Statins, Muscle Disease and Mitochondria
Abstract
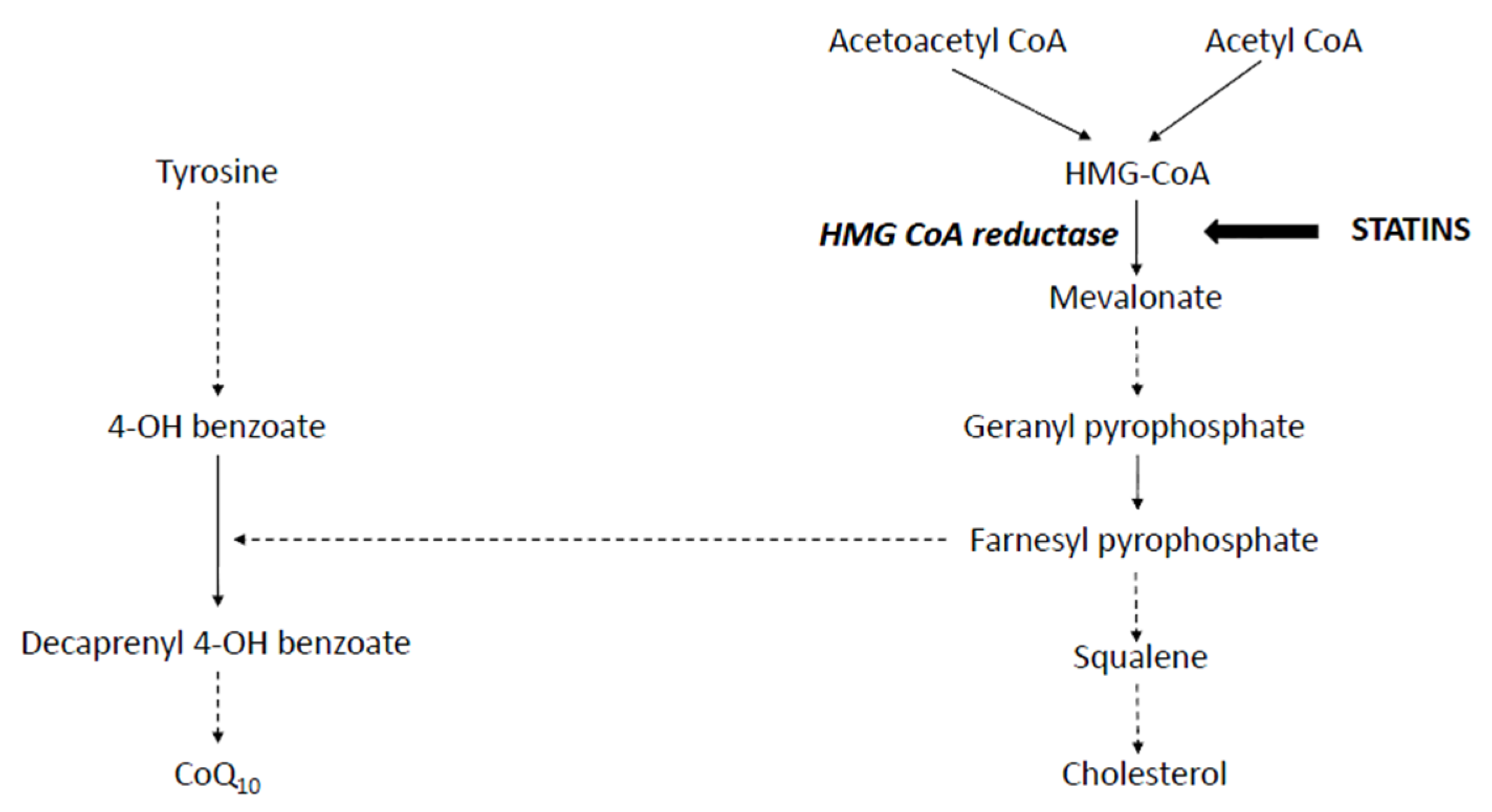
:1. Introduction
2. Statin-Associated Muscle Symptoms
2.1. SAMS Diagnosis
2.2. SAMS Classification
2.3. SAMS Pathobiology
2.3.1. Genetic Predisposition
2.3.2. Mitochondrial Dysfunction
Coenzyme Q10 Deficiency
Mitochondrial Depletion
Inhibition of Mitochondrial Respiratory Chain Complexes
Lactone Toxicity
Impaired Ca2+ Homeostasis
Substrate Overload
3. Statin-Induced Necrotising Inflammatory Myopathy
4. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| CVD | Cardiovascular disease |
| SAMS | Statin associated muscle disease |
| HMG-CoA | 2-Hydroxymethylglutaryl-coenzyme A |
| LDL | Low density lipoprotein |
| FH | Familial Hypercholesterolemia |
| CV | Cardiovascular |
| CK | Creatine Kinase |
| SINIM | Statin induced necrotising inflammatory myopathy |
| CoQ10 | Coenzyme Q10 |
| ATP | Adenosine triphosphate |
| PCSK9 | proprotein convertase subtilisin–kexin type 9 |
| IGF-1 | insulin like growth factor 1 |
| PGC1α | peroxisome proliferator activating receptor gamma co-activator 1 |
| ROS | reactive oxygen species |
| FPP | farnesyl pyrophosphate |
| RYR2 | ryanodine receptor polymorphisms |
References
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic and clinical studies. A Consensus Statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017. [CrossRef]
- Danesh, J.; Erqou, S.; Walker, M.; Thompson, S.G.; Tipping, R.; Ford, C.; Pressel, S.; Walldius, G.; Jungner, I.; Folsom, A.R.; et al. The emerging risk factors collaboration: Analysis of individual data on lipid, inflammatory and other markers in over 1.1 million participants in 104 prospective studies of cardiovascular diseases. Eur. J. Epidemiol. 2007, 22, 839–869. [Google Scholar] [PubMed]
- Steinberg, D. The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part IV: The 1984 coronary primary prevention trial ends it—Almost. J. Lipid. Res. 2006, 47, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, D. Thematic review series: The pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy, part V: The discovery of the statins and the end of the controversy. J. Lipid. Res. 2006, 47, 1339–1351. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; Simes, J.; et al. Efficacy and safety of more intensive lowering of LDL cholesterol: A meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [PubMed]
