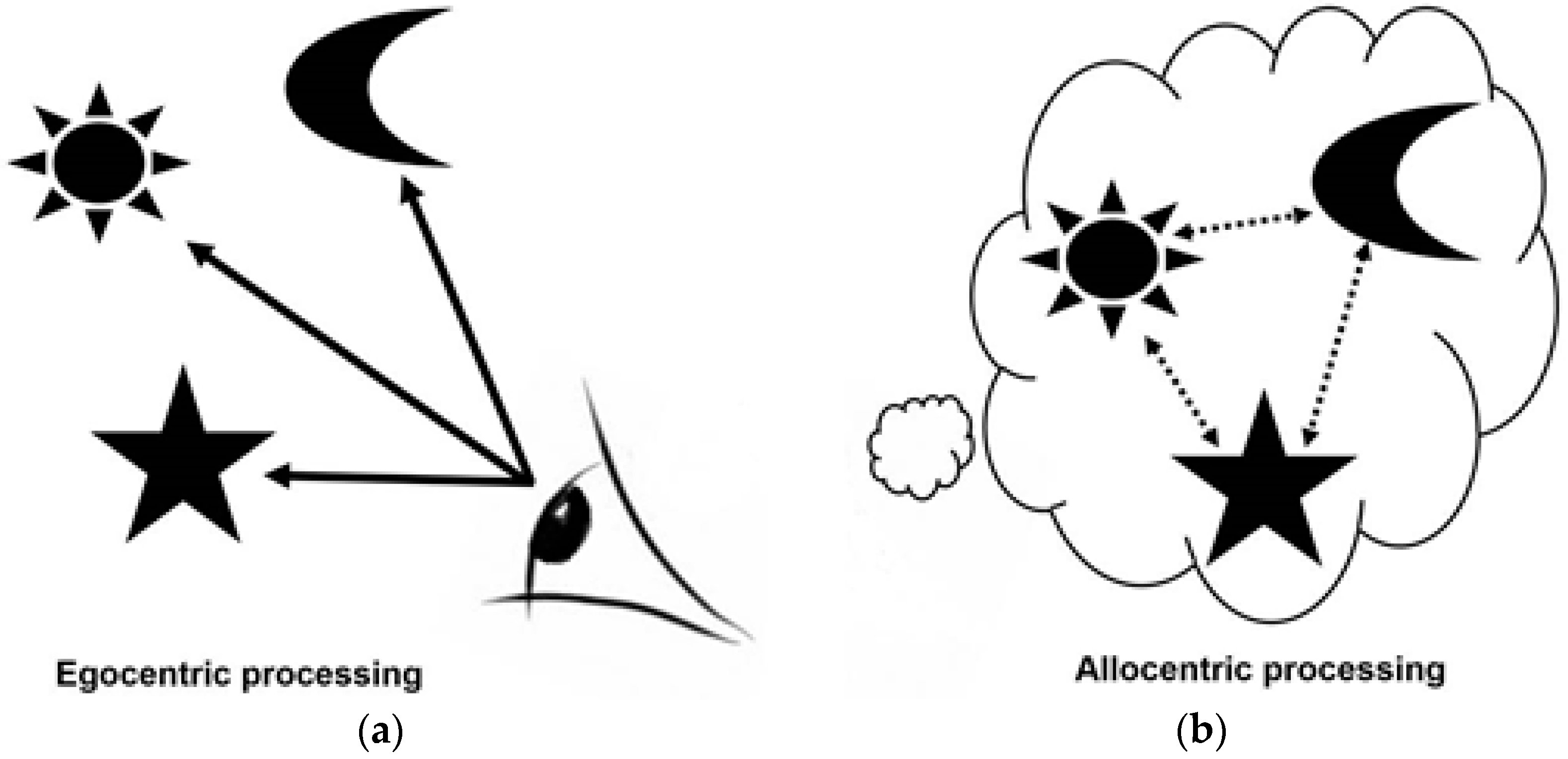
- Egocentric processing is viewpoint dependent and associative, relying on local landmarks in line of sight. This form of processing is not dependent on hippocampal processing: egocentric spatial memory representations are independent of the hippocampus, relying on the striatal circuit, whereas allocentric representations are thought to rely heavily on the hippocampal circuit [2,6,8,10,11,39].
- (ii)
- Allocentric processing enables individuals to construct a viewer-independent representation of the relationship between objects /landmarks/ places in an environment. A spatial “map” [11] is created in which key landmarks are represented in relation to one another rather than in relation to the viewer. This form of representation is particularly important in route planning and is vital for contextualising information during navigation [10,11,28,29]. Allocentric processing, in contrast to egocentric processing, relies heavily on the hippocampal circuit [10,11,39].
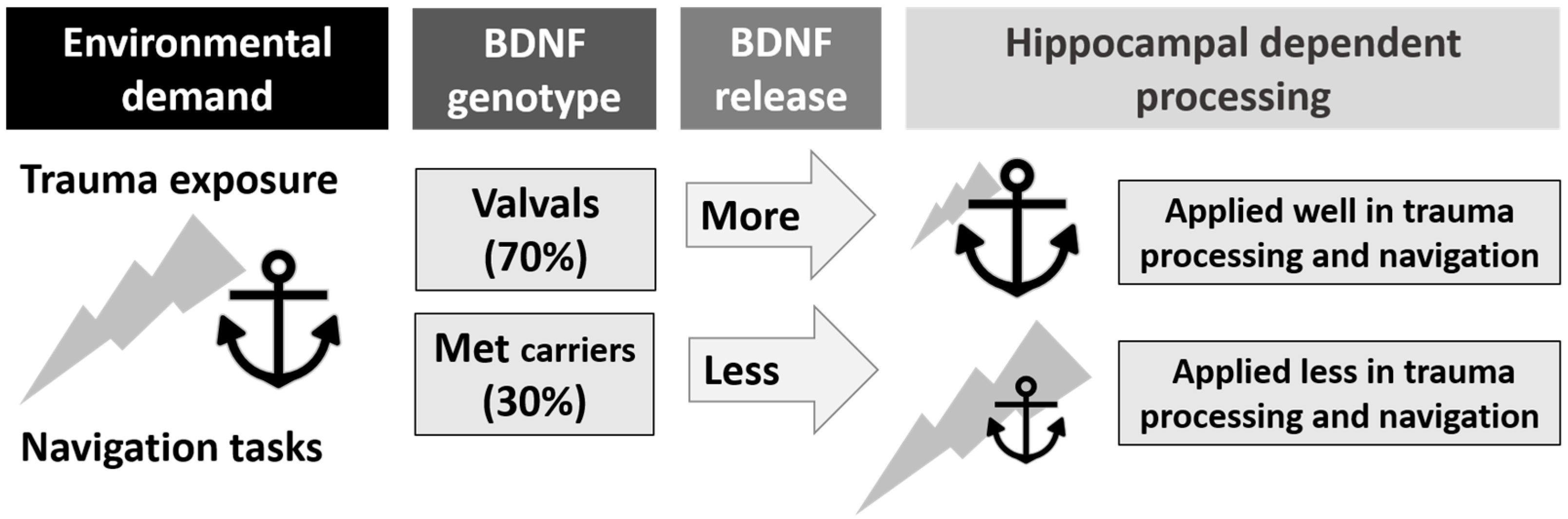
5. Bringing Together Allocentric Spatial Processing, the BDNF Gene and PTSD
- (a)
- (b)
- (c)
6. The Future of BDNF Research
- (a)
- ecologically valid behavioural and subjective tests of navigation, supported by
- (b)
- functional MRI (fMRI) and MRI-assessing activity or volume, possibly pre- and post-trauma exposure, with
- (c)
- adequate neurochemical assessment of activity-dependent hippocampal BDNF release (for example, using blood serum) between genotypes, and
- (d)
- closely matching participants to control for numerous differentiating variables, which may influence hippocampal and broader neuro degradation syndromes, ranging from age and environmental conditions of trauma exposure to epigenetic history and even nutrition [59].