- Stein, E.A.; Bays, H.; O’Brien, D.; Pedicano, J.; Piper, E.; Spezzi, A. Lapaquistat acetate: Development of a squalene synthase inhibitor for the treatment of hypercholesterolemia. Circulation 2011, 123, 1974–1985. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Brugts, J.J.; Fleurence, R.; Ades, A.E. Dose-comparative effects of different statins on serum lipid levels: A network meta-analysis of 256,827 individuals in 181 randomized controlled trials. Eur. J. Prev. Cardiol. 2013, 20, 658–670. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Staffa, J.A.; Shatin, D.; Andrade, S.E.; Schech, S.D.; La, G.L.; Gurwitz, J.H.; Chan, K.A.; Goodman, M.J.; Platt, R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA 2004, 292, 2585–2590. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef] [PubMed]
- The Scandinavian Simvastatin Survival Study (4S) investigators. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The scandinavian simvastatin survival study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
- MRC/BHF Heart Protection Study Investigators. MRC/BHF heart protection study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: A randomised placebo-controlled trial. Lancet 2002, 360, 7–22. [Google Scholar]
- Gehring, P.J. The cataractogenic activity of chemical agents. CRC Crit. Rev. Toxicol. 1971, 1, 93–118. [Google Scholar] [CrossRef] [PubMed]
- Cash, J.; Callender, M.E.; McDougall, N.I.; Young, I.S.; Nicholls, D.P. Statin safety and chronic liver disease. Int. J. Clin. Pract. 2008, 62, 1831–1835. [Google Scholar] [CrossRef] [PubMed]
- Emberson, J.R.; Kearney, P.M.; Blackwell, L.; Newman, C.; Reith, C.; Bhala, N.; Holland, L.; Peto, R.; Keech, A.; Collins, R.; et al. Lack of effect of lowering LDL cholesterol on cancer: Meta-analysis of individual data from 175,000 people in 27 randomised trials of statin therapy. PLoS ONE 2012, 7, e29849. [Google Scholar]
- Collins, G.S.; Altman, D.G. Predicting the adverse risk of statin treatment: An independent and external validation of Qstatin risk scores in the UK. Heart 2012, 98, 1091–1097. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Keech, A.; Kearney, P.M.; Blackwell, L.; Buck, G.; Pollicino, C.; Kirby, A.; Sourjina, T.; Peto, R.; Collins, R.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [PubMed]
- Lessons from Lipitor and the broken blockbuster drug model. Lancet 2011, 378, 1976.