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A. Exploratory Research Data
Appendix A.1. Participants
Appendix A.2. Methods
Appendix A.3. Results
Appendix A.3.1. BDNF and Hippocampal Independent Performance
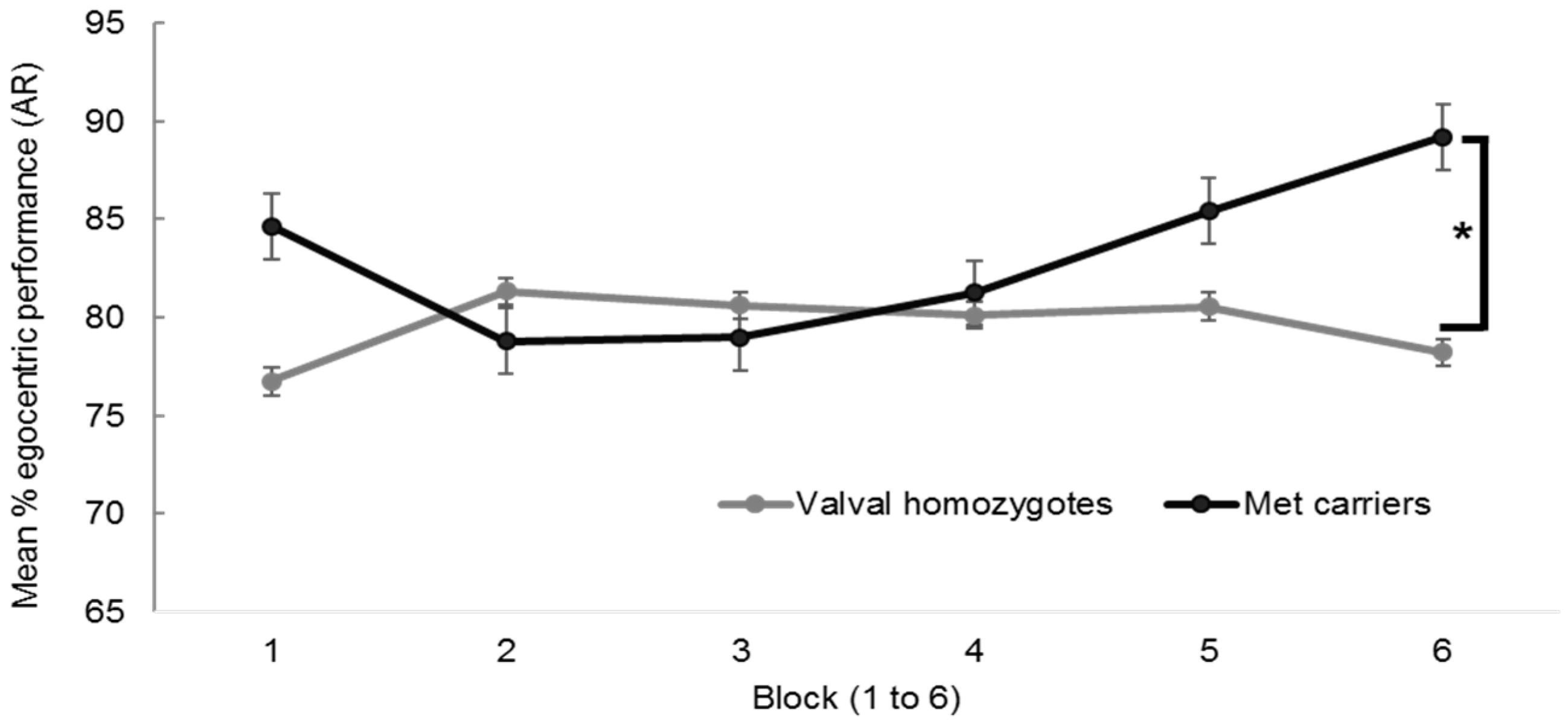
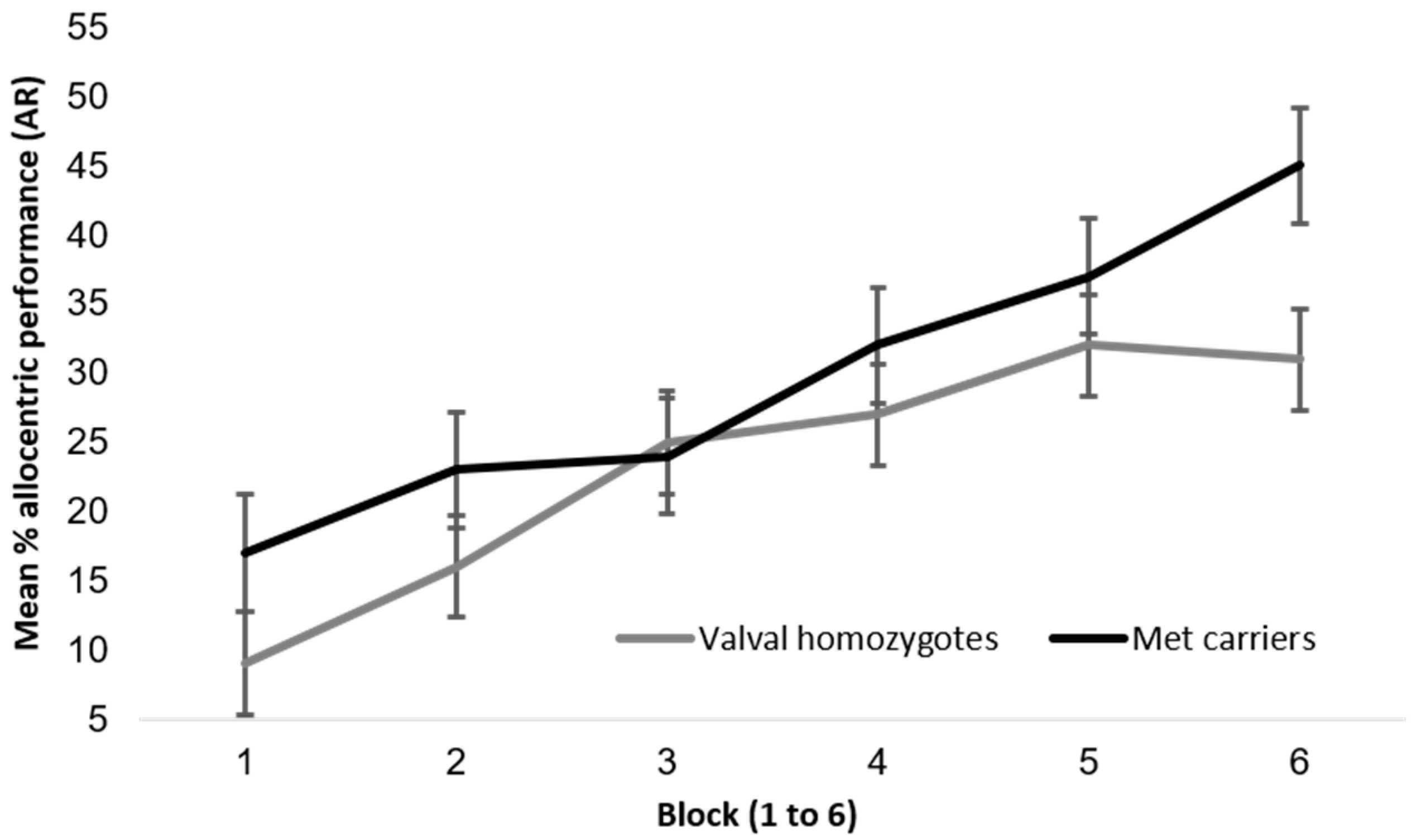
Appendix A.3.2. BDNF and Self-Reported Navigation Competence
| Self-Reported Navigation Competence | Allocentric Performance in BDNF Valval Homozygotes (n = 102) | Allocentric Performance in BDNF Met Carriers (n = 45) |
|---|---|---|
| General competence (SBSOD) | 0.26 * | 0.05 |
| Allocentric competence (QSR) | 0.28 ** | 0.03 |
References
- Koenen, K.C.; Amstadter, A.B.; Nugent, N.R. Gene-environment interaction in post-traumatic stress disorder: An update. J. Trauma. Stress 2009, 22, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Burgess, N.; Brewin, C.R.; King, J.A. Impaired allocentric spatial processing in posttraumatic stress disorder. Neurobiol. Learn. Mem. 2015, 119, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Murphy, D.; Smith, K.V. An adapted imaginal exposure approach to traditional methods used within trauma-focused cognitive behavioural therapy, trialled with a veteran population. Cogn. Behav. Ther. 2016, 9, 10. [Google Scholar] [CrossRef]
- Bisby, J.A.; Horner, A.J.; Hørlyck, L.D.; Burgess, N. Opposing effects of negative emotion on amygdala and hippocampal memory for items and associations. Soc. Cogn. Affect. Neurosci. 2016, 11, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; Wiener, J.M. PTSD recovery, spatial processing, and the val66met polymorphism. Front. Hum. Neurosci. 2014, 8, 100. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.K.; McDougall, S.; Thomas, S.; Wiener, J.M. Impairment in active navigation from trauma and Post-Traumatic Stress Disorder. Neurobiol. Learn. Mem. 2017, 140, 114–123. [Google Scholar] [CrossRef] [PubMed]
- Brewin, C.; Burgess, N. Contextualisation in the revised dual representation theory of PTSD: A response to Pearson and colleagues. J. Behav. Ther. Exp. Psychiatry 2014, 45, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Bisby, J.A.; King, J.A.; Brewin, C.R.; Burgess, N.; Curran, H.V. Acute effects of alcohol on intrusive memory development and viewpoint dependence in spatial memory support a dual representation model. Biol. Psychiatry 2010, 68, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Erwin, E. The Freud Encyclopaedia; Routledge: London, UK, 2003. [Google Scholar]
- Wolbers, T.; Wiener, J.M. Challenges for identifying the neural mechanisms that support spatial navigation: The impact of spatial scale. Front. Hum. Neurosci. 2014, 8, 571. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, J.; Nadel, L. The Hippocampus as a Cognitive Map; Oxford University Press: Oxford, UK, 1978. [Google Scholar]
- Maren, S. Seeking a Spotless Mind: Extinction, Deconsolidation, and Erasure of Fear Memory. Neuron 2011, 70, 830–845. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J. Imagery in therapy: An information-processing analysis of fear. Behav. Ther. 1977, 8, 862–886. [Google Scholar] [CrossRef]
- Eichenmbaum, H. A corticol-hioppocampal system for declarative memory. Nat. Rev. Neurosci. 2000, 1, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Dennis, N.A.; Cabeza, R.; Need, A.C.; Waters-Metenier, S.; Goldstein, D.B.; LaBar, K.S. Brain-derived neurotrophic factor val66met polymorphism and hippocampal activation during episodic encoding and retrieval tasks. Hippocampus 2011, 21, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.; Becker, S.; Burgess, N. Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychol. Rev. 2007, 114, 340–375. [Google Scholar] [CrossRef] [PubMed]
- Glazer, D.A.; Mason, O.; King, J.A.; Brewin, C.R. Contextual memory, psychosis-proneness, and the experience of intrusive imagery. Cogn. Emot. 2013, 27, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hariri, A.R.; Goldberg, T.E.; Mattay, V.S.; Kolachana, B.S.; Callicott, J.H.; Egan, M.F.; Weinberger, D.R. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J. Neurosci. 2003, 23, 6690–6694. [Google Scholar] [PubMed]
- Elzinga, B.M.; Bremner, J.D. Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? J. Affect. Disord. 2002, 70, 1–17. [Google Scholar] [CrossRef]
- Elzinga, B.M.; Molendijk, M.L.; Oude Voshaar, R.C.; Bus, B.A.; Prickaerts, J.; Spinhoven, P.; Penninx, B.J. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology 2011, 214, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Brewin, C.R.; Dalgleish, T.; Joseph, S. A dual representation theory of post-traumatic stress disorder. Psychol. Rev. 1996, 103, 670–686. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.; Dieppa-Perea, L.M.; Melendez, L.M.; Quirk, G.J. Induction of fear extinction with hippocampal-infralimbic BDNF. Science 2010, 328, 1288–1290. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.A.; James, E.L.; Kilford, E.J.; Deeprose, C. Key Steps in Developing a Cognitive Vaccine against Traumatic Flashbacks: Visuospatial Tetris versus Verbal Pub Quiz. PLoS ONE 2010, 5, e13. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, R.; Moriguchi, Y.; Yamashita, F.; Mori, T.; Nemoto, K.; Okada, T.; Hori, H.; Noguchi, H.; Kunugi, H.; Ohnishi, T. Dose-dependent effect of the Val66Met polymorphism of the brain-derived neurotrophic factor gene on memory-related hippocampal activity. Neurosci. Res. 2008, 61, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.M.; Das, D.; Taylor, J.L.; Noda, A.; Yesavage, J.A.; Salehi, A. BDNF polymorphism predicts the rate of decline in skilled task performance and hippocampal volume in healthy individuals. Transl. Psychiatry 2011, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kambeitz, J.P.; Bhattacharyya, S.; Kambeitz-Ilankovic, L.M.; Valli, I.; Collier, D.A.; McGuire, P. Effect of BDNF val(66)met polymorphism on declarative memory and its neural substrate: A meta-analysis. Neurosci. Biobehav. Rev. 2012, 36, 2165–2177. [Google Scholar] [CrossRef] [PubMed]
- Hajek, T.; Kopecek, M.; Hoschl, C. Reduced hippocampal volumes in healthy carriers of brain-derived neurotrophic factor Val66Met polymorphism: Meta-analysis. World J. Biol. Psychiatry 2012, 13, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Lövdén, M.; Schaefer, S.; Noack, H.; Kanowski, M.; Kaufmann, J.; Tempelmann, C.; Bodammer, N.C.; Kühn, S.; Heinze, H.J.; Lindenberger, U.; et al. Performance-related increases in hippocampal N-acetylaspartate (NAA) induced by spatial navigation training are restricted to BDNF Val homozygotes. Cereb. Cortex 2011, 21, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Banner, H.; Bhat, V.; Etchamendy, N.; Joober, R.; Bohbot, V.D. The brain-derived neurotrophic factor Val66Met polymorphism is associated with reduced functional magnetic resonance imaging activity in the hippocampus and increased use of caudate nucleus-dependent strategies in a human virtual navigation task. Eur. J. Neurosci. 2011, 33, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Vidal, L.E.; Do-Monte, F.H.; Sotres-Bayon, F.; Quirk, G.J. Hippocampal—Prefrontal BDNF and Memory for Fear Extinction. Neuropsychopharmacology 2014, 39, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Notaras, M.; Hill, R.; van den Buuse, M. The BDNF gene Val66Met polymorphism as a modifier of psychiatric disorder susceptibility: Progress and controversy. Mol. Psychiatry 2015, 20, 916–930. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, X.-X.; Hu, X.-Z. Post-traumatic stress disorder risk and brain-derived neurotrophic factor Val66Met. World J. Psychiatry 2014, 6, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mandel, A.L.; Ozdener, H.; Utermohlen, V. Identification of pro- and mature brain-derived neurotrophic factor in human saliva. Arch. Oral Biol. 2009, 54, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Doidge, N. The Brain That Changes Itself: Stories of Personal Triumph from the Frontiers of Brain Science; James, H., Ed.; Silberman Books; Penguin: London, UK, 2007. [Google Scholar]