- Naci, H.; Brugts, J.; Ades, T. Comparative tolerability and harms of individual statins: A study-level network meta-analysis of 246,955 participants from 135 randomized, controlled trials. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; Macchi, C.; Morlotti, B.; Sirtori, C.R.; Ruscica, M. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur. J. Intern. Med. 2015, 26, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Alfirevic, A.; Neely, D.; Armitage, J.; Chinoy, H.; Cooper, R.G.; Laaksonen, R.; Carr, D.F.; Bloch, K.M.; Fahy, J.; Hanson, A.; et al. Phenotype standardization for statin-induced myotoxicity. Clin. Pharmacol. Ther. 2014, 96, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babu, S.; Li, Y. Statin induced necrotizing autoimmune myopathy. J. Neurol. Sci. 2015, 351, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, N.; Wierzbicki, A.S. Statin myopathy: Over-rated and under-treated? Curr. Opin. Cardiol. 2016, 31, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Michalska-Kasiczak, M.; Sahebkar, A.; Mikhailidis, D.P.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Kees Hovingh, G.; Farnier, M.; et al. Analysis of vitamin D levels in patients with and without statin-associated myalgia—A systematic review and meta-analysis of 7 studies with 2420 patients. Int. J. Cardiol. 2014, 178, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Khayznikov, M.; Hemachrandra, K.; Pandit, R.; Kumar, A.; Wang, P.; Glueck, C.J. Statin intolerance because of myalgia, myositis, myopathy, or myonecrosis can in most cases be safely resolved by vitamin D supplementation. N. Am. J. Med. Sci. 2015, 7, 86–93. [Google Scholar] [PubMed]
- Hou, T.; Li, Y.; Chen, W.; Heffner, R.R.; Vladutiu, G.D. Histopathologic and biochemical evidence for mitochondrial disease among 279 patients with severe statin myopathy. J. Neuromuscul. Dis. 2017, 4, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Baker, S.K.; Jacobson, T.A.; Kopecky, S.L.; Parker, B.A. An assessment by the statin muscle safety task force: 2014 update. J. Clin. Lipidol. 2014, 8, S58–S71. [Google Scholar] [CrossRef] [PubMed]
- Mancini, G.B.; Tashakkor, A.Y.; Baker, S.; Bergeron, J.; Fitchett, D.; Frohlich, J.; Genest, J.; Gupta, M.; Hegele, R.A.; Ng, D.S. Diagnosis, prevention, and management of statin adverse effects and intolerance: Canadian Working Group Consensus update. Can. J. Cardiol. 2013, 29, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Bruckert, E.; Hayem, G.; Dejager, S.; Yau, C.; Begaud, B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—The PRIMO study. Cardiovasc. Drugs Ther. 2005, 19, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Plutzky, J.; Skentzos, S.; Morrison, F.; Mar, P.; Shubina, M.; Turchin, A. Discontinuation of statins in routine care settings: A cohort study. Ann. Intern. Med. 2013, 158, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.A.; Capizzi, J.A.; Grimaldi, A.S.; Clarkson, P.M.; Cole, S.M.; Keadle, J.; Chipkin, S.; Pescatello, L.S.; Simpson, K.; White, C.M.; et al. Effect of statins on skeletal muscle function. Circulation 2013, 127, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Katz, D.H.; Intwala, S.S.; Stone, N.J. Addressing statin adverse effects in the clinic: The 5 Ms. J. Cardiovasc. Pharmacol. Ther. 2014, 19, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Brinton, E.A.; Ito, M.K.; Jacobson, T.A. Understanding Statin Use in America and Gaps in Patient Education (USAGE): An internet-based survey of 10,138 current and former statin users. J. Clin. Lipidol. 2012, 6, 208–215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banach, M.; Rizzo, M.; Toth, P.P.; Farnier, M.; Davidson, M.H.; Al-Rasadi, K.; Aronow, W.S.; Athyros, V.; Djuric, D.M.; Ezhov, M.V.; et al. Statin intolerance—An attempt at a unified definition. Position paper from an International Lipid Expert Panel. Expert Opin. Drug Saf. 2015, 14, 935–955. [Google Scholar] [CrossRef] [PubMed]