- Egan, M.F.; Kojima, M.; Callicott, J.H.; Goldberg, T.E.; Kolachana, B.S.; Bertolino, A.; Zaitsev, E.; Gold, B.; Goldman, D.; Dean, M.; et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003, 112, 257–269. [Google Scholar] [CrossRef]
- Wang, T. Does BDNF Val66Met Polymorphism Confer Risk for Posttraumatic Stress Disorder. Neuropsychobiology 2015, 71, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Soliman, F.; Glatt, C.E.; Bath, K.G.; Levita, L.; Jones, R.M.; Pattwell, S.S.; Jing, D.; Tottenham, N.; Amso, D.; Somerville, L.H.; et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010, 327, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Andero, R.; Ressler, K.J. Fear extinction and BDNF: Translating animal models of PTSD to the clinic. Genes Brain Behav. 2012, 11, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Dudchenko, P. Why People Get Lost: The Psychology and Neuroscience of Spatial Cognition; Oxford University Press: Oxford, UK, 2010. [Google Scholar]
- Miller, J.K. Lost in Trauma: Post-Traumatic Stress Disorder, Spatial Processing and the Brain-Derived Neurotrophic Factor Gene. Ph.D. Thesis, Bournemouth University, Poole, UK, 2016. Available online: http://eprints.bournemouth.ac.uk/25012/ (accessed on 14 July 2016).
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ozbay, F.; Lappalainen, J.; Kranzler, H.R.; van Dyck, C.H.; Charney, D.S.; Price, L.H.; Southwick, S.; Yang, B.Z.; Rasmussen, A.; et al. Brain-Derived Neurotrophic Factor Gene (BDNF) Variants and Alzheimer’s Disease, Affective Disorders, Posttraumatic Stress Disorder, Schizophrenia and Substance Dependence. Am. J. Med. Genet. 2006, 141B, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, M.; Richardson, A.; Montello, D.; Lovelace, K.; Subbiah, I. Development of a self-report measure of environmental spatial ability. Intelligence 2002, 30, 425–447. [Google Scholar] [CrossRef]
- Pazzaglia, F.; De Beni, R. Strategies of processing spatial information in survey and landmark-centred individuals. Eur. J. Cogn. Psychol. 2001, 13, 493–508. [Google Scholar] [CrossRef]
- Münzer, S.; Hölscher, C. Entwicklung und Validierung eines Fragebogens zu räumlichen Strategien (Development and Validation of a Self-report Measure of Environmental Spatial Strategies). Diagnostica 2011, 57, 111–125. [Google Scholar] [CrossRef]
- Wiener, J.M.; de Condappa, O.; Harris, M.A.; Wolbers, T. Maladaptive bias for extra hippocampal navigation strategies in aging humans. J. Neurosci. 2013, 33, 6012–6017. [Google Scholar] [CrossRef] [PubMed]
- Zoladz, P.R.; Diamond, D.M. Current status on behavioral and biological markers of PTSD: A search for clarity in a conflicting literature. Neurosci. Biobehav. Rev. 2013, 37, 860–895. [Google Scholar] [CrossRef] [PubMed]
- Bonne, O.; Gill, J.M.; Luckenbaugh, D.A.; Collins, C.; Owens, M.J.; Alesci, S.; Neumeister, A.; Yuan, P.; Kinkead, B.; Manji, H.K.; et al. Corticotropin-releasing factor, interleukin-6, brain-derived neurotrophic factor, insulin-like growth factor-1, and substance p in the cerebrospinal fluid of civilians with post- traumatic stress disorder before and after treatment with paroxetine. J. Clin. Psychiatry 2011, 72, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Furman, A.J.; Clements-Stephens, A.M.; Marchette, S.A.; Shelton, A.L. Persistent and stable biases in spatial learning mechanisms predict navigational style. Cogn. Affect. Behav. Neurosci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, D.J.H.; Fergeson, T.; Skelton, R.W. Acute stress switches spatial navigation strategy from egocentric to allocentric in virtual Morris water maze. Neurobiol. Learn. Mem. 2016, 132, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Karnik, M.S.; Wang, L.; Barch, D.M.; Morris, J.; Csernansky, J.G. BDNF polymorphism rs6265, hippocampal structure and memory performance in healthy control subjects. Psychiatry Res. 2010, 178, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Bohbot, V.D.; Lerch, J.; Thorndycraft, B.; Iaria, G.; Zijdenbos, A.P. Gray matter differences correlate with spontaneous strategies in a human virtual navigation task. J. Neurosci. 2007, 27, 10078–10083. [Google Scholar] [CrossRef] [PubMed]
- Joffe, R.T.; Gatt, J.M.; Kemp, A.H.; Grieve, S.; Dobson-Stone, C.; Kuan, S.A.; Schofield, P.R.; Gordon, E.; Williams, L.M. Brain derived neurotrophic factor Val66Met polymorphism, the five factor model of personality and hippocampal volume: Implications for depressive illness. Hum. Brain Mapp. 2009, 30, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Chaieb, L.; Antal, A.; Ambrus, G.G.; Paulus, W. Brain-derived neurotrophic factor: Its impact upon neuroplasticity and inducing transcranial brain stimulation protocols. Neurogenetics 2014, 15, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, L.; Suliman, S.; Malan-Müller, S.; Hemmings, S.; Seedat, S. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism and plasma levels in road traffic accident survivors. Anxiety Stress Coping 2016. [Google Scholar] [CrossRef] [PubMed]