- Stroes, E.S.; Thompson, P.D.; Corsini, A.; Vladutiu, G.D.; Raal, F.J.; Ray, K.K.; Roden, M.; Stein, E.; Tokgozoglu, L.; Nordestgaard, B.G.; et al. Statin-associated muscle symptoms: Impact on statin therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur. Heart J. 2015, 36, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Canestaro, W.J.; Austin, M.A.; Thummel, K.E. Genetic factors affecting statin concentrations and subsequent myopathy: A HuGENet systematic review. Genet. Med. 2014, 16, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Mangravite, L.M.; Engelhardt, B.E.; Medina, M.W.; Smith, J.D.; Brown, C.D.; Chasman, D.I.; Mecham, B.H.; Howie, B.; Shim, H.; Naidoo, D.; et al. A statin-dependent QTL for GATM expression is associated with statin-induced myopathy. Nature 2013, 502, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Luzum, J.A.; Kitzmiller, J.P.; Isackson, P.J.; Ma, C.; Medina, M.W.; Dauki, A.M.; Mikulik, E.B.; Ochs-Balcom, H.M.; Vladutiu, G.D. GATM polymorphism associated with the risk for statin-induced myopathy does not replicate in case-control analysis of 715 dyslipidemic individuals. Cell Metab. 2015, 21, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Tay, S.K.; DiMauro, S.; Pang, A.Y.; Lai, P.S.; Yap, H.K. Myotoxicity of lipid-lowering agents in a teenager with MELAS mutation. Pediatr. Neurol. 2008, 39, 426–428. [Google Scholar] [CrossRef] [PubMed]
- Chariot, P.; Abadia, R.; Agnus, D.; Danan, C.; Charpentier, C.; Gherardi, R.K. Simvastatin-induced rhabdomyolysis followed by a MELAS syndrome. Am. J. Med. 1993, 94, 109–110. [Google Scholar] [CrossRef]
- Thomas, J.E.; Lee, N.; Thompson, P.D. Statins provoking MELAS syndrome. A case report. Eur. Neurol. 2007, 57, 232–235. [Google Scholar] [CrossRef] [PubMed]
- Vladutiu, G.D.; Simmons, Z.; Isackson, P.J.; Tarnopolsky, M.; Peltier, W.L.; Barboi, A.C.; Sripathi, N.; Wortmann, R.L.; Phillips, P.S. Genetic risk factors associated with lipid-lowering drug-induced myopathies. Muscle Nerve 2006, 34, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. Mitochondrial dysfunction and chronic disease: Treatment with natural supplements. Integr. Med. 2014, 13, 35–43. [Google Scholar]
- Du Souich, P.; Roederer, G.; Dufour, R. Myotoxicity of statins: Mechanism of action. Pharmacol. Ther. 2017, 175, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulou, M.; Corsini, A.; Roden, M. The role of mitochondria in statin-induced myopathy. Eur. J. Clin. Investig. 2015, 45, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Heales, S.J.; Mills, K.; Eaton, S.; Land, J.M.; Hargreaves, I.P. Determination of coenzyme Q10 status in blood mononuclear cells, skeletal muscle, and plasma by HPLC with di-propoxy-coenzyme Q10 as an internal standard. Clin. Chem. 2005, 51, 2380–2382. [Google Scholar] [CrossRef] [PubMed]
- Ericsson, J.; Dallner, G. Distribution, biosynthesis, and function of mevalonate pathway lipids. Subcell. Biochem. 1993, 21, 229–272. [Google Scholar] [PubMed]
- Desbats, M.A.; Lunardi, G.; Doimo, M.; Trevisson, E.; Salviati, L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ 10) deficiency. J. Inherit. Metab. Dis. 2015, 38, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Banach, M.; Serban, C.; Sahebkar, A.; Ursoniu, S.; Rysz, J.; Muntner, P.; Toth, P.P.; Jones, S.R.; Rizzo, M.; Glasser, S.P.; et al. Effects of coenzyme Q10 on statin-induced myopathy: A meta-analysis of randomized controlled trials. Mayo Clin. Proc. 2015, 90, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, P.; Mercier, J.; Lacampagne, A. New insights into mechanisms of statin-associated myotoxicity. Curr. Opin. Pharmacol. 2008, 8, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicki, A.S.; Poston, R.; Ferro, A. The lipid and non-lipid effects of statins. Pharmacol. Ther. 2003, 99, 95–112. [Google Scholar] [CrossRef]
- Littarru, G.P.; Langsjoen, P. Coenzyme Q10 and statins: Biochemical and clinical implications. Mitochondrion 2007, 7, S168–S174. [Google Scholar] [CrossRef] [PubMed]
- Paiva, H.; Thelen, K.M.; Van, C.R.; Smet, J.; De, P.B.; Mattila, K.M.; Laakso, J.; Lehtimaki, T.; von, B.K.; Lutjohann, D.; et al. High-dose statins and skeletal muscle metabolism in humans: A randomized, controlled trial. Clin. Pharmacol. Ther. 2005, 78, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Stride, N.; Hey-Mogensen, M.; Hansen, C.N.; Bang, L.E.; Bundgaard, H.; Nielsen, L.B.; Helge, J.W.; Dela, F. Simvastatin effects on skeletal muscle: Relation to decreased mitochondrial function and glucose intolerance. J. Am. Coll. Cardiol. 2013, 61, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, I.P.; Duncan, A.J.; Heales, S.J.; Land, J.M. The effect of HMG-CoA reductase inhibitors on coenzyme Q10, Possible biochemical/clinical implications. Drug Saf. 2005, 28, 659–676. [Google Scholar] [CrossRef] [PubMed]
- Avis, H.J.; Hargreaves, I.P.; Ruiter, J.P.; Land, J.M.; Wanders, R.J.; Wijburg, F.A. Rosuvastatin lowers coenzyme Q10 levels, but not mitochondrial adenosine triphosphate synthesis, in children with familial hypercholesterolemia. J. Pediatr. 2011, 158, 458–462. [Google Scholar] [CrossRef] [PubMed]
- Duncan, A.J.; Hargreaves, I.P.; Damian, M.S.; Land, J.M.; Heales, S.J. Decreased ubiquinone availability and impaired mitochondrial cytochrome oxidase activity associated with statin treatment. Toxicol. Mech. Methods 2009, 19, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Arnadottir, M.; Eriksson, L.O.; Thysell, H.; Karkas, J.D. Plasma concentration profiles of simvastatin 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase inhibitory activity in kidney transplant recipients with and without ciclosporin. Nephron 1993, 65, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Neuvonen, P.J.; Kantola, T.; Kivisto, K.T. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin. Pharmacol. Ther. 1998, 63, 332–341. [Google Scholar] [CrossRef]
- Rabar, S.; Harker, M.; O’Flynn, N.; Wierzbicki, A.S. Lipid modification and cardiovascular risk assessment for the primary and secondary prevention of cardiovascular disease: Summary of updated NICE guidance. BMJ 2014, 349, G4356. [Google Scholar] [CrossRef] [PubMed]
- Stringer, H.A.; Sohi, G.K.; Maguire, J.A.; Cote, H.C. Decreased skeletal muscle mitochondrial DNA in patients with statin-induced myopathy. J. Neurol. Sci. 2013, 325, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Schick, B.A.; Laaksonen, R.; Frohlich, J.J.; Paiva, H.; Lehtimaki, T.; Humphries, K.H.; Cote, H.C. Decreased skeletal muscle mitochondrial DNA in patients treated with high-dose simvastatin. Clin. Pharmacol. Ther. 2007, 81, 650–653. [Google Scholar] [CrossRef] [PubMed]
- Mullen, P.J.; Zahno, A.; Lindinger, P.; Maseneni, S.; Felser, A.; Krahenbuhl, S.; Brecht, K. Susceptibility to simvastatin-induced toxicity is partly determined by mitochondrial respiration and phosphorylation state of Akt. Biochim. Biophys. Acta 2011, 1813, 2079–2087. [Google Scholar] [CrossRef] [PubMed]