- Hemmings, S.M.; Martin, L.I.; Klopper, M.; van der Merwe, L.; Aitken, L.; de Wit, E.; Black, G.F.; Hoal, E.G.; Walzl, G.; Seedat, S. BDNF val66met and drd2taq1a polymorphisms interact to influence PTSD symptom severity: A preliminary investigation in a South African population. Progr. Neuropsychopharmacol. Biol. Psychiatry 2012, 40, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Dror, I.E.; Schmitz-Williams, I.C.; Smith, W. Older adults use mental representations that reduce cognitive load: Mental rotation utilizes holistic representations and processing. Exp. Aging Res. 2005, 31, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Perroud, N.; Courtet, P.; Vincze, I.; Jaussent, I.; Jollant, F.; Bellivier, F.; Leboyer, M.; Baud, P.; Buresi, C.; Malafosse, A. Interaction between BDNF Val66Met and childhood trauma on adult’s violent suicide attempt. Genes Brain Behav. 2008, 7, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M.; Baym, C.L.; Cohen, N.J. Identifying and Characterizing the Effects of Nutrition on Hippocampal Memory. Adv. Nutr. 2014, 5, 337S–343S. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Lee, F.S. Variant BDNF (Val66Met) impact on brain structure and function. Cogn. Affect. Behav. Neurosci. 2006, 6, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Foa, E.B.; Cashman, L.; Jaycox, L.H.; Perry, K. The validation of a self-report measure of PTSD: The PTSD Diagnostic Scale. Psychol. Assess. 1997, 9, 445–451. [Google Scholar] [CrossRef]
- Iaria, G.; Petrides, M.; Dagher, A.; Pike, B.; Bohbot, V.D. Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J. Neurosci. 2003, 23, 5945–5952. [Google Scholar] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, J.K.; McDougall, S.; Thomas, S.; Wiener, J. The Impact of the Brain-Derived Neurotrophic Factor Gene on Trauma and Spatial Processing. J. Clin. Med. 2017, 6, 108. https://doi.org/10.3390/jcm6120108
Miller JK, McDougall S, Thomas S, Wiener J. The Impact of the Brain-Derived Neurotrophic Factor Gene on Trauma and Spatial Processing. Journal of Clinical Medicine. 2017; 6(12):108. https://doi.org/10.3390/jcm6120108
Chicago/Turabian StyleMiller, Jessica K., Siné McDougall, Sarah Thomas, and Jan Wiener. 2017. "The Impact of the Brain-Derived Neurotrophic Factor Gene on Trauma and Spatial Processing" Journal of Clinical Medicine 6, no. 12: 108. https://doi.org/10.3390/jcm6120108