- Bonifacio, A.; Mullen, P.J.; Mityko, I.S.; Navegantes, L.C.; Bouitbir, J.; Krahenbuhl, S. Simvastatin induces mitochondrial dysfunction and increased atrogin-1 expression in H9c2 cardiomyocytes and mice in vivo. Arch. Toxicol. 2016, 90, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Charles, A.L.; Echaniz-Laguna, A.; Kindo, M.; Daussin, F.; Auwerx, J.; Piquard, F.; Geny, B.; Zoll, J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ’mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur. Heart J. 2012, 33, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, R.A.; Garcia-Smith, R.; Bisoffi, M.; Conn, C.A.; Trujillo, K.A. Ubiquinol rescues simvastatin-suppression of mitochondrial content, function and metabolism: Implications for statin-induced rhabdomyolysis. Eur. J. Pharmacol. 2013, 711, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sandri, M.; Lin, J.; Handschin, C.; Yang, W.; Arany, Z.P.; Lecker, S.H.; Goldberg, A.L.; Spiegelman, B.M. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl. Acad. Sci. USA 2006, 103, 16260–16265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, C.A.; Pol, D.; Zacharewicz, E.; Lee-Young, R.S.; Snow, R.J.; Russell, A.P.; McConell, G.K. Statin-induced increases in atrophy gene expression occur independently of changes in PGC1alpha protein and mitochondrial content. PLoS ONE 2015, 10, e0128398. [Google Scholar] [CrossRef] [PubMed]
- Westwood, F.R.; Bigley, A.; Randall, K.; Marsden, A.M.; Scott, R.C. Statin-induced muscle necrosis in the rat: Distribution, development, and fibre selectivity. Toxicol. Pathol. 2005, 33, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Westwood, F.R.; Scott, R.C.; Marsden, A.M.; Bigley, A.; Randall, K. Rosuvastatin: Characterization of induced myopathy in the rat. Toxicol. Pathol. 2008, 36, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Holden, C. Peering under the hood of Africa’s runners. Science 2004, 305, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.P.; Teshima, Y.; Akao, M.; Marban, E. Simvastatin attenuates oxidant-induced mitochondrial dysfunction in cardiac myocytes. Circ. Res. 2003, 93, 697–699. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Torok, M.; Zahno, A.; Waldhauser, K.M.; Brecht, K.; Krahenbuhl, S. Toxicity of statins on rat skeletal muscle mitochondria. Cell. Mol. Life Sci. 2006, 63, 2415–2425. [Google Scholar] [CrossRef] [PubMed]
- Sirvent, P.; Bordenave, S.; Vermaelen, M.; Roels, B.; Vassort, G.; Mercier, J.; Raynaud, E.; Lacampagne, A. Simvastatin induces impairment in skeletal muscle while heart is protected. Biochem. Biophys. Res. Commun. 2005, 338, 1426–1434. [Google Scholar] [CrossRef] [PubMed]
- Bjorkhem-Bergman, L.; Lindh, J.D.; Bergman, P. What is a relevant statin concentration in cell experiments claiming pleiotropic effects? Br. J. Clin. Pharmacol. 2011, 72, 164–165. [Google Scholar] [CrossRef] [PubMed]
- Phillips, P.S.; Haas, R.H.; Bannykh, S.; Hathaway, S.; Gray, N.L.; Kimura, B.J.; Vladutiu, G.D.; England, J.D. Statin-associated myopathy with normal creatine kinase levels. Ann. Intern. Med. 2002, 137, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Arenas, J.; Fernandez-Moreno, M.A.; Molina, J.A.; Fernandez, V.; del Hoyo, P.; Campos, Y.; Calvo, P.; Martin, M.A.; Garcia, A.; Moreno, T.; et al. Myoglobinuria and COX deficiency in a patient taking cerivastatin and gemfibrozil. Neurology 2003, 60, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Mans, R.A.; McMahon, L.L.; Li, L. Simvastatin-mediated enhancement of long-term potentiation is driven by farnesyl-pyrophosphate depletion and inhibition of farnesylation. Neuroscience 2012, 202, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, D.; Wassenaar, W. Rhabdomyolysis and cerivastatin: Was it a problem of dose? CMAJ 2002, 167, 737. [Google Scholar] [PubMed]
- Subramanian, R.; Fang, X.; Prueksaritanont, T. Structural characterization of in vivo rat glutathione adducts and a hydroxylated metabolite of simvastatin hydroxy acid. Drug Metab. Dispos. 2002, 30, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Schirris, T.J.; Renkema, G.H.; Ritschel, T.; Voermans, N.C.; Bilos, A.; van Engelen, B.G.; Brandt, U.; Koopman, W.J.; Beyrath, J.D.; Rodenburg, R.J.; et al. Statin-induced myopathy is associated with mitochondrial complex III inhibition. Cell Metab. 2015, 22, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Yada, T.; Kuriyama, M.; Osame, M. Cytosolic Ca2+ increase and cell damage in L6 rat myoblasts by HMG-CoA reductase inhibitors. Biochem. Biophys. Res. Commun. 1994, 202, 1579–1585. [Google Scholar] [CrossRef] [PubMed]
- Inoue, R.; Tanabe, M.; Kono, K.; Maruyama, K.; Ikemoto, T.; Endo, M. Ca2+-releasing effect of cerivastatin on the sarcoplasmic reticulum of mouse and rat skeletal muscle fibers. J. Pharmacol. Sci. 2003, 93, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Guis, S.; Figarella-Branger, D.; Mattei, J.P.; Nicoli, F.; Le Fur, Y.; Kozak-Ribbens, G.; Pellissier, J.F.; Cozzone, P.J.; Amabile, N.; Bendahan, D. In vivo and in vitro characterization of skeletal muscle metabolism in patients with statin-induced adverse effects. Arthrit. Rheum. 2006, 55, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Marciante, K.D.; Durda, J.P.; Heckbert, S.R.; Lumley, T.; Rice, K.; McKnight, B.; Totah, R.A.; Tamraz, B.; Kroetz, D.L.; Fukushima, H.; et al. Cerivastatin, genetic variants, and the risk of rhabdomyolysis. Pharmacogenet. Genom. 2011, 21, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Draeger, A.; Sanchez-Freire, V.; Monastyrskaya, K.; Hoppeler, H.; Mueller, M.; Breil, F.; Mohaupt, M.G.; Babiychuk, E.B. Statin therapy and the expression of genes that regulate calcium homeostasis and membrane repair in skeletal muscle. Am. J. Pathol. 2010, 177, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Hedenmalm, K.; Granberg, A.G.; Dahl, M.L. Statin-induced muscle toxicity and susceptibility to malignant hyperthermia and other muscle diseases: A population-based case-control study including 1st and 2nd degree relatives. Eur. J. Clin. Pharmacol. 2015, 71, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, J.E.; Constantin-Teodosiu, D.; Glaves, P.D.; Martin, E.A.; Davies, W.J.; Westwood, F.R.; Sidaway, J.E.; Greenhaff, P.L. Pharmacological activation of the pyruvate dehydrogenase complex reduces statin-mediated upregulation of FOXO gene targets and protects against statin myopathy in rodents. J. Physiol. 2012, 590, 6389–6402. [Google Scholar] [CrossRef] [PubMed]
- Hafizi Abu Bakar, M.; Kian Kai, C.; Wan Hassan, W.N.; Sarmidi, M.R.; Yaakob, H.; Zaman, H.H. Mitochondrial dysfunction as a central event for mechanisms underlying insulin resistance: The roles of long chain fatty acids. Diabetes Metab. Res. Rev. 2015, 31, 453–475. [Google Scholar] [CrossRef] [PubMed]
- Needham, M.; Fabian, V.; Knezevic, W.; Panegyres, P.; Zilko, P.; Mastaglia, F.L. Progressive myopathy with up-regulation of MHC-I associated with statin therapy. Neuromuscul. Disord. 2007, 17, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Grable-Esposito, P.; Katzberg, H.D.; Greenberg, S.A.; Srinivasan, J.; Katz, J.; Amato, A.A. Immune-mediated necrotizing myopathy associated with statins. Muscle Nerve 2010, 41, 185–190. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramachandran, R.; Wierzbicki, A.S. Statins, Muscle Disease and Mitochondria. J. Clin. Med. 2017, 6, 75. https://doi.org/10.3390/jcm6080075
Ramachandran R, Wierzbicki AS. Statins, Muscle Disease and Mitochondria. Journal of Clinical Medicine. 2017; 6(8):75. https://doi.org/10.3390/jcm6080075
Chicago/Turabian StyleRamachandran, Radha, and Anthony S. Wierzbicki. 2017. "Statins, Muscle Disease and Mitochondria" Journal of Clinical Medicine 6, no. 8: 75. https://doi.org/10.3390/jcm6080075




